Abstract
Intracellular bacterial pathogens evade the bactericidal functions of mammalian cells by physical escape from their phagosome and replication into the cytoplasm or through the modulation of phagosome maturation and biogenesis of a membrane-bound replicative organelle. Here, we detail in murine primary macrophages the intracellular life cycle of Francisella tularensis, a highly infectious bacterium that survives and replicates within mammalian cells. After transient interactions with the endocytic pathway, bacteria escaped from their phagosome by 1 h after infection and underwent replication in the cytoplasm from 4 to 20 h after infection. Unexpectedly, the majority of bacteria were subsequently found to be enclosed within large, juxtanuclear, LAMP-1-positive vacuoles called Francisella-containing vacuoles (FCVs). FCV formation required intracytoplasmic replication of bacteria. Using electron and fluorescence microscopy, we observed that the FCVs contained morphologically intact bacteria, despite fusing with lysosomes. FCVs are multimembranous structures that accumulate monodansylcadaverine and display the autophagy-specific protein LC3 on their membrane. Formation of FCVs was significantly inhibited by 3-methyladenine, confirming a role for the autophagic pathway in the biogenesis of these organelles. Taken together, our results demonstrate that, via autophagy, F. tularensis reenters the endocytic pathway after cytoplasmic replication, a process thus far undescribed for intracellular pathogens.
Keywords: macrophage, pathogenesis, tularemia, trafficking
Intracellular bacterial pathogens have devised various strategies for circumventing the microbicidal functions of mammalian cells, in order to survive and multiply intracellularly (1, 2). The two canonical strategies used by these pathogens are (i) physical escape from the degradative endocytic compartments by means of lysis of the phagosomal membrane and replication within the cytoplasm, often accompanied by actin-based motility, as exemplified by Listeria, Shigella, or Rickettsia spp.; or (ii) maintenance inside a pathogen-tailored, membrane-bound compartment stalled along, or segregated from, the endocytic compartment, as is the case for Salmonella, Mycobacterium, Chlamydia, Brucella, or Legionella spp. (1–3).
Francisella tularensis is a Gram-negative, highly infectious, facultative intracellular pathogen that causes tularemia, a zoonotic disease affecting humans and other mammals with significant mortality (4). Given its high infectivity and lethality, F. tularensis has raised concerns as a potential bioterrorism agent; however, little is known about its pathogenesis. F. tularensis is capable of infecting various mammalian cell types, among which macrophages constitute a survival and replication niche essential to the virulence of this bacterium. Early studies in rodent macrophages have suggested that intracellular Francisella resides inside a phagosome that does not fuse with lysosomes and whose acidification is essential for intracellular survival (5, 6). However, recent evidence has challenged these results by demonstrating the phagosomal escape of virulent and attenuated Francisella strains into the cytoplasm of various murine or human primary macrophages or macrophage-like cells, followed by bacterial replication (7–9). Phagosomal escape occurs 2–4 h postinfection (p.i.), suggesting some early interactions with the endocytic pathway. After replication, bacterial egress is thought to occur via the induction of programmed cell death (4, 10). It has also recently been proposed that Francisella-induced cell death is an innate immune macrophage response to cytoplasmic bacteria aimed at restricting bacterial multiplication (11). Here, we have investigated Francisella interactions with the endocytic compartment in a synchronized infection model of murine bone-marrow-derived macrophages (BMM). We show that, after limited interactions with the endocytic pathway, phagosomal disruption occurs rapidly. Moreover, we describe a postreplication stage of Francisella intracellular trafficking whereby bacteria reenter the endocytic compartment via an autophagy-mediated process to reside in large fusogenic vacuoles. This finding represents a unique trafficking event for an intracellular bacterium and suggests that bacterial pathogens can cycle through different host cell compartments during their intracellular cycle.
Results and Discussion
Francisella Rapidly Disrupts Its Phagosomal Membrane to Access the Macrophage Cytoplasm.
To examine early interactions of Francisella with the endocytic compartment of murine BMMs, we synchronized bacterial uptake by BMMs as described in Materials and Methods. Five minutes after infection, intracellular bacteria colocalized with the early endosome marker early endosome antigen-1 (EEA-1; Fig. 1A), indicating interactions with early endosomes. Such interactions were transient and were rapidly followed by the acquisition of the late endosomal/lysosomal marker lysosomal-associated membrane protein 1 (LAMP-1; Fig. 1A), indicating a progressive maturation of the Francisella-containing phagosome along the endocytic pathway. The percentage of LAMP-1-positive phagosomes peaked at 20 min p.i. (52 ± 5.7%; all data are given as mean ± SD) and then decreased to 7.7 ± 2.9% at 60 min p.i. (Fig. 1A), suggesting significant phagosomal escape by 60 min p.i., earlier than previously reported in murine (7) or human (8) phagocytes. To determine whether the loss of LAMP-1 colocalization with intracellular bacteria was due to phagosomal disruption, we developed a fluorescence microscopy assay of phagosomal integrity, based on the sequential use of digitonin and saponin to differentially label bacteria that are cytoplasmic or within a compromised phagosome and those enclosed within an intact phagosome (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). In an infection of BMMs with F. tularensis live vaccine strain (LVS), 36 ± 5.7% and 95 ± 3.1% of intracellular bacteria were detectable at 20 min and 60 min p.i., respectively, by cytoplasmically delivered antibodies (Fig. 1B; and Fig. 6E, which is published as supporting information on the PNAS web site), indicating that these bacteria had significantly escaped from their phagosome or compromised their phagosomal membrane. Consistently, infected BMMs analyzed by transmission electron microscopy (TEM) contained an increasing percentage of bacteria surrounded by degraded membranes from 30 min to 2 h p.i. (55% at 30 min, 75% at 1h, and 90% at 2 h p.i.; Fig. 1 B and C). Most phagosomes displayed >75% of degraded membranes by 1 h p.i. This finding demonstrates that F. tularensis LVS rapidly disrupts its phagosome after uptake by murine BMMs and reaches the cytoplasm. Thereafter (Fig. 1B and data not shown), >95% of the intracellular bacteria were cytoplasmic, and replication occurred from 4 h onward, as reported previously (7–9).
Fig. 1.
Phagosomal escape of F. tularensis LVS occurs rapidly in murine BMMs. (A) Confocal microscopy images and quantitation of endocytic marker acquisition by phagosomes during early trafficking events. BMMs were infected with LVS for the indicated times, fixed, and processed for immunofluorescence using anti-Francisella and either EEA-1 or LAMP-1 antibodies. Colocalization of bacteria with either EEA-1 or LAMP-1 was scored for 100 bacteria per condition. Arrows indicate areas magnified in Insets. (Scale bars: 10 μm; Insets, 2 μm.) (B) Quantitation of Francisella escape into the cytoplasm. LVS-infected BMMs were processed for the phagosomal integrity assay, TEM, or LAMP-1 and Francisella immunofluorescence staining. Phagosomal escape was measured as the percentage of intracellular bacteria labeled after digitonin permeabilization (filled circles, cytoplasmic bacteria) or as the percentage of bacteria surrounded by degraded membranes (open squares, representative TEM analysis), and LAMP-1 colocalization with 100 bacteria per time point was scored (open circles). At least 50 bacteria per time point were analyzed by TEM in each experiment. (C) Representative TEM micrograph of an LVS-infected BMM at 1 h p.i. The bacterium is surrounded by degraded membranes, which indicates phagosomal disruption. (Scale bar: 0.5 μm.)
Intracellular Francisella Localize to Large Vacuoles After Intracytoplasmic Replication.
While examining the fate of replicating cytoplasmic bacteria, we unexpectedly detected clusters of bacteria that were not accessible to cytoplasmically delivered antibodies at 24 h p.i. (Fig. 2A). Immunostaining of infected BMMs for the late endosomal/lysosomal marker LAMP-1 revealed that these bacterial clusters were surrounded by a LAMP-1-positive membrane (Fig. 2B), demonstrating that bacteria were enclosed within large endocytic vacuoles. These organelles, named “Francisella-containing vacuoles” (FCVs), were juxtanuclear, heterogeneous in size (2–15 μm in diameter) and numbers of enclosed bacteria (data not shown) and were present in cells that also contained cytoplasmic bacteria. Formation of FCVs increased with time, with the percentage of FCV-containing BMMs reaching 46 ± 8.1% at 24 h p.i. and 81 ± 8.4% at 36 h p.i. (Fig. 2C). The kinetics and extent of FCV formation did not depend significantly on the apparent moi used, although higher infection rates favored FCV formation (Fig. 7, which is published as supporting information on the PNAS web site). FCV formation occurred after the net intracellular growth of Francisella (20 h p.i.; Fig. 2C), suggesting that FCV formation is a postreplication event requiring a specific intracellular bacterial load. Importantly, the virulent F. tularensis subsp. holarctica strain FSC200 and F. tularensis subsp. tularensis strain Schu S4 also formed FCVs in murine BMMs, with comparable kinetics after cytoplasmic replication (Fig. 8, which is published as supporting information on the PNAS web site), indicating that this phenomenon is not due to the attenuation of LVS.
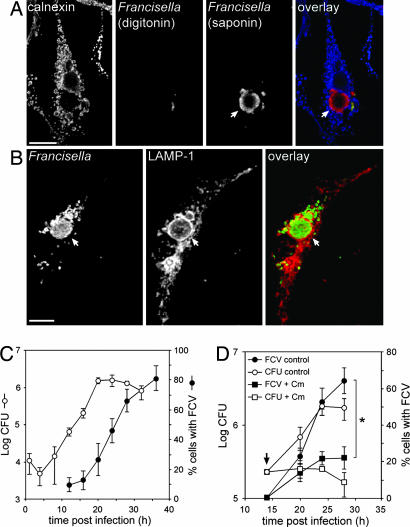
Fig. 2.
Intracellular Francisella become enclosed in large vacuoles after intracytoplasmic replication. (A) Confocal micrographs of an LVS-infected BMM at 24 h p.i., subjected to the phagosomal integrity assay. Cytoplasmic bacteria (red and green, appearing yellow in the overlay) are labeled after digitonin permeabilization, whereas clustered bacteria are detected only after saponin permeabilization (red). Calnexin staining (blue) allows detection of digitonin-permeabilized cells. (B) Confocal micrographs of an LVS-infected BMM at 24 h p.i. BMMs were infected with LVS, fixed, and processed for immunofluorescence with Francisella LPS and LAMP-1 antibodies. Bacterial clusters (green) are enclosed in LAMP-1-positive, membrane-bound compartments (red) termed Francisella-containing vacuoles (FCVs). Arrows indicate FCVs. (Scale bars: 10 μm.) (C) Kinetics of intracellular replication and FCV formation. BMMs were infected with LVS for the indicated times. Intracellular bacteria were enumerated from cfus, and FCV formation was measured as the percentage of infected cells harboring LAMP-1-positive FCVs. (D) Effect of inhibition of bacterial protein synthesis on FCV formation and replication. BMMs were infected with LVS and left untreated or treated at 14 h p.i. with 10 μg/ml chloramphenicol (indicated by arrow), and FCV formation (filled shapes) or intracellular growth (open shapes; cfu) was measured. The asterisk indicates statistically significant differences between control and chloramphenicol-treated BMMs at 28 h p.i. (P < 0.05, two-tailed unpaired Student’s t test).
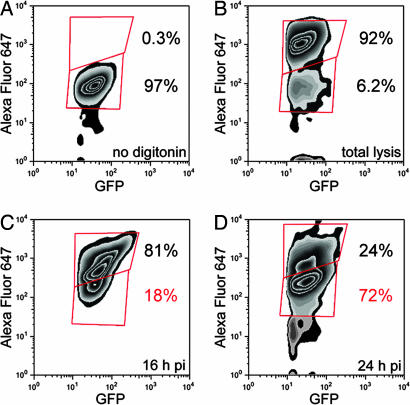
We next quantified the proportion of cytoplasmic and vacuolar bacteria recovered from infected BMMs by flow cytometry analysis. In these experiments, digitonin permeabilization was used to specifically label cytoplasmic bacteria, while all bacteria were detectable through GFP expression, as described in Materials and Methods. We first controlled for the specificity of labeling cytoplasmic bacteria. In the absence of cytoplasmic delivery of Alexa Fluor 647-conjugated anti-Francisella antibodies, no intracellular bacteria were labeled (Fig. 3A), whereas the majority of intracellular bacteria (92%) were labeled after BMM lysis (Fig. 3B). At 16 h p.i., 81% of intracellular GFP-expressing LVS were detected by cytoplasmically delivered Alexa Fluor 647-conjugated anti-Francisella antibodies (Fig. 3C) and hence were cytoplasmic, in agreement with the low frequency of FCV in BMMs at this time point (Fig. 2C). In contrast, 72% of bacteria were inaccessible to cytoplasmically delivered antibodies at 24 h p.i. (Fig. 3D) and hence were vacuolar, demonstrating that the majority of intracellular Francisella are located within FCVs at this time point.
Fig. 3.
Flow-cytometry-based quantitation of cytoplasmic and vacuolar Francisella at 16 and 24 h p.i. BMMs were infected with GFP-expressing LVS, and cytoplasmic bacteria were labeled using AlexaFluor 647-conjugated anti-Francisella antibodies after digitonin permeabilization. (A) Negative control of AlexaFluor 647 labeling of cytoplasmic, GFP-expressing bacteria when BMMs infected for 24 h were processed without digitonin permeabilization. (B) Positive control of AlexaFluor 647 labeling of GFP-expressing bacteria recovered from BMMs infected for 24 h. The majority (92%) were labeled after total lysis. (C) Analysis of GFP-expressing bacteria recovered from BMMs infected for 16 h, showing that the majority (81%) were labeled with Alexa Fluor 647 and hence are cytoplasmic. (D) Analysis of GFP-expressing bacteria recovered from BMMs infected for 24 h, showing that the majority (72%) were not labeled with AlexaFluor 647 and hence are vacuolar. Percentages shown in red refer to the proportions of vacuolar bacteria. Data are from one experiment representative of three.
To assess whether FCV formation requires metabolically active bacteria, we blocked bacterial protein synthesis by adding chloramphenicol at 14 h p.i. and examined FCV formation thereafter. Chloramphenicol treatment stopped bacterial multiplication without significant killing (Fig. 2D) and significantly prevented FCV formation (22 ± 6.1% at 28 h p.i.) compared with untreated BMMs (64 ± 7.1%, P < 0.01; Fig. 2D). This phenomenon was reversible, inasmuch as bacterial replication and FCV formation resumed when chloramphenicol was chased at 20 h p.i. (data not shown). Hence, bacterial protein synthesis, or the full extent of replication, is required for FCV formation.
Induction of programmed cell death has been associated with Francisella replication (10). To examine whether FCVs originated from phagocytosed bodies from neighboring Francisella-infected BMMs that had undergone cell death, we measured cell death in LVS-infected BMMs via the lactate dehydrogenase release assay and the TUNEL assay. Under our infection conditions, cell death was very low at 24 h p.i. (< 15%; Fig. 9, which is published as supporting information on the PNAS web site). We also quantitated FCV formation either in C57BL/6 BMMs treated with the pan-caspase inhibitor Z-VAD (Biomol, Plymouth Meeting, PA) to inhibit programmed cell death or in BMMs derived from ASC−/− mice, which do not undergo programmed cell death when infected with Francisella because of a defect in the inflammasome-associated adaptor protein ASC (11). In both conditions, FCV formation was not affected compared with untreated C57BL/6 BMMs (Fig. 9). Collectively, these results rule out an exogenous, cell-death-related origin for FCVs.
Next, FCV ultrastructure was examined by TEM. At 24 h p.i., many individual bacteria or groups of bacteria were enclosed in membrane-bound compartments (Fig. 4A and B), a feature that was not observed at earlier time points (data not shown). In most cases, FCVs were large membrane-bound vacuoles filled with undegraded bacteria (Fig. 4 B and E). Interestingly, these compartments displayed double-membrane structures reminiscent of autophagic vacuoles (Fig. 4 C and D, arrows).
Fig. 4.
FCVs are double membrane-bound vacuoles containing intact bacteria. BMMs were infected with LVS and processed for TEM at 24 h p.i. (A and B) TEM micrographs showing individual bacteria or groups of bacteria enclosed by double membranes (indicated by arrows). (C and D) Magnifications of the boxed areas in A and B, respectively, showing double membranes (arrows) surrounding bacteria. (E) Ultrastructure of a typical FCV showing clustered, intact bacteria enclosed by a membrane. (Scale bars: A, B, and E, 0.5 μm; C and D, 0.2 μm.)
FCV Formation Requires Autophagy.
Because the ultrastructure of FCVs suggested an autophagic nature, we sought to determine whether these organelles displayed additional autophagic features. We first examined whether FCVs accumulated the autophagic probe monodansylcadaverine (MDC). At 24 h p.i., 74 ± 8.9% of the LAMP-1-positive FCVs strongly labeled with MDC (Fig. 5A and C), indicating an autophagic origin for FCVs. To confirm this result, we expressed in BMMs a GFP fusion with the autophagosomal membrane-associated protein LC3 (12) that specifically labels autophagosomes and examined GFP-LC3 recruitment on FCVs. GFP-LC3 was enriched on the majority of FCVs at 24 h p.i. (62 ± 9.6% of LAMP-1-positive FCVs; Fig. 5 B and C). GFP-LC3 enrichment was dependent on LC3 association with membranes because the GFP-LC3ΔC22, G120A mutant form, which cannot be processed and conjugated to autophagosomal membranes (12), was not significantly recruited to FCVs (8.8 ± 1.9%; Fig. 5 B and C). Taken together, these results demonstrate the autophagic origin of FCVs. To extend these findings, we examined the effect of autophagy inhibition on FCV formation. Infected BMMs were treated at 14 h p.i. with 5 mM 3-methyladenine (3-MA), and FCV formation was analyzed at 24 h p.i. In uninfected cells, such treatment inhibited autophagosome formation by 76% upon amino acid starvation (Fig. 5D). 3-MA significantly reduced FCV formation such that only 18 ± 4.5% of BMMs contained FCVs, compared with 43.3 ± 11.1% of control BMMs (P = 0.04; Fig. 4D), yet treatment did not significantly affect the intracellular yield of bacteria (Fig. 10, which is published as supporting information on the PNAS web site). Collectively, these results demonstrate that FCVs display autophagic features and require autophagy for their formation but are not involved in either intracellular proliferation or killing.
Fig. 5.
FCVs display autophagic features, and their biogenesis requires autophagy. (A) LVS-infected BMMs were labeled with MDC (blue) before fixation at 24 h p.i. and immunofluorescence staining of Francisella (green) and LAMP-1 (red). (B) BMMs were transduced to express GFP-LC3 or GFP-LC3ΔC22, G120A (GFP-LC3ΔC22) and infected with LVS for 24 h p.i. before fixation and immunostaining of Francisella (blue), GFP (green), and LAMP-1 (red). Insets are single-channel fluorescence images of FCVs. Arrows indicate FCVs. (C) Quantitation of MDC accumulation and recruitment of GFP-LC3 or GFP-LC3ΔC22, G120A on LAMP-1-positive FCVs at 24 h p.i. For each condition, 100 FCVs were scored per experiment. (D) Effect of autophagy inhibition on FCV formation. (Left) LVS-infected BMMs were treated with 5 mM 3-MA at 14 h p.i., and FCV formation was scored at 24 h p.i. As a positive control for autophagy inhibition, uninfected, GFP-LC3-expressing BMMs were left untreated or pretreated with 3-MA for 1 h, then starved for 4 h to induce autophagosome formation. (Right) Autophagy was then scored as the percentage of cells containing GFP-LC3-positive vesicles. Asterisks indicate data significantly different from untreated controls (P < 0.05, two-tailed unpaired Student’s t test).
FCVs Interact with Late Endocytic/Lysosomal Compartments.
Given the presence of LAMP-1 on FCV membranes, we tested whether FCVs are mature, fusogenic autolysosomes. With live cell imaging, 74 ± 2.9% of FCVs formed by GFP-expressing LVS at 24 h p.i. accumulated Alexa Fluor 568-dextran, a fluorescent fluid-phase marker preloaded into lysosomes (Fig. 11 A and C, which is published as supporting information on the PNAS web site). Consistently, 76 ± 11% of FCVs contained the luminal lysosomal hydrolase cathepsin D (Fig. 11 B and C), confirming that FCVs fuse with lysosomes, and 73 ± 5.6% of FCVs also accumulated the acidotropic probe LysoTracker Red DND-99 (Fig. 11 C and D), indicating that they are acidic organelles. Hence, FCVs are fusogenic, matured autolysosomes.
Autophagy in mammalian cells has been associated with innate defense mechanisms against intracellular pathogens, retrieving cytoplasmic bacteria for degradation (13, 14) or resuming the maturation of phagosomes stalled along the endocytic pathway (15). Predictably, some cytoplasmic pathogens, such as Shigella, have evolved strategies to avoid autophagy (16), whereas membrane-bound pathogens such as Legionella pneumophila, Porphyromonas gingivalis, Coxiella burnetii, and Brucella abortus may take advantage of this mammalian process (17–20). Here, we show that, after cytoplasmic replication, F. tularensis reenters the endocytic pathway via the autophagic machinery of primary murine macrophages. In addition to uncovering a distinctive stage of the Francisella intracellular cycle, we demonstrate that an intracellular pathogen with a cytoplasmic replication phase can subsequently resume interactions with the endocytic compartment.
FCV formation could be a host- or bacterial-driven process. Given the role of autophagy in innate defense against intracellular pathogens, FCVs could result from a macrophage response to the intracellular bacterial load, aimed at restricting bacterial multiplication. This hypothesis is, nonetheless, questioned by the fact that autophagy is not induced earlier when Francisella initially reach the cytoplasm, inasmuch as autophagy occurs rapidly in response to other cytoplasmic bacteria, including Group A Streptococcus (13), Salmonella (14), or Listeria rendered metabolically inactive with chloramphenicol (21). In contrast, chloramphenicol treatment of Francisella-infected macrophages during (Fig. 2D) or before replication (4 h p.i., data not shown) did not induce the autophagic uptake of bacteria. Instead, Francisella remained cytoplasmic and intact for up to 16 h posttreatment (data not shown). Because FCV biogenesis requires active bacterial protein synthesis and/or substantial replication of bacteria, FCV formation could be a temporally regulated, bacterially driven process. Francisella appears to prevent a macrophage autophagic response before and during bacterial replication. Once replication is completed, the macrophage autophagic response develops or is actively triggered by the bacteria, inducing their autophagic uptake and allowing FCV formation. The biogenesis of such organelles, which is obviously a significant event of the Francisella intracellular cycle, involves the majority of bacteria and occurs with both attenuated and highly virulent strains. Additionally, the report of bacteria enclosed by double membranes within peritoneal cells of mice infected with LVS (6) potentiates the significance of FCVs by suggesting that they form in vivo.
The reentry of Francisella into the endocytic degradative pathway after cytoplasmic replication is a unique phenomenon in the trafficking of intracellular pathogens. Because FCVs possess features of autolysosomes, intravacuolar Francisella likely encounter a lysosomal environment. Yet no sign of bacterial degradation was observed in most FCVs, suggesting that Francisella can resist phagolysosomal bactericidal conditions once it reenters the endocytic pathway. The role of FCVs in the intracellular cycle and pathogenesis of Francisella remains to be elucidated. FCV formation does not affect intracellular survival and replication, suggesting that the vacuoles are not involved in bacterial proliferation. As a postreplication stage, FCVs may subject the bacteria to environmental cues required to induce a subset of genes involved in egress and/or reinfection. Given their interactions with the endocytic compartment, FCVs might also provide intracellular Francisella with access to the macrophage membrane trafficking functions, promoting bacterial egress through exocytosis. Studies of the biogenesis and dynamics of FCVs could address the role of these intracellular organelles in Francisella pathogenesis. Given the increasing prominence of autophagy in bacterial pathogenesis, future studies of FCVs may also provide much-needed information about this important mammalian response.
Materials and Methods
Bacterial Strains and Culture Conditions.
F. tularensis subsp. holarctica strains LVS (ATCC 29684) and FSC200 (22) were obtained from Francis Nano (University of Victoria, Victoria, BC, Canada) and Anders Sjöstedt (Umea University, Umea, Sweden), respectively. F. tularensis subsp. tularensis strain Schu S4 (23) was obtained from Rick Lyons (University of New Mexico, Albuquerque, NM). To generate GFP-expressing bacteria, the plasmid pFNLTP6groE-gfp (24) was introduced by electroporation into the LVS strain, as described in ref. 24. Bacteria were grown on cysteine heart agar (Becton Dickinson, Sparks, MD) supplemented with 9% heated sheep blood (CHAB), and kanamycin (10 μg/ml) when required, for 3 days at 37°C in 7% CO2. For infections with LVS, two to three fresh colonies were inoculated into tryptic soy broth (Becton Dickinson) supplemented with 0.1% l-cysteine (TSB-C) and grown overnight at 37°C with shaking to an OD600 ≈ 1.5. Virulent holarctica or tularensis strains were scraped off freshly streaked CHAB plates and resuspended in TSB-C before infection. To enumerate viable intracellular bacteria, infected macrophages were lysed in sterile distilled water, and serial dilutions were plated on CHAB plates.
Macrophage Culture and Infection.
To generate BMMs, bone marrow cells were collected from dissected femurs of 6- to 12-week-old C57BL/6 female mice (Harlan, Indianapolis, IN), and macrophages were derived in 150-mm non-tissue-culture-treated dishes, as described in ref. 25. After 5 days, loosely adherent BMMs were washed with PBS, harvested by incubation in chilled cation-free PBS on ice for 10 min, resuspended in complete medium, and seeded onto 12-mm glass coverslips in 24-well plates (immunofluorescence, 1 × 105 per well) or WillCo-dish glass-bottomed 35-mm dishes (live cell imaging, 1 × 105 per dish; WillCo Wells BV, Amsterdam, The Netherlands). BMMs were further cultured for 2 days before infection. BMMs derived from ASC−/− knockout mice were kindly provided by David Weiss and Denise Monack (Stanford University, Stanford, CA).
BMM infections were performed at an moi of 50 by centrifuging bacteria suspended in complete medium onto prechilled macrophages at 400 × g for 10 min at 4°C. BMMs were then rapidly warmed to 37°C for 2 min in a water bath to trigger phagocytosis and further incubated for a total of 20 min at 37°C in 7% CO2. BMMs were extensively washed with DMEM to remove extracellular bacteria and incubated for 40 min in complete medium and then for 60 min in 100 μg/ml gentamicin-containing medium to kill the remaining extracellular bacteria. Thereafter, infected BMMs were incubated in gentamicin-free medium. Such conditions allowed for a synchronized entry of bacteria, leading to 29 ± 3.8% of BMMs infected with one to two bacteria (Fig. 7). At each time point, BMMs were washed three times with PBS before processing. When required, chloramphenicol (Sigma, St. Louis, MO) was added (10 μg/ml). Autophagy was induced by incubating cells in Hank’s balanced salt solution (Mediatech, Herndon, VA) supplemented with 1 g/liter d-glucose for 4 h to mimic amino acid starvation conditions. Autophagy was inhibited by treating BMMs with 5 mM 3-MA (Fluka/Sigma–Aldrich, St. Louis, MO), and the percentage of GFP-LC3-expressing BMMs containing LC3-positive vesicles was scored.
Fluorescence Microscopy.
BMMs were fixed with 3% paraformaldehyde in PBS, pH 7.4, for 10 min at 37°C and processed for immunofluorescence staining as described in ref. 20. The primary antibodies used were mouse monoclonal anti-F. tularensis LPS (US Biologicals, Swampscott, MA), rat monoclonal anti-mouse LAMP-1 (1D4B; developed by J. T. August; obtained from the Developmental Studies Hybridoma Bank; developed under the auspices of the National Institute of Child Health and Human Development; and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA), rabbit polyclonal anticalnexin and mouse monoclonal antiprotein disulfide isomerase (PDI; Stressgen Biotechnologies, Victoria, BC, Canada), rabbit polyclonal anti-human cathepsin D (provided by Stuart Kornfeld, Washington University, St. Louis, MO), goat polyclonal anti-EEA1 (N-19; Santa Cruz Biotechnologies, Santa Cruz, CA), and rabbit polyclonal anti-GFP (Molecular Probes, Eugene, OR). The secondary antibodies used were Alexa Fluor 488-conjugated and Alexa Fluor 568-conjugated (Molecular Probes) and cyanin-5-conjugated (Jackson ImmunoResearch, West Grove, PA) donkey anti-mouse, anti-rat, anti-rabbit, and anti-goat antibodies. To label autophagosomes, BMMs were incubated with 50 μM MDC for 1 h, followed by a 30-min chase before fixation. Samples were observed on either a Nikon (Melville, NY) Eclipse E800 epifluorescence microscope for quantitative analysis or a Carl Zeiss (Thornwood, NY) LSM 510 confocal laser scanning microscope for quantitative analysis and image acquisition. MDC fluorescence was imaged by epifluorescence using a DAPI filter and a Carl Zeiss Axiocam digital camera mounted on the LSM 510 confocal microscope, concomitant with the confocal acquisitions of the other fluorescent emissions. Confocal images of 1,024 × 1,024 pixels were acquired and assembled using Adobe Photoshop CS software (Adobe Systems, San Jose, CA).
Flow Cytometry Quantitation of Vacuolar Francisella.
To evaluate the proportions of cytoplasmic and vacuolar Francisella, BMMs in six-well plates (5 × 105 per well) were infected with GFP-expressing LVS and washed with KHM buffer (110 mM potassium acetate/20 mM Hepes/2 mM MgCl2, pH 7.3), and their plasma membranes were selectively permeabilized with 50 μg/ml digitonin in KHM buffer for 1 min at room temperature. After washing with KHM buffer, mouse anti-Francisella LPS antibodies conjugated to Alexa Fluor 647 (Molecular Probes) were specifically delivered to the macrophage cytoplasm (see Supporting Materials and Methods) for 15 min at 37°C to label cytoplasmic bacteria. After washing in KHM buffer, BMMs were lysed in water and lysates were centrifuged at 200 × g for 5 min to remove cellular debris. Supernatants containing all intracellular bacteria were analyzed by using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) for GFP and Alexa Fluor 647 fluorescence. Data were analyzed with FlowJo software, version 6.3.2 (Tree Star, Ashland, OR). Under these conditions, cytoplasmic bacteria were detected as both GFP- and Alexa Fluor 647-positive, whereas vacuolar bacteria were only GFP-positive. At least 90% of intracellular bacteria were GFP-positive after a 24-h infection (data not shown), confirming that the analysis was performed on the majority of the bacterial population.
TEM.
Infected BMMs on 12-mm Aclar coverslips were processed as described in ref. 26, except that BMMs were fixed for 24 h and postfixed in 1% OsO4. Samples were viewed in a Hitachi (Tokyo, Japan) H7500 TEM at 80 kV, fitted with a Hamamatsu (Bridgewater, NJ) CCD camera C4742–95 and Advantage HR/HR-B digital image software (AMT, Danvers, MA) for imaging. To assess phagosomal membrane integrity, 50–100 phagosomes were analyzed per condition in two independent experiments. FCV ultrastructural analysis was performed on ≈50 vacuoles in three independent experiments.
Statistical Analyses.
All data are given as mean ± SD from at least three independent experiments, unless otherwise stated. Statistical analyses were performed by using either a one-way ANOVA with Tukey posttest or an unpaired, two-tailed Student t test. A P value < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Leigh Knodler, Rey Carabeo, and Samantha Gruenheid for critical reading of the manuscript; Francis Nano, Anders Sjöstedt, Rick Lyons, Tamotsu Yoshimori, Stuart Kornfeld, David Weiss, and Denise Monack for providing strains, plasmids, antibodies and macrophages; Holger Lorenz for technical advice on digitonin permeabilization; and Ron Messer for advice and help with flow cytometry. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Abbreviations
- BMM
bone marrow-derived macrophage
- FCV
Francisella-containing vacuole
- LAMP-1
lysosomal-associated membrane protein 1
- LVS
live vaccine strain
- MDC
monodansylcadaverine
- p.i.
postinfection
- TEM
transmission electron microscopy
- 3-MA
3-methyladenine.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Knodler LA, Celli J, Finlay BB. Nat Rev Mol Cell Biol. 2001;2:578–588. doi: 10.1038/35085062. [DOI] [PubMed] [Google Scholar]
- 2.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel JP. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 3.Amer AO, Swanson MS. Curr Opin Microbiol. 2002;5:56–61. doi: 10.1016/s1369-5274(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 4.Oyston PC, Sjostedt A, Titball RW. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 5.Anthony LD, Burke RD, Nano FE. Infect Immun. 1991;59:3291–3296. doi: 10.1128/iai.59.9.3291-3296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortier AH, Leiby DA, Narayanan RB, Asafoadjei E, Crawford RM, Nacy CA, Meltzer MS. Infect Immun. 1995;63:1478–1483. doi: 10.1128/iai.63.4.1478-1483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens DL, Lee BY, Horwitz MA. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santic M, Molmeret M, Abu Kwaik Y. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 10.Lai XH, Golovliov I, Sjostedt A. Infect Immun. 2001;69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariathasan S, Weiss DS, Dixit VM, Monack DM. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, et al. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 14.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 17.Amer AO, Swanson MS. Cell Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Infect Immun. 2001;69:5698–5708. doi: 10.1128/IAI.69.9.5698-5708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 20.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich KA, Burkett C, Webster P. Cell Microbiol. 2003;5:455–468. doi: 10.1046/j.1462-5822.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansson A, Berglund L, Eriksson U, Goransson I, Wollin R, Forsman M, Tarnvik A, Sjostedt A. J Clin Microbiol. 2000;38:22–26. doi: 10.1128/jcm.38.1.22-26.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eigelsbach HT, Braun W, Herring RD. J Bacteriol. 1951;61:557–569. doi: 10.1128/jb.61.5.557-569.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier TM, Havig A, Casey M, Nano FE, Frank DW, Zahrt TC. Appl Environ Microbiol. 2004;70:7511–7519. doi: 10.1128/AEM.70.12.7511-7519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Chastellier C, Frehel C, Offredo C, Skamene E. Infect Immun. 1993;61:3775–3784. doi: 10.1128/iai.61.9.3775-3784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockey DD, Fischer ER, Hackstadt T. Infect Immun. 1996;64:4269–4278. doi: 10.1128/iai.64.10.4269-4278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.