Abstract
We investigated the effect of antagonists of growth hormone-releasing hormone (GHRH) MZ-J-7-138 and JV-1-92 on H460 human non-small cell lung carcinoma (NSCLC) xenografted orthotopically into nude mice. Treatment with MZ-J-7-138 or JV-1-92 inhibited orthotopic growth of H460 NSCLC by 52–65% (P < 0.001) and was associated with a significant decrease in protein expression of K-Ras, cyclooxygenase-2 (Cox-2) and phospho-Akt (pAkt). In other experiments, treatment with MZ-J-7-138 or docetaxel reduced tumor volume of s.c. xenografted H460 human NSCLC by 30–36% (P < 0.01). The combination of MZ-J-7-138 and docetaxel resulted in a synergistic growth inhibition of H460 NSCLC xenografts of 63%. MZ-J-7-138 alone or in combination with docetaxel significantly reduced protein levels of K-Ras, Cox-2, and pAkt by 56–63%. Docetaxel given singly diminished the protein levels only of Cox-2 and did not affect K-Ras and pAkt. High-affinity binding sites, mRNA, and protein expression of pituitary GHRH receptors and its splice variant (SV) 1 were found in H460. H460 NSCLC cells contained GHRH peptide, and its growth was significantly inhibited in vitro by 10 μM MZ-J-7-138 (P < 0.001). Serum insulin-like growth factor 1 (IGF1) was not reduced by either GHRH antagonists. These findings suggest that antiproliferative effects of GHRH antagonists in H460 NSCLC are associated with down-regulation of K-Ras, Cox-2, and pAkt. In conclusion, GHRH antagonists in combination with docetaxel synergistically inhibit growth of H460 NSCLC and the expression of K-ras, Cox-2, and pAkt, which might abrogate the signal transduction pathways for cell growth stimulation and therapeutic resistance.
Keywords: cyclooxygenase-2, K-Ras, non-small cell lung cancer, pAkt, chemotherapeutic substance
Lung carcinoma is the leading cause of cancer-related deaths in both men and women worldwide (1). Non-small cell lung carcinoma (NSCLC) represents 75–80% of all lung cancers. Recent studies have shown that modern chemotherapeutics may have reached the ceiling of their clinical efficacy because the 5-yr survival rate for NSCLC has leveled at 15% (2). Activation of prosurvival pathways mediated through mutant Ras and cyclooxygenase-2 (Cox-2), as well as Akt, might be one of the limiting factors in reducing the efficacy of chemotherapeutic drugs (3–5). Thus, targeting Ras, Cox-2, or Akt, which are frequently expressed in NSCLC tumors, is an appealing strategy in combined-modality treatment protocols that might improve the response to chemotherapy (5–7). In an endeavor to develop a new class of anticancer agents, antagonistic analogues of growth hormone-releasing hormone (GHRH) were synthesized in our institute (8). Experimental studies in vivo and in vitro demonstrated high efficacy of these antagonists in suppressing the proliferation of a wide variety of transformed human cancers, including NSCLC (8). We also showed that GHRH antagonists can inhibit tumor growth by indirect as well as direct mechanisms. The indirect mechanism operates through a suppression of the growth hormone release from the pituitary and the resulting inhibition of the production of insulin-like growth factor 1 (IGF1) in the liver (9). Direct effects of antagonists on growth of various cancers imply the presence of specific receptors for GHRH and GHRH antagonists on tumors (10). Recently, our group demonstrated that some human normal and malignant tissues, including NSCLC cell lines, express mRNA and protein for the human pituitary GHRH receptor (pGHRH-R) and its four truncated splice variants (SVs) (11–15). The physiological and pathophysiological significance of the coexpression of pGHRH-R and its SVs is not yet clear, but both are considered as potential targets for cancer therapy based on GHRH antagonists. Ras proteins (H-, N-, and K-Ras) are involved in many aspects of cell growth, mediating mitogenic and differentiation signals and apoptotic signals. K-Ras point mutations, which occur in 10–30% of lung adenocarcinomas, cause constitutive activation of the protein product p21ras, which results in an excessive activation of its downstream pathways mainly Raf/MEK/ERK1/2 and phosphatidylinositol 3-OH kinase (PI3K)/Akt, both being involved in proliferative and survival signals triggered by Ras (16, 17). Besides the up-regulation of K-Ras, recent evidence suggests a potential role of Cox-2 in the development of some lung cancers (18). Two isoforms of COX have been described: a constitutively expressed enzyme COX-1, present in most cell lines, and an inducible form, COX-2, expressed in response to cytokines, tumor promoters, and growth factors (19). Tumor cells with elevated COX-2 levels are highly angiogenic, invasive, suppressive of host immunity, and resistant to apoptosis, (20–25). Akt is a cytosolic signal transduction protein kinase that plays an important role in cell survival pathways (5). To date, three isoforms of Akt have been identified: Akt1, Akt2, and Akt3 (5). Induction of Akt activity is primarily dependent on the PI3K pathway. For full activation, Akt must be phosphorylated at two sites, one within the activation loop (T-308) and one within the C terminus (S-473) (5). In addition to activation by receptor tyrosine kinase (RTK), G protein-coupled receptors (GPCRs), and K-Ras, Akt can also be activated by many forms of cellular stress as can be observed under treatment with chemotherapeutic substances (5). Once active, Akt controls cellular functions such as apoptosis, cell cycle, gene transcription, and protein synthesis through the phosphorylation of downstream substrates (5). It has been shown that the activation of the pGHRH-R produces a phosphorylation of MAPK in a Ras-dependent manner (26). Thus, in our study, we tested the hypothesis whether the ability of GHRH antagonists to arrest growth of H460 NSCLC in an orthotopic lung model could also be intrinsically linked to the inhibition of oncogenic-ras and ras-dependent steps, including Cox-2 and Akt/phospho-Akt (pAkt). To test the rationale for a new combination treatment for NSCLC, we also investigated the effects of GHRH antagonists alone or in combination with docetaxel on the tumor growth and expression levels of K-Ras, Cox-2, and Akt/pAkt in H460 human NSCLC xenografts.
Results
Effect of GHRH Antagonists on the Orthotopic Growth of H460 Human NSCLC in Nude Mice.
To study the effect of GHRH antagonists against human NSCLC H460 tumors growing in an orthotopic environment, we used a model in which tumor cells were surgically introduced into the pulmonary interstitium. Treatment with GHRH antagonists MZ-J-7-138 at doses of 10 and 20 μg/day or JV-1-92 at a dose of 10 μg/day was initiated on day 4 after intrapulmonary injection of tumor cells and continued for a total of 21 days. Mice were killed under deep anesthesia, and the lungs were removed, checked for tumor growth, and weighed. In all animals, tumor growth could be observed (take rate 100%). Therapy with GHRH antagonists caused a significant reduction of the mean lung weight because of diminished tumor growth (Table 1). Daily treatment with MZ-J-7-138 at dosages of 10 or 20 μg/day significantly reduced the mean lung weights (P < 0.001) by 52% and 65%, respectively, as compared with those in control animals. JV-1-92 at a concentration of 10 μg/day reduced the mean lung weight by 56%. As can be seen in Fig. 1, untreated orthotopic H460 tumors (Fig. 1b) showed a different growth pattern compared with treated ones (Fig. 1c). Untreated tumors grew more extensively and metastasized more often to the contralateral side. Thus, in untreated animals, virtually no functional lung parenchyma was visible, leading to symptoms of dyspnea like elevated breathing rates. Although there were no significant differences in body weight among the groups before the start of treatment, control mice showed a significant (P < 0.001) reduction in body weight as compared with mice treated with 10 or 20 μg of MZ-J-7-138 or JV-1-92 (Table 1).
Table 1.
Effect of therapy with GHRH antagonists MZ-J-7-138 and JV-1-92 on the lung weight (lung block) and body weight of nude mice xenografted orthotopically with H460 human NSCLC
| Groups | Lung block, mg | % Inhibition vs. control | BW (initial), g | BW (final), g |
|---|---|---|---|---|
| Control | 850 ± 57 | 20.60 ± 0.74 | 18.61 ± 0.42 | |
| MZ-J-7-138 (10 μg) | 408 ± 80*** | 52 | 20.50 ± 0.51 | 20.63 ± 0.38*** |
| MZ-J-7-138 (20 μg) | 300 ± 53*** | 65 | 20.44 ± 0.52 | 21.69 ± 0.12*** |
| JV-1-92 (10 μg) | 371 ± 45*** | 56 | 20.62 ± 0.84 | 21.67 ± 0.25*** |
BW, body weight;
***, P < 0.001 vs. control.
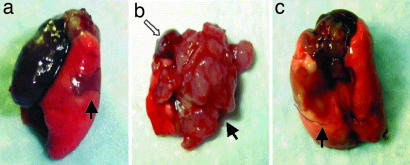
Fig. 1.
Macroscopic findings of lungs on day 4 after orthotopic implantation of H460 human NSCLC (a; view from left, lateral) and 21 days after therapy with GHRH antagonist MZ-J-7-138 given at a dose of 20 μg/day (c; view from dorsal) and untreated (b; view from fronto-basal). Tumors were marked each with filled arrows. As can be seen in b, untreated tumors grew extensively on the implantation side and metastasized to the contralateral right side displacing the heart (hollow arrow) further proximal.
Serum IGF1 and Intracellular GHRH Measurements with RIA.
Serum IGF1 levels were determined in control mice and those treated with GHRH antagonists. There was no significant decrease in serum IGF1 levels after treatment with 10 μg of MZ-J-7-138 (240.75 ± 14 ng/ml), although there was a tendency for lowering serum IGF1 levels under treatment with 20 μg of MZ-J-7-138 (208.3 ± 15 ng/ml) and 10 μg of JV-1-92 (207.8 ± 14 ng/ml) as compared with controls (234 ± 16 ng/ml). GHRH levels were measured in the cytosolic fractions of H460 cells. Significant amounts of GHRH were found (19.7–23.5 ng/mg protein). Medium without cells used as negative control did not contain any detectable levels of GHRH.
Analysis of pGHRH-R and GHRH-R SV1 to -4 and GHRH in Orthotopic H460 Xenografts.
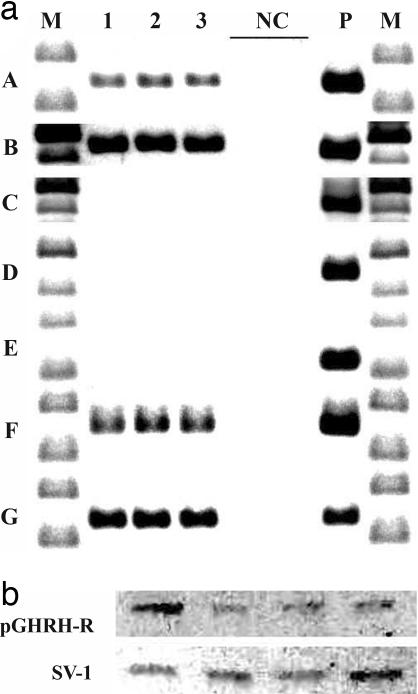
We used real-time PCR to amplify a product of 145, 523, 524, 245, 120, 150, and 140 bp for pGHRH-R, SV1, SV2, SV3, SV4, GHRH, and β-actin, respectively. For the detection of proteins of pGHRH-R (1:2,000) and SV1 to -4 (1:2,000), membranes were incubated with antisera raised and affinity-purified in our laboratory (27). As can be seen in Fig. 2a, we could amplify the mRNA for pGHRH-R and SV1 and the GHRH hormone in orthotopic xenografts of H460 and were able to detect the corresponding protein for pGHRH-R and SV1 at 40 kDa and 39 kDa in H460 NSCLC orthotopic tumors by Western blotting (Fig. 2b). In accordance with results obtained by RT-PCR and Western blotting, binding studies confirmed high-affinity binding sites for hGHRH analog [His1,Nle27]hGHRH(1–32)NH2 and GHRH antagonist JV-1-42 in the membrane preparations of H460 NSCLC tumors from the control groups (Kd = 6.80 ± 0.2 nM and Bmax = 104.0 ± 11.9 fmol/mg protein; and Kd = 1.51 ± 0.2 nM and Bmax = 319.7 ± 17.9 fmol/mg protein, respectively).
Fig. 2.
RT-PCR (a) and Western blot (b) analysis of pGHRH-R (aA) and SV1 (aB), SV2 (aC), SV3 (aD), SV4 (aE), GHRH (aF), and β-actin (aG) in representative samples of xenografted tumor tissue of H460 human NSCLC (lanes 1, 2, and 3) and normal human pituitary (P). DNA molecular weight marker is presented in lane M. Negative controls with no RNA in reverse transcription reaction and no cDNA in real-time PCR are shown in lane NC. In Fig. 2b all 4 lanes show representative H460 tumor samples
Effect of GHRH and GHRH Antagonist MZ-J-7-138 on the Proliferation of Human NSCLC H460 in Vitro.
H460 NSCLC cells cultured in vitro were exposed to various concentrations of GHRH(1–29) NH2 or GHRH antagonist MZ-J-7-138, and the effect on the proliferation was followed by crystal violet assay (data not shown). GHRH(1–29) NH2 at 0.1–10 μM did not affect the growth of H460 NSCLC cells in vitro. GHRH antagonist MZ-J-7-138 at a concentration of 10 μM significantly (P < 0.001) inhibited the proliferation of H460 cells by 41 ± 6% as compared with controls. Lower concentrations MZ-J-7-138 (0.1–1 μM) had no effect.
Effect of Antagonists of GHRH on the Expression of K-Ras, Cox-2, and Akt/pAkt, in Orthotopic Xenografts of H460.
Significant immunoreactive bands to polyclonal antibodies to Cox-2, Akt1/2, and pAkt (Ser-473) and monoclonal antibodies to Cox-2 and K-Ras were detected with specific antisera (Fig. 3). The bands were submitted to densitometric analyses and normalized to β-actin levels. Levels of expression of these proteins are shown as a percentage of control (Table 2). Controls were accepted as 100%. A significant decrease in the expression of K-Ras (43–78%), Cox-2 (27–68%), and pAkt (48–68%) was observed in orthotopic xenografts of H460 NSCLC after treatment with antagonists of GHRH MZ-J-7-138 and JV-1-92 as compared with control groups. No significant inhibitory effect on the expression of Akt1/2 protein was detected after treatment with GHRH antagonists.
Fig. 3.
Western blot for protein expression of K-Ras, Cox-2, Akt1/2, and pAkt (S-473) in orthotopic xenografts of H460 human NSCLC after treatment with GHRH antagonists MZ-J-7-138 (10 or 20 μg/day), JV-1-92 (10 μg/day), or untreated. Four representative tumors from each group are shown, and the experiments were repeated at least three times. Protein levels were normalized to β-actin protein (42 kDa) and are expressed as percentage of control values as shown in Table 2.
Table 2.
Protein levels of K-Ras, Cox-2, Akt1/2, and pAkt in orthotopic xenografts of H460 human NSCLC after treatment with GHRH antagonists MZ-J-7-138 and JV-1-92
| Protein levels (controls accepted as 100%), % |
|||
|---|---|---|---|
| MZ-J-7-138 (10 μg) | MZ-J-7-138 (20 μg) | JV-1-92 (10 μg) | |
| K-Ras | 57 ± 13** | 27 ± 8*** | 22 ± 10*** |
| Cox-2 | 73 ± 9* | 32 ± 2*** | 42 ± 12*** |
| Akt1/2 | 83 ± 14 | 80 ± 12 | 105 ± 16 |
| pAkt | 50 ± 12** | 52 ± 11** | 32 ± 7*** |
Values are means ± SE. Protein levels of 5–8 tumors in each group were quantified by densitometric analysis, and the data were normalized to β -actin values.
*, P < 0.05;
**, P < 0.01;
***, P < 0.001 vs. controls.
Effect of GHRH Antagonist MZ-J-7-138, Docetaxel, and Their Combination on Growth of H460 Human NSCLC Tumors Xenografted s.c. into Nude Mice.
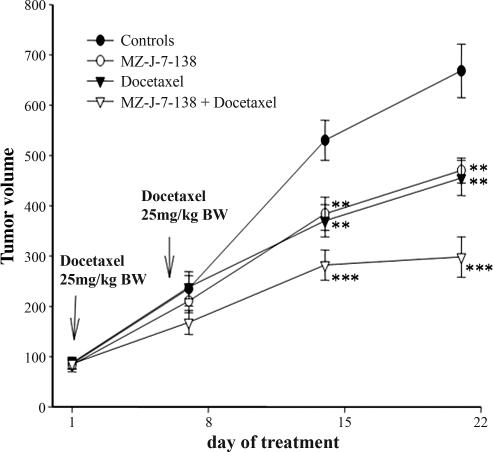
The efficacy of MZ-J-7-138 and docetaxel was assessed in H460 tumors xenografted s.c. into nude mice. Daily treatment with 10 μg of MZ-J-7-138 or docetaxel at a dose of 25 mg/kg body weight (BW) administered i.p. on day 1 and 6, significantly (P < 0.001) inhibited the growth of H460 xenografts (Fig. 4). MZ-J-7-138 significantly decreased tumor volume and tumor weight by 30%, whereas docetaxel reduced tumor volume by 36% and tumor weight by 26% (Table 3). Both compounds, MZ-J-7-138 (P < 0.05) and docetaxel (P < 0.01), significantly prolonged tumor volume doubling time (Table 3). Combined treatment with MZ-J-7-138 and docetaxel caused a highly significant (P < 0.001) tumor inhibition after 14 days, followed by tumor stasis during the third week of treatment (P < 0.001) (Fig. 4). Thus, 21 days after starting the treatment, the combination reduced the tumor volume of H460 xenografts by 63% and tumor weight by 49%. Tumor doubling time was also significantly prolonged from 6.51 to 15.49 days in tumors, which had been treated with the combination of MZ-J-7-138 and docetaxel. At the end of both experiments, no significant differences in body weights or weights of various organs were observed (data not shown).
Fig. 4.
Effect of treatment with GHRH antagonist MZ-J-7-138 given s.c. at a dose of 10 μg/day, docetaxel given i.p. at a concentration of 25 mg/kg BW at day 1 and 6, or the combination of MZ-J-7-138 with docetaxel on the tumor volume of H460 human NSCLC xenografted s.c. into nude mice. Vertical bars indicate SE. ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. control.
Table 3.
Effect of therapy with GHRH antagonist MZ-J-7-138, docetaxel, and its combination on the growth of H460 human NSCLC xenografted s.c. into nude mice
| Groups | Tumor weight, g (% inhibition) | Tumor volume, mm3 (% inhibition) | Tumor volume doubling time, days | |
|---|---|---|---|---|
| Initial | Final | |||
| Control | 1,166 ± 60 | 85 ± 10 | 668 ± 53 | 6.51 ± 0.43 |
| MZ-J-7-138 (10 μg) | 823 ± 50*** (30) | 83 ± 14 | 470 ± 25** (30) | 11.45 ± 0.42* |
| Docetaxel2× 25 mg/kg BW | 871 ± 65** (25) | 87 ± 12 | 455 ± 35** (32) | 12.10 ± 0.35** |
| MZ-J-7-138 + docetaxel | 596 ± 40*** (49) | 86 ± 11 | 298 ± 40*** (65) | 15.49 ± 2.1*** |
Values show the mean ± SE.
*, P < 0.05;
**, P < 0.01;
***, P < 0.001 vs. control (one-way ANOVA test).
Effect of GHRH Antagonist MZ-J-7-138, docetaxel and Its Combination on the Expression of K-Ras, Cox-2, Akt1/2, and pAkt in H460 NCSLC Xenografted s.c. into Nude Mice.
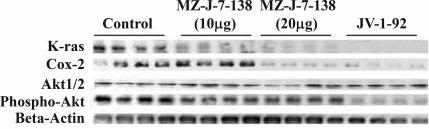
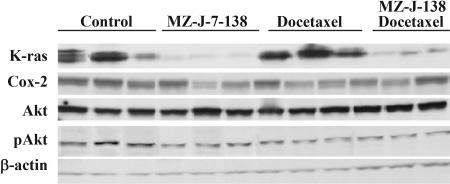
Protein expression of K-Ras, Cox-2, Akt1/2, and pAkt was assessed by Western blot analysis in H460 NSCLC xenografts (Fig. 5). The levels of expression of these proteins are shown as a percentage of control (Table 4), the controls being accepted as 100%. The treatment with MZ-J-7-138 at a dose of 10 μg/day reduced expression of K-Ras by 63% (P < 0.001), whereas docetaxel alone given at a dose of 25 mg/kg BW i.p at day 1 and 6 left K-Ras expression unaffected. Combined treatment reduced protein levels of K-Ras up to 72% as compared with controls. Therapy with MZ-J-7-138 or docetaxel as single agents diminished protein levels of Cox-2 by 56% and 29%, respectively. Protein expression of Cox-2 was down-regulated by 60% when both substances were combined. MZ-J-7-138 and docetaxel did not alter expression of Akt1/2 when given alone, whereas combination treatment caused a significant decrease of Akt1/2 up to 40%. pAkt was lowered by MZ-J-7-138 by 58%, whereas docetaxel had no significant effect on phosphorylated Akt (P = 0.236). Combination of docetaxel with MZ-J-7-138 again reduced pAkt up to 49%.
Fig. 5.
Western blot analysis of protein expression of K-Ras, Cox-2, Akt1/2, and pAkt in H460 human NSCLC xenografts after treatment with MZ-J-7-138 given s.c. at a dose of 10 μg/day, docetaxel given i.p. at a concentration of 25 mg/kg BW at day 1 and 6, or its combination. Three representative tumors from each group are shown, and the experiments were repeated at least three times.
Table 4.
Effect of treatment with GHRH antagonists MZ-J-7-138, docetaxel, and its combination on protein levels of K-Ras, Cox-2, Akt1/2, and pAkt in tumors of H460 human NSCLC xenografted s.c. into nude mice
| Protein levels (Controls accepted as 100%), % |
|||
|---|---|---|---|
| MZ-J-7-138 | Docetaxel | MZ-J-7-138 + docetaxel | |
| K-Ras | 27 ± 5*** | 80 ± 9 | 28 ± 4*** |
| Cox-2 | 44 ± 4*** | 71 ± 6*** | 40 ± 2*** |
| Akt1/2 | 99 ± 4 | 90 ± 8 | 60 ± 2*** |
| pAkt | 42 ± 8*** | 76 ± 13 | 51 ± 3*** |
Values are means ± SE. Protein levels of three tumors in each group were quantified by densitometric analysis, and the data were normalized to β -actin values.
***, P < 0.001 vs. controls.
Discussion
Although progress has been made in the past 10 yr in the management of patients with NSCLC, the 5-yr relative survival rates have not improved substantially (2). A new approach to the therapy of lung cancer is mandatory.
GHRH antagonists have already been shown to be effective in vivo against human NSCLC and a wide range of other human tumors xenografted s.c. into nude mice (8). Nevertheless, organ microenvironment influences the phenotype of tumor cells and is essential for optimal growth of tumors, as originally enunciated by Paget's “seed and soil” hypothesis and confirmed by others (28, 29). Consequently, we wanted to demonstrate the effect of GHRH antagonists against human NSCLC H460 in the clinically more relevant orthotopic model. We selected the H460 NSCLC cell line because, after xenografting into nude mice, these cells form rapidly growing tumors and express mutant K-Ras as well as Cox-2 and Akt (4). After 3 weeks of treatment with GHRH antagonists, the mean lung weights of animals treated with MZ-J-7-138 or JV-1-92 were significantly reduced up to >65% as compared with those of the controls. In accordance with this reduction in lung weight, which was due to inhibition of tumor cell growth, treated animals showed a dramatically better performance status with lack of weight loss or symptoms of dyspnea like elevated breathing rates. The indirect mechanism of action of GHRH antagonists, which is based on the suppression of the pituitary GH-hepatic IGF1 axis, could not be observed in our setting because we could not find any decreases in serum IGF1 levels in animals treated with GHRH antagonists. Nevertheless, our findings from RT-PCR, Western blot, and binding assays showed that H460 cells express receptors for both the pGHRH-R as well as the splice variant SV1. Thus, the main action of GHRH antagonists MZ-J-7-138 and JV-1-92 used in our study is probably exerted directly on receptors for GHRH on tumoral cells. H460 NSCLC expresses and produces GHRH, as could be observed in our study by real-time PCR and RIA. Administration of GHRH antagonist MZ-J-7-138 at a dose of 10 μM also inhibited proliferation of H460 NSCLC cells in vitro. Although we could not show the stimulation of H460 cells by GHRH(1–29)NH2 in vitro, GHRH seems to fulfill some of the parameters for an autocrine growth factor in human H460 NSCLC xenografts. The lack of stimulation by exogenous GHRH in our in vitro study might be explained by the fact that H460 cells may already produce GHRH at levels that can cause maximal stimulation of this cell line in vitro. It is also possible that other peptides from the vasoactive intestinal peptide (VIP) family that show high homology to GHRH and that were present in the serum-containing media activated GHRH-receptors, preventing a further response to exogenous GHRH.
The aim of new therapy is selectively to affect the pathways deregulated in tumor cells. Several genetic alterations have been recognized to play a role in the development and promotion of lung cancer, such as gene mutations of the ras gene or overexpression of Cox-2 and constitutively activated Akt (4, 6). It has been shown in CHO-4 cells transfected with the pGHRH-R that GHRH leads to an activation of MAP kinases in a Ras-dependent manner (26). Thus, we evaluated the protein expression of K-Ras p21ras by Western blotting. We could show that treatment with GHRH antagonists decreased p21ras in a dose-dependent fashion up to 77% compared with controls. It has been demonstrated that loss of mutant K-Ras expression abolishes tumor formation in athymic nude mice (30). A recent study also reported that GHRH stimulates proliferation of MDA-MB-231 breast cancer cells in a dose-dependent manner, that the activation of MAPK is necessary for this effect, and that the signaling by GHRH to MAPK occurs through Ras and Raf proteins (31). Thus, the activation of GHRHR operates through a pathway that requires phosphorylation of Ras, Raf, and MAPK (31). Metaanalysis of recent literature showed that Ras gene alteration and/or protein overexpression is a poor prognostic factor for survival of patients with NSCLC in univariate analysis (32). Thus, the ras family of genes has been identified as potential targets for therapeutic intervention with farnesyltransferase inhibitors (FTIs) (33). A recent phase II study reported clinical activity of the FTI lonafarnib in combination with paclitaxel on patients with taxane-refractory/resistant metastatic NSCLC (6). Inhibition of K-ras activity was shown to enhance radiosensitivity (34) and probably could overcome resistance to treatment with single-agent EGF Receptor (EGFR) inhibitors because patients with K-Ras mutations showed poorer clinical outcomes when treated with EGFR-inhibitor erlotinib and chemotherapy than patients lacking oncogenic ras (35).
Targeting Cox-2, a marker of poor prognosis in stage I NSCLC, is also promising in therapy of NSCLC (36). Thus, Cox-2 inhibition by selective Cox-2 inhibitors enhanced the response to chemotherapeutic regimens and led to a prolonged progression-free survival when used in combination with radiotherapy (7, 37). As can be seen in Table 2, treatment of orthotopic xenografts of H460 NSCLC with GHRH antagonists led to a significant dose-dependent down-regulation of the protein expression of Cox-2 up to 68%. A possible downstream pathway involved in this negative regulation of Cox-2 by GHRH antagonists could be PKC. PKC was shown to enhance Cox-2 expression in a Ras-dependent manner and could be inhibited by GHRH antagonists (38, 39). Decreased Cox-2 levels could also be due to down-regulation of K-Ras because this oncogene previously has been implicated in the positive regulation of Cox-2 (40).
Multiple growth factors such as IGF1-, VEGF-, and EGF-related growth factors contribute to the development and progression of NSCLC by activating pathways leading to cell survival and therapeutic resistance. The identification and abrogation of the activity of those kinases would then be effective, acting at the point of convergence of growth factor-induced cell signaling. Akt is probably the best-characterized kinase known to promote cellular survival downstream of growth factor activation. Among the multiple inductors of the phosphatidylinositol 3-OH kinase (PI3K)/Akt pathway are receptor tyrosine kinases (RTKs) or G protein-coupled receptors (GPCRs) and oncogenic ras (5). We reported previously that GHRH antagonist treatment was followed by a down-regulation of tyrosine kinase receptors as IGF1 receptor, VEGF receptors, and EGF receptors in lung carcinomas, prostate cancer, and glioblastomas (11, 41–43). Thus, the negative regulation of RTKs, as well as impaired levels of K-Ras, might contribute to the decrease in pAkt levels of up to 68% after treatment with GHRH antagonists, as could be seen in our study.
It has been shown that mutant Ras, Cox-2, and activated Akt contribute to tumor cell survival and resistance to chemotherapeutic agents (3, 4, 5). Furthermore, it was reported that chemotherapy increases Cox-2 and Akt activity (5, 44, 45). Thus, we wanted to elucidate whether a combination of GHRH with chemotherapeutic agent docetaxel would alter docetaxel-induced growth inhibition and protein expression of K-Ras, Cox-2, and Akt/pAkt in s.c. xenografts of H460 NSCLC. As can be seen in Fig. 4, the combination of GHRH antagonist MZ-J-7-138 with docetaxel synergistically augmented inhibition of NSCLC tumors and led to a significant down-regulation in the expression levels of K-Ras, Cox-2, pAkt, and Akt1/2 (Fig. 5 and Table 4). An increase in the protein expression of Cox-2 and pAkt, reported for treatment with taxanes (5, 44, 45), was not seen in our study. This discrepancy might be explained by the fact that increases in Cox-2 or pAkt might be only transient and could not be observed in this setting because docetaxel was given only in the first week of treatment.
In conclusion, this study demonstrates that growth inhibition of orthotopic H460 NSCLC by GHRH antagonists is associated with a marked down-regulation in the protein expression of K-Ras, Cox-2, and pAkt. Furthermore, a combination of GHRH antagonist MZ-J-7-138 with docetaxel was demonstrated to produce a synergistic growth inhibition of H460 human NSCLC xenografts and was similarly accompanied by a marked decrease in the protein expression of K-Ras, Cox-2, and pAkt. Thus, a combination of GHRH antagonists with current chemotherapeutic regimens could improve the management of lung cancers.
Materials and Methods
Peptides and Chemicals.
GHRH(1–29) and GHRH antagonists MZ-J-7-138 and JV-1-92 were synthesized in our laboratory by solid-phase methods (46–48). Docetaxel was purchased from ChonTech (Waterford, CT). Matrigel (phenol red-free) was obtained from Sigma (St. Louis, MO). For daily injection, GHRH antagonists were dissolved in 0.1% DMSO in 10% aqueous propylene glycol solution (vehicle solution).
Cell Line, Animals, and Cancer Xenografts.
The NCI-H460 human NSCL cell line obtained from American Type Culture Collection (ATCC) was grown in RPMI medium 1640 supplemented with 1 μM sodium pyruvate. Trypan blue staining was used to assess cell viability, and only single-cell suspensions of viability >90% were used for the intrapulmonary injection.
Five- to six-week-old female athymic nude mice (Ncr nu/nu) were obtained from the National Cancer Institute (NCI, Bethesda, MD). The animals were housed in laminar air-flow cabinets under pathogen-free conditions with a 12-hr light/12-hr dark schedule, and fed autoclaved standard chow and water ad libitum.
The surgery procedure for the orthotopic xenograft model as reported by Doki et al. (49) was used with modification. Under deep anesthesia with isoflourane, a 5-mm skin incision in the left chest was made ≈4 mm (tail side) from the scapula. Fat and muscle were separated from costal bones. On observing left lung motion through the pleura, a 27-gauge needle attached to a 50-μl Hamilton syringe was directly inserted through the sixth intercostal space into the lung to a depth of 3 mm. Human NCI-H460 cells (5 × 105), suspended in 20 μl of HBSS containing Matrigel (1:1 vol/vol), were injected into the lung parenchyma. After injection, a cotton-tipped applicator was pressed on the injection site to stop any bleeding, and the skin incision was closed with a surgical skin clip. A pilot study showed that all animals (n = 20) developed lung cancer with macroscopically visible tumor nodes within 4 days after intrapulmonary injection (Fig. 1a). Thus, 4 days after transplantation of tumors, mice were randomized and treated s.c. with vehicle, MZ-J-7-138 (10 μg/day per animal), MZ-J-7-138 (20 μg/day per animal), and JV-1-92 (at a dose of 10 μg/day per animal). Body weight was measured weekly. There was no significant intergroup difference in body weight at the beginning of the treatment as can be seen in Table 1. After 21 days of treatment when mice started to become moribund with symptoms of weight loss (>20% of initial weight) and elevated breathing rates, they were killed under deep anesthesia by exsanguination through the vena cava inferior. After removal of the heart, the whole lung block was weighed. Half of the macroscopical tumor was fixed in 10% buffered formalin for histological examination; the other one was snap-frozen and stored at −70°C until further analyses. Histopathological examination of each specimen was undertaken to confirm the presence of cancer with minimal admixed nonmalignant tissue (<20%) before Western blotting studies.
Combination studies with docetaxel were exerted in a s.c. xenograft model, which was initiated as described (50). When tumors reached a volume of ≈85 mm3, mice were randomized into the following treatment groups (n = 10): group 1, control, vehicle injections; group 2, s.c. treatment with MZ-J-7-138 at a dose of 10 μg/day; group 3, on day 1 and 6 single i.p. administration of docetaxel (25 mg/kg BW); and group 4, combination of GHRH antagonist MZ-J-7-138 and docetaxel (at the same dosages and frequencies of each as alone). Tumor volume and body weight were measured weekly. Three weeks after start of treatment, mice were killed under anesthesia and necropsy was performed. Tumors and organs were removed and weighed. Tumor volume and tumor volume doubling time between the start and the end of the treatment were calculated as described (51, 52). All experiments were reviewed by the Veterans Affairs Medical Center's institutional animal care and use committee and were performed in accordance with institutional guidelines for animal care.
RIA for Serum IGF1 and Intracellular GHRH.
The methods used for determination of IGF1 serum levels and detection of intracellular GHRH have been described in detail (53, 54).
Total RNA Extraction and Real-Time PCR.
Total RNA extraction, isolation, and amplification of the cDNAs for human pGHRH-R; SV1, SV2, SV3, and SV4 of GHRH-R; GHRH hormone; and β-actin were performed as described (15).
Radioligand-Binding Studies.
Radioiodinated derivatives of GHRH antagonist JV-1-42 and hGHRH analog [His1, Nle27]hGHRH(1–32)NH2 were prepared by the chloramine-T method as described (13, 54). Preparation of membrane fractions from xenografted tumor samples was performed as reported (13, 54). Binding characteristics of receptors for GHRH were determined by in vitro ligand competition assays based on the binding of radiolabeled JV-1-42 and [His1,Nle27]hGHRH(1–32)NH2 to tumor membrane fractions (13, 54). Binding affinities (Kd) and capacities (Bmax) were calculated by the LIGAND-PC computerized curve-fitting software and by Scatchard analysis (13, 54).
Western Blotting Assays.
H460 tumors (orthotopic and s.c. xenografts) were homogenized, and 20 μg of protein from each sample were separated by 7.5–15% SDS/PAGE Tris·HCl Criterion Precasted Gels (Bio-Rad, Hercules, CA), depending on the molecular weight of the selected protein. The membranes were incubated for 3–5 h at room temperature in 5% nonfat dry milk in TBS-Tween, followed by incubation with the specific polyclonal antisera (1:1,000) to Cox-2 (C-20), Akt1/2 (N-19), and pAkt (Ser-473), and monoclonal antibodies (1:1,000) to Cox-2 (29) and K-Ras (F234). All antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), except the pAkt (Ser-473), which was purchased from Cell Signaling Technology (Danvers, MA). For detection of pGHRH-R (1:2,000) and SV1 (1:2,000), membranes were incubated with antisera raised and affinity-purified in our laboratory (27). The blots were probed at 4°C overnight with the specific antisera, and the signal for the immunoreactive proteins was developed with peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) and visualized by exposure to the chemiluminescence substrate (Amersham Pharmacia Bioscience, Piscataway, NJ). The protein bands were quantified by normalizing the signals of different proteins to β-actin signal (1:2,000; Santa Cruz Biotechnology) by using the Kodak (Rochester, NY) EDAS 290 imaging system with Kodak 1D Image Analysis Software.
In Vitro Studies.
The effect of GHRH and MZ-J-7-138 on the growth of human H460 cells cultured in vitro was evaluated by crystal violet assay as described (50).
Statistical Analyses.
The SigmaStat Software (Jandel Scientific, San Raphael, CA) was used for the statistical analysis of data. Results are presented as means ± SE and were evaluated by a one-way ANOVA test.
Abbreviations
- NSCLC
non-small cell lung carcinoma
- GHRH
growth hormone-releasing hormone
- pGHRH-R
pituitary growth hormone-releasing hormone receptor
- SV
splice variant
- Cox-2
cyclooxygenase-2
- IGF1
insulin-like growth factor 1
- BW
body weight
- pAkt
phospho-Akt.
Footnotes
Conflict of interest statement: Tulane University has applied for a patent on the GHRH antagonists listed in this article, and J.L.V. and A.V.S. are coinventors on that patent.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Subbaramaiah K, Hart JC, Norton L. J Biol Chem. 2000;275:14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 4.Brognard J, Clark AS, Ni Y, Dennis PA. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 5.West KA, Castillo SS, Dennis PA. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim ES, Kies MS, Fossella FV, Glisson BS, Khuri FR. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 7.Altorki NK, Keresztes RS, Port JL. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 8.Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 9.Pinski J, Schally AV, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 10.Kineman RD. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanashiro CA, Schally AV, Groot K, Armatis P, Bernardino ALF, Varga JL. Proc Natl Acad Sci USA. 2003;100:15836–15841. doi: 10.1073/pnas.2536558100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halmos G, Schally AV, Varga JL, Plonowski A, Rekasi Z, Czompoly T. Proc Natl Acad Sci USA. 2000;97:10555–10560. doi: 10.1073/pnas.180313097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z. J Clin Endocrinol Metab. 2002;87:4707–4714. doi: 10.1210/jc.2002-020347. [DOI] [PubMed] [Google Scholar]
- 14.Rekasi Z, Czompoly T, Schally AV, Halmos G. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Köster F, Kovitz K, Groot K, et al. Proc Natl Acad Sci USA. 2005;202:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moodie SA, Willumsem BM, Weber MJ, Wolfman A. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Viciana P, Warne PH, Dhand R. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 18.Brown JR, Dubois RN. Clin Cancer Res. 2004;10:4266–4269. doi: 10.1158/1078-0432.CCR-040014. [DOI] [PubMed] [Google Scholar]
- 19.Koki A, Khan NK, Woerner BM. Adv Exp Med Biol. 2002;507:177–184. doi: 10.1007/978-1-4615-0193-0_28. [DOI] [PubMed] [Google Scholar]
- 20.Tsujii M, DuBois RN. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 21.Uefuji K, Ichikura T, Mochizuki H. Clin Cancer Res. 2000;6:135–138. [PubMed] [Google Scholar]
- 22.Dohadwala M, Luo J, Zhu L. 27. 2001;6:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohadwala M, Batra RK, Luo J. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolina M, Sharma S, Lin Y. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 25.Datta SR, Brunet A, Greenberg ME. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 26.Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Endocrinology. 2000;141:2113–2119. doi: 10.1210/endo.141.6.7513. [DOI] [PubMed] [Google Scholar]
- 27.Toller GL, Horvath JE, Schally AV, Halmos G, Varga JL, Groot K, Chism D, Zarandi M. Proc Natl Acad Sci USA. 2004;101:15160–15165. doi: 10.1073/pnas.0406348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paget S. Lancet. 1889;I:571–573. [Google Scholar]
- 29.Wilmanns C, Fan D, O'Brian DA, Bucana CD, Fidler IJ. Int J Cancer. 1992;52:98–104. doi: 10.1002/ijc.2910520118. [DOI] [PubMed] [Google Scholar]
- 30.Baba I, Shirasawa S, Iwamoto R. Cancer Res. 2000;60:6886–6889. [PubMed] [Google Scholar]
- 31.Siriwardana G, Bradford A, Coy D, Zeitler P. Mol Endocrinol. 2006 Apr 13; doi: 10.1210/me.2005-0001. 10.1210/me.2005–0001. [DOI] [PubMed] [Google Scholar]
- 32.Mascaux C, Iannino N, Martin B, Sculier JP. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BE, Heymach JV. Clin Cancer Res. 2004;10:4254–4256. [Google Scholar]
- 34.Brunner TB, Gupta AK, Shi Y, Hahn SM, Muschel RJ, Mckenna WG, Bernhard EJ. Int J Radiat Biol. 2003;79:569–576. doi: 10.1080/09553000310001610196. [DOI] [PubMed] [Google Scholar]
- 35.Eberhard DA, Johnson BE, Hillan KJ. J Clin Oncol. 2005;23:1–14. [Google Scholar]
- 36.Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Xu XC. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 37.Liao Z, Koaki R, Milas L, Cox JD. Clin Cancer Res. 2005;11:3342–3348. doi: 10.1158/1078-0432.CCR-04-1741. [DOI] [PubMed] [Google Scholar]
- 38.Chang MS, Chen BC, Yu MT, Lin CH. Cell Signalling. 2005;17:299–310. doi: 10.1016/j.cellsig.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Kanashiro CA, Schally AV, Zarandi M, Hammann BD, Varga JL. Int J Cancer. 2004;112:570–576. doi: 10.1002/ijc.20437. [DOI] [PubMed] [Google Scholar]
- 40.Van Putten V, Refaat Z, Dessev C, Nemenoff RA. J Biol Chem. 2001;276:1226–1232. doi: 10.1074/jbc.M003581200. [DOI] [PubMed] [Google Scholar]
- 41.Kiaris H, Schally AV, Varga JL. Neoplasia. 2000;2:242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanashiro CA, Schally AV, Varga JL, Hammann BD, Halmos G, Zarandi M. Cancer Lett. 2005;226:123–131. doi: 10.1016/j.canlet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Stangelberger A, Schally AV, Varga JL, Hammann BD, Groot K, Halmos G, Cai RZ, Zarandi M. Prostate. 2005;64:303–315. doi: 10.1002/pros.20262. [DOI] [PubMed] [Google Scholar]
- 44.Altorki NK, Port JL, Zhang F, Dannenberg AJ. Clin Cancer Res. 2005;11:4191–4197. doi: 10.1158/1078-0432.CCR-05-0108. [DOI] [PubMed] [Google Scholar]
- 45.Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. J Biol Chem. 2003;278:37637–37647. doi: 10.1074/jbc.M301481200. [DOI] [PubMed] [Google Scholar]
- 46.Varga JL, Schally AV, Csernus VJ, Zarandi M, Halmos G, Rekasi Z. Proc Natl Acad Sci USA. 1999;96:692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varga JL, Schally AV, Horvath JE, Kovacs M, Halmos G, Groot K, Toller GL, Rekasi Z, Zarandi M. Proc Natl Acad Sci USA. 2004;101:1708–1713. doi: 10.1073/pnas.0307288101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarandi M, Kovacs M, Horvath J, Toth K, Halmos G, Groot K, Nagy A, Kele Z, Schally AV. Peptides. 1997;18:423–430. doi: 10.1016/s0196-9781(96)00344-0. [DOI] [PubMed] [Google Scholar]
- 49.Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. 1999;79:1121–1126. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keller G, Schally AV, Groot K, Toller GL, Havt A, Köster F, Armatis P, Halmos G, Zarandi M, Varga JL, Engel JB. Proc Natl Acad Sci USA. 2005;102:10628–10633. doi: 10.1073/pnas.0504102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiaris H, Schally AV, Nagy A, Sun B, Armatis P, Szepeshazi K. Br J Cancer. 1999;81:966–971. doi: 10.1038/sj.bjc.6690794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koppan M, Halmos G, Arencibia JM, Lamharzi N, Schally AV. Cancer. 1998;83:1335–1343. doi: 10.1002/(sici)1097-0142(19981001)83:7<1335::aid-cncr10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 53.Busto R, Schally AV, Braczkowski R, Plonowski A, Krupa M, Groot K, Armatis P, Varga JL. Regul Pept. 2002;108:47–53. doi: 10.1016/s0167-0115(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 54.Halmos G, Rekasi Z, Szoke B, Schally AV. Receptor. 1993;3:87–97. [PubMed] [Google Scholar]