Abstract
The vgf gene has been identified as an energy homeostasis regulator. Vgf encodes a 617-aa precursor protein that is processed to yield an incompletely characterized panel of neuropeptides. Until now, it was an unproved assumption that VGF-derived peptides could regulate metabolism. Here, a VGF peptide designated TLQP-21 was identified in rat brain extracts by means of immunoprecipitation, microcapillary liquid chromatography–tandem MS, and database searching algorithms. Chronic intracerebroventricular (i.c.v.) injection of TLQP-21 (15 μg/day for 14 days) increased resting energy expenditure (EE) and rectal temperature in mice. These effects were paralleled by increased epinephrine and up-regulation of brown adipose tissue β2-AR (β2 adrenergic receptor) and white adipose tissue (WAT) PPAR-δ (peroxisome proliferator-activated receptor δ), β3-AR, and UCP1 (uncoupling protein 1) mRNAs and were independent of locomotor activity and thyroid hormones. Hypothalamic gene expression of orexigenic and anorexigenic neuropeptides was unchanged. Furthermore, in mice that were fed a high-fat diet for 14 days, TLQP-21 prevented the increase in body and WAT weight as well as hormonal changes that are associated with a high-fat regimen. Biochemical and molecular analyses suggest that TLQP-21 exerts its effects by stimulating autonomic activation of adrenal medulla and adipose tissues. In conclusion, we present here the identification in the CNS of a previously uncharacterized VGF-derived peptide and prove that its chronic i.c.v. infusion effected an increase in EE and limited the early phase of diet-induced obesity.
Keywords: autonomic nervous system, β adrenergic receptor, MALDI-TOF, neuropeptide, peroxisome proliferator-activated receptor δ
Energy homeostasis is a complex physiological function that is coordinated at multiple levels. Stimulated by the discovery of leptin and the pandemic diffusion of obesity and type-2 diabetes, the regulation of energy homeostasis has received increasing attention (1–4). New players are being continuously identified and screened as molecular candidates to counteract obesity (5–10). Vgf, initially identified as a nerve growth factor-responsive gene, is also robustly induced by BDNF and neurotrophin 3 and marginally induced by epidermal and fibroblast growth factors, IL-6, and insulin (11–13). Vgf received great attention after the observation that VGF-deficient mice are lean, hypermetabolic, and resistant to various types of obesity (14, 15). In the rat brain, VGF is abundant in the cortex, hypothalamus, hippocampus, and olfactory system and in a number of thalamic, septal, amygdaloid, and brainstem nuclei, with the local availability of neurotrophins for receptor occupation being the critical parameter in determining its selective expression (12, 13). Changes in vgf expression also increase in the arcuate nucleus of fasted rats (14) and hamsters that are exposed to a short or long day’s length (16). However, up until now, it was still unproved that VGF-derived peptides are metabolic neuromodulators (13). Vgf encodes a 617-aa protein in rodents that, upon processing by prohormone convertase (PC) 1/3 and PC2, yields several peptides that are stored in dense core granules and secreted through the regulated pathway (13, 17). Based on the phenotype of VGF−/− mice (14, 15), we predicted that VGF-derived peptides are anabolic neuropeptides. Here, we identified in rat brain extracts a previously uncharacterized VGF-derived peptide, designated as TLQP-21, which spans from residue 556 to residue 576 of the precursor sequence. Contrary to our prediction, TLQP-21 increases energy expenditure (EE) and blocks the early phase of high-fat diet-induced obesity.
Results
Identification of TLQP-21.
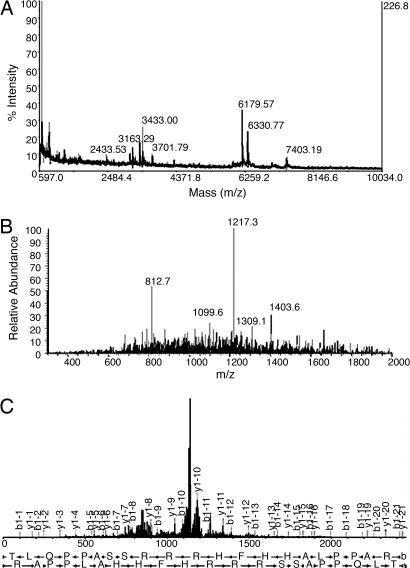
VGF peptides were immunopurified and characterized from rat brain extracts by means of an antiserum raised against the N terminus of TLQP-62 (residues 556–617), a major VGF form in the CNS (18). MALDI-TOF MS analysis of immunoprecipitates revealed one pseudomolecular ion (MH+) at a mass-to-charge ratio (m/z) of 2,433.53 (Fig. 1a) corresponding to the peptide at position 556–576 of the VGF sequence, designated TLQP-21. In addition, the singly charged as well as the doubly charged ions of TLQP-62 were detected at m/z = 7,403.19 and 3,701.79, respectively (Fig. 1a). TLQP-21 was unambiguously identified by microcapillary liquid chromatography–tandem MS (MS/MS) analysis of the immunoprecipitates and sequence database searching algorithms. TLQP-21 was separated and detected as a doubly charged ion at m/z = 1,217.27 (Fig. 1b). On the basis of the MS/MS spectra resulting from low-energy collision-induced dissociation of the precursor ion (Fig. 1c), an automated SEQUEST search against the National Center for Biotechnology Information nonredundant database indicated a cross-correlation value (Xcorr) of 1.75 and a Δ correlation value (dCn) of 0.09 (Table 1, which is published as supporting information on the PNAS web site). Manual interpretation of the MS/MS analysis by a MASCOT search in the Sequence Query mode led to the identification of the TLQPPASSRRRHFHHALPPAR peptide with a high confidence (ion score = 62; P = 0.0083). Furthermore, TLQP-21 was identified as a triply charged ion by SEQUEST in a second independent experiment (Xcorr = 2.98; dCn = 0.44; data not shown).
Fig. 1.
TLQP-21 indentification. (a) MALDI-TOF MS spectrum-immunoselected VGF peptides. (b) MicroLC MS/MS full mass spectrum recorded at a retention time of 21 min and 41 sec, showing the doubly charged ion corresponding to TLQP-21 at an m/z of 1,217.3. (c) Fragment ion spectrum.
TLQP-21 Upsets Energy Balance.
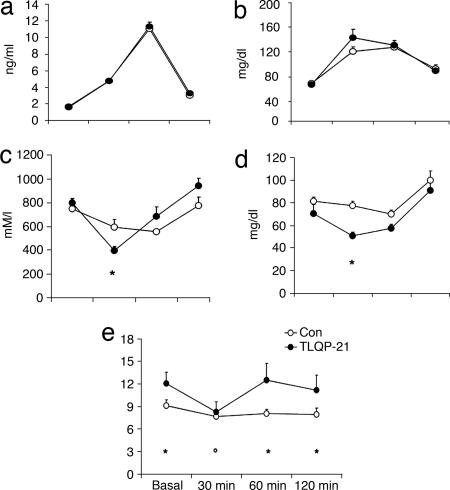
Fourteen-day i.c.v. TLQP-21 treatment increased EE at rest (+12%; P < 0.05) but less so when animals showed moderate (+7%) or high (+4%) activity level (F(1,10) = 5.6; P < 0.05; Fig. 2a). TLQP-21 also increased rectal temperature (T) (Fig. 2b; U(8,8) = 10; P < 0.05). Increased EE and T were paralleled by increased serum epinephrine (E) and decreased norepinephrine (NE) levels (Fig. 2 c and d; E, U(16,18) = 75.5, P < 0.05; NE, U(17,18) = 93, P < 0.05) independent of locomotor activity and free triiodothyronine and free thyroxine serum levels (Fig. 7 and Table 2, which are published as supporting information on the PNAS web site). Brown adipose tissue (BAT)-NE was slightly increased in TLQP-21-treated mice (control, 13.2 ± 1.2 ng/mg; TLQP-21, 15.1 ± 1.2 ng/mg), and white adipose tissue (WAT)-NE was inversely correlated with relative WAT/body weight (WAT/bw) (r = −0.6; P < 0.01). T and E values were positively correlated (r = 0.56; P < 0.05). TLQP-21 also increased adrenal gland weight, whereas corticosterone level remained unaffected (U(17,18) = 89; P < 0.05; Table 2). Finally, body weight and food intake were unaffected, whereas WAT/bw and circulating leptin were slightly reduced (Fig. 7 and Table 2).
Fig. 2.
Physiological effects in mice receiving TLQP-21. (a) EE (n = 6 per group). (b) Rectal temperature (n = 8 per group). (c) E levels. (d) NE levels. (e) TG levels. (f) FFA/TG ratio. Unless otherwise stated, n = 16–18 per group. ∗, P < 0.05.
TLQP-21 treatment also lowered circulating triglycerides (TGs) but not free fatty acids (FFAs) or glucose; therefore, an increased FFA/TG ratio was observed (Fig. 2 e and f; TG, U(17,18) = 66, P < 0.01; FFA/TG, U(17,18) = 72, P < 0.01). TG also was positively correlated with WAT/bw (r = 0.35; P < 0.05).
In the glucose tolerance test, TLQP-21 treatment reduced TG levels (F(1,9) = 6.1, P < 0.05; post hoc, P < 0.05) and, to a lesser extent, FFA levels (F(3,27) = 6.4, P < 0.01; post hoc, P = 0.079) 30 min after glucose load (Fig. 3c and d), leading to a transient normalization in the FFA/TG ratio (Fig. 3e; F(3,27) = 3.1, P < 0.05; control vs. TLQP-21 at baseline, 60 min, and 120 min, P < 0.05; TLQP-21 at 30 min vs. baseline, 60 min, and 120 min, P < 0.05).
Fig. 3.
Glucose tolerance test. Basal levels and levels 30, 60, and 120 min after oral glucose (3 mg/kg) treatment are shown for the following: insulin (a), glucose (b), FFAs (c), TGs (d), and FFA/TG ratio (e). n = 6 per group; ∗, P < 0.05 control vs. TLQP-21; °, TLQP-21 at 30 min vs. TLQP-21 at baseline, 60 min, and 120 min.
TLQP-21 Up-Regulates β Adrenergic Receptor (β-AR), Peroxisome Proliferator-Activated Receptor δ (PPAR-δ), and Uncoupling Protein 1 (UCP1) Gene Expression in the Adipose Tissue.
Hypothalamic expression of agouti-related peptide, neuropeptide Y, melanocyte-concentrating hormone, proopiomelanocortin, and corticotropin-releasing hormone was unaffected by TLQP-21 (Table 3, which is published as supporting information on the PNAS web site).
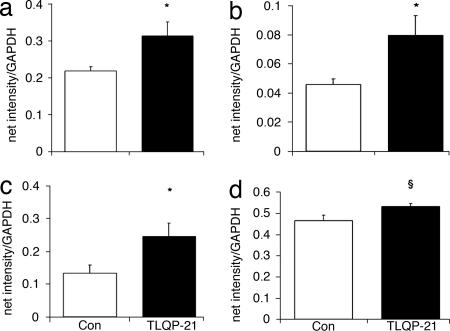
TLQP-21 determined major changes in the adipose tissues. β2-AR mRNA increased in the BAT (Fig. 4a; U(5,6) = 3; P < 0.05) and was positively correlated with serum E (r = 0.68; P < 0.05) and FFA/TG (r = 0.7; P < 0.05). In the WAT, TLQP-21 increased PPAR-δ and UCP1 mRNAs, whereas β3-AR expression slightly increased (Fig. 4 b–d; PPAR-δ, U(5,5) = 3, P < 0.05; UCP1, U(4,6) = 3, P = 0.05; β3-AR, U(5,6) = 5, P = 0.06). β3-AR positively correlated with PPAR-δ (r = 0.77; P < 0.05) and FFA/TG ratio (r = 0.61; P < 0.05) but negatively correlated with serum NE (r = −0.62; P < 0.05), WAT/bw (r = −0.66; P < 0.05), and TG levels (r = −0.8; P < 0.01).
Fig. 4.
β2-AR mRNA in the BAT (a) and PPAR-δ (b), UCP1 (c), and β3-AR (d) mRNA in the WAT. Data are presented as net intensity/GAPDH expression. n = 4–6 per group; ∗, P < 0.05; §, P = 0.06.
TLQP-21 Confers Resistance to the Early Phase of Diet-Induced Obesity.
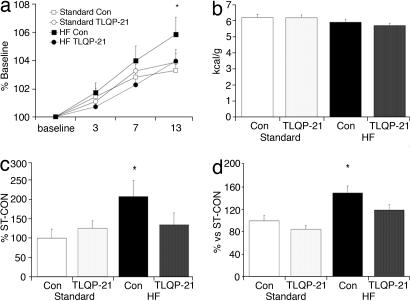
We investigated whether TLQP-21 could prevent the early phase of obesity induced by 14 days of a high-fat diet (20% lard addition to rodent chow; HF). As expected, control animals (HF-CON) showed increased body weight gain (Fig. 5a; post hoc, day 13, P < 0.01), caloric efficiency (Fig. 5c; diet, F(1,51) = 4.3, P < 0.05; diet X treatment, F(1,51) = 4.3, P = 0.08), and WAT/bw (Fig. 5d; treatment, F(1,54) = 5.7, P < 0.05; diet, F(1,54) = 18.6, P < 0.0001) when compared with mice receiving a standard diet (ST-CON and ST-TLQP-21). TLQP-21 conferred resistance to obesity: HF-TLQP-21 mice showed almost unaffected body and WAT weight and caloric efficiency (Fig. 5). These effects occurred despite the fact that HF- and ST-fed mice ingested a similar number of kilocalories and showed similar locomotor activity (Fig. 5b; see also Figs. 7 and 8, which are published as supporting information on the PNAS web site).
Fig. 5.
Physiological and behavioral parameters in mice receiving a high-fat (HF) diet. Shown are body weight changes from baseline (a), food intake (b), caloric efficiency (c), and WAT/bw (d). Standard, standard chow; Con, mice treated with aCSF. n = 11–12 per group; ∗, P < 0.05.
As expected, HF-CON mice had increased serum leptin (Fig. 6a; diet, F(1,34) = 13.8; P < 0.0001) and decreased ghrelin (diet, F(1,38) = 10.9; P < 0.001). On the contrary, HF-TLQP-21 mice showed half the rise of leptin of HF-CON mice and a normalization of ghrelin. TG levels, but not FFA or glucose levels, decreased in both HF-treated groups compared with ST-CON, whereas HF-TLQP-21 did not differ from ST-TLQP-21 (Fig. 6b; TG, diet, F(1,52) = 21.3, P < 0.001; diet X treatment, F(1,52) = 13.7, P < 0.001). Accordingly, HF resulted in up-regulation of the FFA/TG ratio in both HF groups when compared with ST-CON. HF-CON, and slightly so HF-TLQP-21 (P = 0.06), also differed from ST-TLQP-21 mice for the same parameter (Fig. 6b; diet, F(1,52) = 28.6, P < 0.0001; treatment X diet, F(1,52) = 2.7, P < 0.05; see Table 4, which is published as supporting information on the PNAS web site, for further results).
Fig. 6.
Hormones and serological parameters in mice receiving a high-fat (HF) diet. (a) Serum levels of leptin and ghrelin. (b) Levels of TGs, FFAs, and glucose and FFA/TG ratio. Standard, standard chow; Con, mice treated with aCSF. n = 11–12 per group; ∗∗, P < 0.01.
Discussion
TLQP-21 Is a Neuropeptide That Upsets Energy Balance.
Ever since the discovery that targeted deletion of the Vgf gene determined a lean, hypermetabolic, obesity-resistant phenotype (14, 15), it has been crucial to know the molecular basis of Vgf’s activity on energy balance. The present study shows the immunoprecipitation from rat brain extracts of a previously uncharacterized VGF peptide designated TLQP-21 and its identification by MS/MS and database search engines. TLQP-21 appears to derive from further processing of TLQP-62, an abundant VGF C-terminal peptide that is produced inside dense core vesicles by prohormone convertase 1/3 (18). The lean and hypermetabolic phenotype of VGF−/− mice led us to predict that VGF peptides are anabolic neuropeptides. On the contrary, we demonstrated that chronic i.c.v. treatment of TLQP-21 increased resting EE, rectal temperature, and serum E without affecting body weight or food intake. Increased EE was not paralleled by changes in locomotor activity or thyroid hormones. In addition, it was not paralleled by increased gene expression of β3-AR or UCP1 expression in the BAT and was accompanied by only a slight increase of BAT-NE. Therefore, activation of BAT thermogenesis does not appear to be the primary mediator of the effects observed (19, 20). However, increased BAT β2-AR expression (42% increase) might contribute to EE and hyperthermia (4, 19–21).
On the contrary, increased EE and T may be attributed to the permissive role of increased E (22) and to changes occurring in the WAT. WAT receives sympathetic innervation downstream of the paraventricular nucleus and ventromedial hypothalamus, the nucleus of the solitary tract, the intermediolateral cell group, and the central autonomic nucleus of the spinal cord (23–25). Sympathetic stimulation determines lipolysis and EE primarily by means of β-AR stimulation (26). In agreement with our proposal, TLQP-21 treatment mildly decreased WAT weight, whereas β3-AR expression was slightly (15% increase) but significantly up-regulated within the same tissue; the two parameters were inversely correlated. WAT weight was also negatively correlated with tissue NE level. TLQP-21 treatment also increased PPAR-δ mRNA (74% increase) in the WAT. Overall, this effect may contribute to the observed increase in EE by determining fatty acid oxidation and energy uncoupling (2, 8, 21, 25).
TLQP-21 effected increased serum E and BAT-NE levels, which are consistent with other physiological effects, whereas the reduced serum NE level seems at odds. Circulating NE mainly derives from synaptic leakage after exocytotic release from nerve terminals, and sympathetic-stimulated catabolism results in increased tissue reuptake of NE (27, 28). Therefore, decreased serum NE might result from increased tissue reuptake. Alternatively, TLQP-21 may differentially regulate functionally specific autonomic outputs innervating different tissues (28, 29).
TLQP-21 also increased UCP1 in the WAT (84% increase). Fat pads are a mix of brown (expressing UCP1) and white (not expressing UCP1) adipocytes (19, 30). The epididymal WAT is mainly composed of white adipocytes. Increased UCP1 mRNA would then imply transdifferentiation of brown adipocytes (3, 31). Interestingly, increased expression of catabolic mediators in the WAT was paralleled by a reduction of serum TG levels and high FFA/TG ratio. A reappraisal of published data has revealed a high FFA/TG ratio after treatments (e.g., β3-AR agonists) or gene overexpression increasing lipolysis and blocking obesity (e.g., refs. 5–7 and 32). Therefore, TLQP-21 effect mimics β-agonist activity and further points to sustained sympathetic stimulation of adipose tissue. We have also observed that TLQP-21 induces contractions of gastric fundus strips (G.L.C., C.S., R.P., and A.L., unpublished work). This effect is inhibited by the cyclooxygenase inhibitors indomethacine and naproxen, and, accordingly, an increased production of prostaglandin E2 (PGE2) follows TLQP-21 administration. Central induction or treatment with PGE2 induces hyperthermia and increases EE (33–35). Therefore, chronic TLQP-21-induced upset of energy balance is compatible with a mechanism of action involving central PGE2 production.
TLQP-21 also determined a stronger decline of FFA and TG in the glucose tolerance test when compared with controls. This transient decrease (30 min after glucose intake) determined a momentary normalization of the FFA/TG ratio. Increased liver and adipose tissue sensitivity to the lipostatic action of insulin due to sustained parasympathetic stimulation after glucose overload may explain this result (24, 36).
Although we identified TLQP-21 in brain tissue extract, its regional localization and its concentration within the CNS are not known yet, and the identification of its receptor remains elusive, too. Presently, whether the effects we observed after 15-μg/day infusion of the peptide are physiological or pharmacological is only speculative. However, the concentration used seems compatible with a physiological range based on independent studies (37–39). Another relevant issue is the identification of the brain structure(s) mediating TLQP-21 action. Anabolic (agouti-related peptide, neuropeptide Y, and melanocyte-concentrating hormone) and catabolic (proopiomelanocortin and corticotropin-releasing hormone) hypothalamic peptides were not grossly affected by TLQP-21. Although whole hypothalamic semiquantitative RT-PCR is not informative of regional differences in gene expression, it is often used to make a preliminary evaluation of changes in mRNA (40–42). Even considering this limitation, our results would rule out a primary role of the hypothalamus in mediating the effects of TLQP-21 while being compatible with the activation of brainstem nuclei downstream of the hypothalamus. This conclusion agrees with the proposal that VGF would function in the outflow pathways downstream of hypothalamic melanocortin 4 receptors (Mc4Rs) that project by means of the autonomic nervous system to peripheral metabolic tissues and regulate energy homeostasis (13, 15). Recent evidence (43) in Mc4R−/− mice suggests a divergent melanocortin pathway in the control of food intake (hypothalamic/amygdaloid nuclei) and EE [possibly brainstem/spinal cord neurons where Mc4R colocalizes with pseudorabies virus injected in the inguinal WAT (44)]. TLQP-21, affecting EE but not affecting feeding or hypothalamic peptides, may modulate one such Mc4R-sensitive extrahypothalamic site controlling EE.
TLQP-21 Prevents the Effects of High-Fat Diet.
In mice that were fed a standard diet, TLQP-21 upset energy balance but did not determine an overall shift in energy homeostasis.o We hypothesized that energy homeostasis could have been positively boosted by challenging mice with a high-fat diet. TLQP-21 almost completely blocked the early phases of diet-induced obesity. Despite TLQP-21-treated mice ingesting a similar number of kilocalories with respect to controls, both body weight and visceral WAT showed only a modest increase. Consistent with these effects, HF-TLQP-21 mice showed half the rise in leptin that was observed in HF-CON mice and a normalization of ghrelin. Fig. 5a shows that body weight of HF-CON and HF-TLQP-21 mice at the end of the 2-week treatment phase (time constrained by the limited capacity of the osmotic pumps) substantially diverged. We can predict that, by increasing HF exposure and peptide delivery, the curves will spread out more conspicuously. TLQP-21-induced increase of EE, T, and adipose tissue catabolic mediators is compatible with the block of weight gain and adiposity (1, 4, 20). These results suggest that TLQP-21 can be a promising target for a pharmacological intervention aimed at limiting adiposity by increasing EE (9, 10).
Does VGF Encode for Several Metabolically Active Peptides?
Surprisingly, the profile of TLQP-21 treated mice closely matches the phenotype of VGF−/− mice. Both VGF−/− and TLQP-21-treated mice showed hyperthermia, increased lipolysis, EE and tissue sensitivity to insulin (13–15, 46), and even similar molecular changes in BAT and WAT (13). Moreover, both VGF deletion and TLQP-21 treatment blunted diet-induced obesity (15). It is not unusual that results from constitutive gene knockout and pharmacological studies result in contrasting findings (47, 48). However, an intriguing hypothesis would clarify the apparent contradiction of the present study and studies by Hahm et al. (14, 15): One, or more, VGF-derived peptide should have an anabolic role that positively affects energy homeostasis and opposes TLQP-21 effects. Indeed, vgf encodes for a precursor protein that yields a number of peptides, which have not been fully characterized (13). Three VGF peptides were shown to possess biological activity: TLQP-62 and AQEE-30 increase the synaptic charge in hippocampal neurons (18), and AQEE-30 and LQEQ-19 facilitate penile erection (49). In addition, we showed that TLQP-21 induces contraction of rat gastric longitudinal muscle (G.L.C., C.S., R.P., and A.L., unpublished work) and modulates formalin pain (R.R., A.B., A.M., F.R.D., R.P, A.L., and F.P., unpublished work). If our hypothesis is confirmed, VGF should be considered a polypeptide precursor that encodes for different metabolically active neuropeptides in analogy with others (e.g., proopiomelanocortin). Site-specific and/or time-dependent regulation of VGF processing would then account for its function, depending on different physiological signals.
Conclusion
This study shows the identification in brain extracts of a previously uncharacterized neuropeptide designated TLQP-21. Results discussed address a role for this peptide in centrally stimulating the autonomic nervous system, possibly by means of central prostaglandin induction, peripheral adrenomedullary activity, and adipose tissue catabolism, to upset energy balance. By virtue of its effect, TLQP-21 also limited weight gain and adiposity associated with a high-fat diet.
Materials and Methods
Brain Extracts.
Brains of Dark Agouti male rats were dissected, minced, boiled for 10 min in 10 ml of H2O per gram of tissue, and subsequently incubated at room temperature for 10 min. The supernatant was cleared at 22,000 × g for 1 h at 4°C and kept on ice until prompt use to inactivate proteases and enrich extraction of low-molecular-weight peptides.
Immunopurification of VGF Peptides.
The antiserum used for immunoaffinity purification was raised in rabbits against residues 556–576 of rat VGF (C.B. and G.L.F., unpublished work). Extracts were adjusted to 20 mM Tris·HCl (pH 7.5) (binding buffer) followed by binding onto protein G Sepharose beads for 3 h at room temperature. Antigen Ig-bound beads were washed three times with binding buffer and recovered. Elution was performed into 40% acetonitrile (ACN)/0.2% TFA solution in H2O, and samples were stored at 4°C.
MALDI-TOF MS.
Samples were mixed 1:1 with a saturated solution of α-cyano-4-hydroxycinnamic acid matrix in 50% ACN/0.1% TFA and deposited onto the stainless-steel target (Perseptive Biosystems, Foster City, CA). Spectra were obtained essentially as described in ref. 50.
Microcapillary Liquid Chromatography MS/MS.
Analysis was performed essentially as described in ref. 51. The following gradient was applied: 10% buffer B (80% acetonirile/0.2% formic acid) at 3 min, 60% B at 53 min, 90% buffer B at 63 min, 90% buffer B at 68 min, 0% buffer B at 70 min, and 0% buffer B at 90 min. Spectra were acquired in an automated mode, consisting of a full MS spectrum followed by three data-dependent MS/MS scans on the three most intense peaks of each full MS spectrum.
Peptide Identification.
MS/MS results were submitted to SEQUEST 2.0 screening against a nonredundant protein database downloaded from the National Center for Biotechnology Information home page (www.ncbi.nlm.nih.gov). To confirm the identification of TLQP-21, MASCOT 2.1 Sequence Query was used against the SwissProt Rattus database. Query inputs were adopted based on the daughter ions list detected by a SEQUEST search (see Table 1): 217.27 tag (1,495.7, RRH, 1,046.2), tag (553.7, [IL], 440.5); enzyme, none; fixed/variable modifications, none; peptide tolerance, ±2 Da; MS/MS tolerance, ±0.6 Da; peptide charge, +1, +2, and +3. Average mass was selected.
Central TLQP-21 Administration.
Animals.
Male CD1 mice (Charles River, Calco, Italy) were housed in an environmentally controlled room (temperature of 21–24°C, light on at 0700 and light off at 1900), with food (3.4 kcal/g Mucedola) and water available ad libitum. Experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC).
Experimental procedure.
All experiments consisted of a 4-day baseline followed by a 14-day treatment phase consisting of continuous i.c.v. injection by means of osmotic pumps of TLQP-21 in artificial cerebrospinal fluid (aCSF). For all of the experimental phase, mice were individually housed, and locomotor activity was monitored. Body weight and food and water intake were determined (between 0900 and 1000) on the day of osmotic pump positioning (basal), on day 1, and every second day. Animals were killed by decapitation after brief CO2 exposure between 0900 and 1100.
Peptide delivery.
Synthetic TLQP-21 peptide (TLQPPASSRRRHFHHALPPAR; Primm, Milan, Italy) at a dose of 1 mM dissolved in aCSF or aCSF alone was i.c.v.-delivered through microosmotic pumps (model 1002; Alzet, Palo Alto, CA; flow rate of 0.25 μl/h, 15 μg/day) connected to the Alzet brain infusion kit 1. Pumps were filled and incubated overnight at 37°C. TLQP-21 thermostability was ascertained by measuring with HPLC (1 mM solution) before and after 14 days at 37°C (data not shown). The dose was selected based on previous studies in which the acute effect of TLQP-21 on restraint-induced catecholamine release (A.B., T.P., and A.M., unpublished work), hind paw formalin-induced licking behavior (A.B., R.P., and F.P., unpublished work), and penile erection (49) was assessed. The dose was also consistent with other VGF-derived peptides (37) and neuropeptides infused by means of osmotic minipumps (38, 39).
Surgery.
Alzet pumps and brain infusion kits were implanted under anesthesia (100 mg/kg ketamine i.p. and 5 mg/kg xylazine i.p.). Cannulae were implanted according to the stereotaxic coordinates (anteroposterior, −0.1 mm; mediolateral, ±1.0 mm; dorsoventral, 3 mm from bregma) (52). Cannulae were fixed to the mouse skull with polycarbonate cement (Shofu, Kyoto, Japan).
High-Fat Diet.
In the baseline phase, mice were fed with powdered pellets suspended in water to obtain a dense consistency. In the treatment phase, mice were fed with a high-fat diet consisting of diluted powdered rodent chow plus 20% lard (calculated energy content of the dry diet was 4.33 kcal/g).
Oral Glucose Tolerance Test.
Mice were fasted for 7 h (from 0730 to 1430) and orally injected with glucose at a dose of 3 g/kg. Blood samples were obtained from the tail vein before (baseline) and 30, 60, and 120 min after glucose load. Insulin, glucose, FFA, and TG levels were analyzed as described below.
Home Cage Locomotor Activity.
Activity was scored by means of an automated infrared system (Activiscope; New Behavior, Zürich, Switzerland).
Indirect Calorimetry.
EE was determined by indirect calorimetry with an Oxymax system (Columbus Instruments, Columbus, OH) with an air flow of 0.75 liter/min. O2 and CO2 gas content was recorded every 15 min for 21 h, starting at 1000. Mice had food and water ad libitum. The respiratory exchange ratio (VCO2/VO2) and EE were calculated by using Oxymax 5.9. Calculated data were analyzed according to mouse activity level that was determined by infrared sensors as follows: rest, 0 cpm; moderate, 1–80 cpm; high, >80 cpm.
Rectal Temperature.
Temperature was determined between 0900 and 1100 (room temperature was 22 ± 1°C) with a rectal probe (Physitemp Instruments, Clifton, NJ) connected to a portable thermometer (Hanna Instruments, Padua, Italy).
Serum Measurements.
Ghrelin, fee triiodothyronine, free thyroxine, total corticosterone, and insulin were measured with RIA. Leptin was measured with ELISA. Glucose was measured by the esokinase method. TG and FFA levels were measured with a colorimetric reaction kit. Serum NE and E levels were determined with HPLC (for details, see Supporting Materails and Methods which is published as supporting information on the PNAS web site).
RT-PCR.
Interscapular BAT, epididymal WAT, and hypothalami (landmarks: optic chiasma, lateral sulci, mammillary bodies, and third ventricle) were quickly dissected, collected in RNAlater (Sigma-Aldrich, St. Louis, MO), and stored at −80°C. RT-PCR was performed as described in ref. 40 (Table 5, which is published as supporting information on the PNAS web site).
Statistical Analysis.
Data from standard diet-fed groups were analyzed with Mann–Whitney’s U test or multivariate ANOVA [followed by Tukey’s honestly significant difference (HSD) post hoc tests, which can be used also with nonsignificant F values (53)] where appropriate. Data from high-fat diet groups were analyzed with two- or three-way ANOVA followed by Tukey’s HSD test. Correlations were performed with a nonparametric Spearman’s test.
Supplementary Material
Acknowledgments
We thank Prof. J. Feldon, C. Rösli, Dr. B. Roschitzki, and the team at the Functional Genomics Centre Zürich (Zürich, Switzerland). This work was supported by Fondo per gli Investimenti della Ricerca di Base Grants RBNE01JKLF_004 (to A.M.), RBNE01JKLF_006 (to R.P.), RBNE013XSJ_004 (to R.P.), RBNE01JKLF_008 (to V.L.), and RBNE01JKLF_01 (to E.E.M.); Instituto Zooprofilattico Sperimentale Grant SA 002/02 (to R.P.); the Fondo di Ateneo per la Ricerca of the University of Milan–Bicocca (A.T. and V.L.); the Fondo Integrativo Speciale per La Ricerca–Neurobiotecnologie and Telethon E0830 (to A.L.); European Molecular Biology Organization Fellowship ASTF 35-2005 (to G.L.C.); and Swiss National Science Foundation PIIB 109067/1 (to G.L.C.).
Abbreviations
- BAT
brown adipose tissue
- β-AR
β adrenergic receptor
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- i.c.v.
intracerebroventricular
- PPAR-δ
peroxisome proliferator-activated receptor δ
- MS/MS
tandem MS
- EE
energy expenditure
- NE
norepinephrine
- E
epinephrine
- WAT/bw
WAT/body weight
- TG
triglyceride
- FFA
free fatty acid
- aCSF
artificial cerebrospinal fluid.
Footnotes
Conflict of interest statement: No conflicts declared.
In a recent meeting abstract, Jethwa et al. (45) confirmed our observations by showing that subchronic central TLQP-21 treatment may reduce body and adipose tissue weight in the Siberian hamster.
References
- 1.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Barish GD, Narkar VA, Evans RM. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman JM. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 4.Lowell BB, Spiegelman BM. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 5.Cederberg A, Grønning LM, Ahren B, Tasken K, Carlsson P, Enerback S. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 6.Riu E, Ferre T, Hidalgo A, Mas A, Franckhauser S, Otaegui P, Bosch F. FASEB J. 2003;17:1715–1717. doi: 10.1096/fj.02-1163fje. [DOI] [PubMed] [Google Scholar]
- 7.Solveva V, Graves RA, Rasenick MM, Spiegelman BM, Ross SR. Mol Endocrinol. 1997;11:27–38. doi: 10.1210/mend.11.1.9870. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Lee C-H, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 9.Crowley VEF, Yeo GSH, O'Rahilly S. Nat Rev Drug Discov. 2002;1:276–286. doi: 10.1038/nrd770. [DOI] [PubMed] [Google Scholar]
- 10.Jandacek RJ, Woods SC. Drug Discov Today. 2004;9:874–880. doi: 10.1016/S1359-6446(04)03244-1. [DOI] [PubMed] [Google Scholar]
- 11.Levi A, Eldridge JD, Paterson BM. Science. 1985;229:393–395. doi: 10.1126/science.3839317. [DOI] [PubMed] [Google Scholar]
- 12.Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Possenti R, Levi A. Front Neuroendocrinol. 2000;21:199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- 13.Levi A, Ferri GL, Watson E, Possenti R, Salton SR. Cell Mol Neurobiol. 2004;24:517–533. doi: 10.1023/b:cemn.0000023627.79947.22. [DOI] [PubMed] [Google Scholar]
- 14.Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, et al. Neuron. 1999;23:537–548. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- 15.Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN, Lee C, Elmquist JK, Lechan RM, Mobbs CV, Salton SRJ. J Neurosci. 2002;22:6929–6938. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross AW, Bell LM, Littlewood PA, Mercer JG, Barrett P, Morgan PJ. Endocrinology. 2005;146:1940–1947. doi: 10.1210/en.2004-1538. [DOI] [PubMed] [Google Scholar]
- 17.Trani E, Giorni A, Canu N, Amadoro G, Rinaldi AM, Halban PA, Ferri GL, Possenti R, Schinina ME, Levi A. J Neurochem. 2002;81:565–574. doi: 10.1046/j.1471-4159.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- 18.Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB. J Neurosci. 2003;23:10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon B, Nedegaard J. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lowell BB, Flier JS. Ann Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 21.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 22.Silva JE. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 23.Rayner DV. Proc Nutr Soc; 2001. pp. 357–364. [DOI] [PubMed] [Google Scholar]
- 24.Fliers E, Kreier F, Voshol PJ, Havekes LM, Sauerwein HP, Kalsbeek A, Buijs RM, Romijn JA. J Neuroendocrinol. 2003;15:1005–1010. doi: 10.1046/j.1365-2826.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 25.Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 26.Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WHM, Astrup A. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 27.Garofano MA, Kettelhut IC, Rosolino JE, Migliorini RH. J Auton Nerv Syst. 1996;60:206–208. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DS. The Autonomic Nervous System in Health and Disease. New York: Dekker; 2001. [Google Scholar]
- 29.Morrison SF. Am J Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 30.Cinti S. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Tiraby C, Langin D. Trends Endocrinol Metab. 2003;14:439–441. doi: 10.1016/j.tem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Ito M, Tanaka T, Tokita S, Moriya M, et al. Am J Physiol. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- 33.Heleniak EP, Aston B. Med Hypot. 1989;28:13–33. doi: 10.1016/0306-9877(89)90149-7. [DOI] [PubMed] [Google Scholar]
- 34.Amir S, Schiavetto A. Brain Res. 1990;528:138–142. doi: 10.1016/0006-8993(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, et al. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uyama N, Geerts A, Reynaert H. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:808–820. doi: 10.1002/ar.a.20086. [DOI] [PubMed] [Google Scholar]
- 37.Pan H, Nanno D, Che F-H, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA. Biochemistry. 2005;44:4939–4948. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- 38.Baran K, Preston E, Wilks D, Cooney GJ, Kraegen EW, Sainsbury A. Diabetes. 2002;51:152–158. doi: 10.2337/diabetes.51.1.152. [DOI] [PubMed] [Google Scholar]
- 39.Overton JM, Williams DT, Chambers JB, Rashotte ME. Hypertension. 2001;37:663–669. doi: 10.1161/01.hyp.37.2.663. [DOI] [PubMed] [Google Scholar]
- 40.Bresciani E, Nass R, Torsello A, Gaylinn B, Avallone R, Locatelli V, Thorner MO, Muller EE. Neuroendocrinology. 2004;80:52–59. doi: 10.1159/000080793. [DOI] [PubMed] [Google Scholar]
- 41.Ziotopulou M, Mantzoros CS, Hileman SM, Flier JS. Am J Physiol. 2000;279:E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Wang P, Zheng H, Smith RG. Proc Natl Acad Sci USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Song CK, Jackson RM, Harris RBS, Richard D, Bartness TJ. Am J Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 45.Jethwa PH, Warner A, Brameld JM, Keyte JW, Nilaweera NK, Morgan PJ, Barrett P, Francis JP. Front Neuroendocrinol. 2006;27:5–6. [Google Scholar]
- 46.Watson E, Hahm S, Mizuno TM, Windsor J, Montgomery C, Scherer PE, Mobbs CV, Salton SR. Endocrinology. 2005;146:5151–5163. doi: 10.1210/en.2005-0588. [DOI] [PubMed] [Google Scholar]
- 47.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crebbe JC, Nestler EJ, Hen R. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 48.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 49.Succu S, Cocco C, Mascia MS, Melis T, Melis MR, Possenti R, Levi A, Ferri GL, Argiolas A. Eur J Neurosci. 2004;20:3035–3040. doi: 10.1111/j.1460-9568.2004.03781.x. [DOI] [PubMed] [Google Scholar]
- 50.Scheurer SB, Rybak JN, Roesli C, Brunisholz RA, Potthast F, Schlapbach R, Neri D, Elia G. Proteomics. 2005;5:2718–2728. doi: 10.1002/pmic.200401163. [DOI] [PubMed] [Google Scholar]
- 51.Scheurer SB, Roseli C, Neri D, Elia G. Proteomics. 2005;5:3035–3039. doi: 10.1002/pmic.200402069. [DOI] [PubMed] [Google Scholar]
- 52.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. London: Academic; 1997. [Google Scholar]
- 53.Wilcox RR. New Statistical Procedures for Social Science. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.