Abstract
Gβγ, a ubiquitous second messenger, relays external signals from G protein-coupled receptors to networks of intracellular effectors, including voltage-dependent calcium channels. Unlike high-voltage-activated Ca2+ channels, the inhibition of low-voltage-activated Ca2+ channels is subtype-dependent and mediated selectively by Gβ2-containing dimers. Yet, the molecular basis for this exquisite selectivity remains unknown. Here, we used pure recombinant Gβγ subunits to establish that the Gβ2γ2 dimer can selectively reconstitute the inhibition of α1H channels in isolated membrane patches. This inhibition is the result of a reduction in channel open probability that is not accompanied by a change in channel expression or an alteration in active-channel gating. By exchanging residues between the active Gβ2 subunit and the inactive Gβ1 subunit, we identified a cluster of amino acids that functionally distinguish Gβ2 from other Gβ subunits. These amino acids on the β-torus identify a region that is distinct from those regions that contact the Gα subunit or other effectors.
Keywords: α-1H channels, channel regulation, Gβγ dimers
Low-voltage-activated (LVA) Ca2+ currents carried by α1H Ca2+ channels play well documented roles in the regulation of neuronal excitability (1). α1H currents are important in the perception and transmission of noxious stimuli (2) and in the development of neuropathic pain after peripheral axonal injury (3), and the α1H gene (CACNA1H) is a susceptibility locus for childhood absence epilepsy (4). α1H channels are regulated by kinases (5), redox potential (6), and the activation of G protein-coupled receptors that generate membrane-delimited signals (7–9). For example, in neurons of the dorsal root ganglion, GABAB (10), adenosine A1 (11), and dopamine (7) receptor activation produce a steady-state inhibition of α1H whole-cell currents. Current inhibition is reproduced by photoreleased GTPγS and blocked by pertussis toxin pretreatment (10) and can be voltage-independent. Thus, dopamine-mediated inhibition is not temporarily reversed by a strong depolarizing pulse and is replicated in excised membrane patches, manifesting as a decrease in the probability of opening of LVA α1H channels (7). Analogously, Gβ2γ2 dimers elicit a voltage-independent inhibition of α1H whole-cell currents carried by recombinant channels by binding directly to the II-III loop of the channel protein (9).
To date, 7 Gβ and 12 Gγ subunits have been identified in mammalian systems, producing a large number of potentially unique dimers that could differ in their interactions among effectors (12). However, the complexity of these signals is mitigated by the reality that the Gβ1–4 subunits are >85% identical in amino acid sequence (13). Thus, although there are differences in potency and efficacy, Gβγ dimers containing any of the Gβ1–4 subunits can regulate many effectors, such as G protein-activated inwardly rectifying K (GIRK) K+ channels (13, 14) and high-voltage-activated Ca2+ channels (15–18). In surprising contrast, only Gβγ dimers containing Gβ2 decrease whole-cell currents carried by recombinant α1H channels; dimeric isoforms containing Gβ(1,3–4) are not modulatory (9).
In this study, we took advantage of this remarkable differential modulation to understand how this selectivity in Gβ signaling is achieved. Our results identify a cluster of amino acids that functionally distinguish Gβ2 from other Gβ subunits and define a region that is distinct from those regions on the β-torus that make contact with the α-subunit or other effectors.
Results
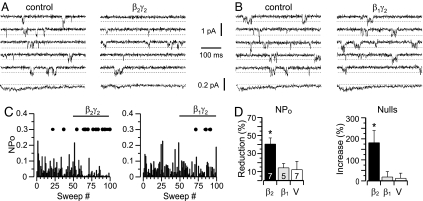
Gβ2-containing dimers selectively inhibit the activity of α1H channels and do not inhibit the α1G channel, a T-type Ca2+ channel homolog (9). Because the II-III loop of α1H channels preferentially binds Gβ2-containing subunits and confers current inhibition to unregulated α1G channels (9), we hypothesized that modulation of α1H channel gating by Gβ2γ2 was mediated by the direct binding of Gβγ dimers to the channel protein and, thus, would be membrane-delimited. To test this possibility, we measured single-channel currents in membrane patches excised from HEK-293 cells stably expressing α1H Ca2+ channels, comparing the effects of pure, recombinant Gβ2γ2 and Gβ1γ2 dimers (Fig. 1). ICa was activated by a depolarizing pulse to −35 mV, and control activity was monitored for 5–6 min before direct application of either Gβγ isoform. α1H Ca2+ channels active at −35 mV displayed diverse patterns of small openings (Fig. 1 A and B) and full steady-state potential-dependent inactivation (data not shown). After the application of Gβ2γ2 to the bath (x̄ = 2 nM), α1H channels opened less frequently, decreasing the average ensemble current (105 sweeps, concatenated from three cells) to 45.7% of control (Fig. 1A). By contrast, bath application of Gβ1γ2 (x̄ = 2 nM) did not change the frequency of single-channel opening or decrease the average ensemble current (Fig. 1B).
Fig. 1.
Recombinant Gβ2γ2, but not Gβ1γ2, inhibits α1H channel activity in patches excised from HEK-293 cells. (A and B) Shown are records and analysis of single-channel ICa currents elicited by repeated (6-sec) test pulses to −35 mV from −90 mV. Five consecutive sweeps and the ensemble average of 105 sweeps concatenated from three cells (bottom trace) recorded before (Left) and after (Right) reconstitution of 1 nM Gβ2γ2 (A) or Gβ1γ2 (B) into the patch. (C) Time course of NPo from two representative patches before and after adding Gβγ. Sequential exposure to vehicle (sweeps 1–50) and in sweeps 51–100 Gβγ dimer: Gβ2γ2 (Left) or Gβ1γ2 (Right). Blank/inactive sweeps (Nulls) are indicated by filled circles arbitrarily drawn at 0.3 (NPo) for clarity. (D) Bar graphs plot mean ± SEM of the percent reduction in NPo (Left) or percent increase in nulls (Right) elicited by Gβ2γ2 or Gβ1γ2 relative to vehicle exposure for each patch. Numbers are patches recorded for each condition. ∗, P < 0.05 compares vehicle with treatment groups by ANOVA.
Fig. 1C illustrates the time course of channel modulation by Gβ2γ2 from an exemplar recording of an excised patch with at least two active α1H channels. For each sweep, a value of channel open probability (NPo) was calculated from the ratio of the total open time and the test pulse duration. Gβ2γ2 markedly decreased NPo and concomitantly increased the number of silent sweeps (denoted by a solid circle, Fig. 1C). The decrease in NPo ranged from 72% to 27% in seven patches and was accompanied by a 181 ± 57% increase in the number of null sweeps (Fig. 1D). This degree of inhibition was close to maximal, because a 10-fold-higher concentration of Gβ2γ2 (x̄ = 20 nM) only decreased NPo to 70.9 ± 7.6% (n = 3). Moreover, the Gβ2γ2-induced decrease in NPo was not accompanied by an increase in the latency to first opening (49.7 ± 4.3 ms (control), 48.7 ± 5.8 ms (β2γ2), n = 3 cells, not significant) or by a change in the single-channel conductance of 6.4 pS (Fig. 5C, which is published as supporting information on the PNAS web site). The amplitude distribution of the test-pulse currents before and after the addition of Gβ2γ2 yielded average midpoints for the primary conductance state (70% of openings) that were not different from each other (−0.419 ± 0.003 pA (vehicle); −0.389 ± 0.004 pA (β2γ2), at −35 mV). Nor did Gβ2γ2 alter the open-state distribution of dwell times, which remained well fitted to the sum of two exponentials with unchanged proportions (τ1 = 0.44 ± 0.07 ms (68%); τ2 = 2.86 ± 0.15 ms (32%), Fig. 5 A and B). Therefore, a Gβ2γ2-elicited decrease in α1H whole-cell current is the apparent consequence of a decrease in the number of channel openings. By contrast, Gβ1γ2 (x̄ = 2 nM) had no effect on NPo in five patches showing macroscopic currents (Fig. 1B and D), consistent with its failure to modulate whole-cell current in published overexpression studies (9). Finally, we tested for the ability of Gβ2γ2 to inhibit α1H(GII-III) unitary currents. In concert with its whole-cell behavior, Gβ2γ2 failed to decrease the open probability of α1H(GII-III) chimeric channels (117 ± 13%, n = 4) in which the II-III loop is replaced with that from unregulated α1G channels (9). Taken together, these data, with recombinant protein delivered at low nanomolar concentrations, establish a modulation that is membrane-delimited, and selective for Gβ2, and clarify the elementary events underlying the Gβ2γ2-elicited inhibition of whole-cell current. This modulation differs fundamentally from the inhibition of N-, P/Q-, and R-type Ca2+ channels that is mediated by all Gβγ subtypes (13, 17, 19).
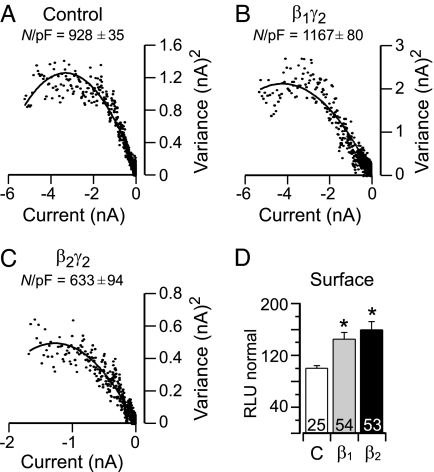
Because of the difficulty of obtaining patches expressing a single recombinant channel, our analysis of single-channel currents precluded a distinction between a Gβ2γ2-mediated change in N, the number of active channels, and Po, the probability of finding the channel open. Therefore, we used nonstationary noise analysis to analyze the fluctuations in macroscopic current caused by the opening and closing of α1H channels to determine and compare the effect of Gβ2γ2 with that of Gβ1γ2 (20, 21). We conducted variance analysis of the current elicited upon repolarization to −70 mV after a test pulse to +30 mV that was optimized to produce maximal channel opening with no apparent macroscopic inactivation. Fitted parameters from variance-mean current plots (Fig. 2A–C), indicate no change in single-channel current current (i) or the maximal activity (Pomax) of an active channel but rather, a Gβxγ2-elicited change in the number (N) of active channels in the membrane. Notably, the expression of Gβ1γ2 increased the number of active channels in the membrane, in contrast to Gβ2γ2, which reduced it.
Fig. 2.
Gβ1 and Gβ2 increase surface channel expression, but only Gβ2 reduces active channel number. (A–C) Variance analysis of ICa whole-cell currents. Tail currents were elicited from HEK-293 α1H-stable cells coexpressing GFP and empty vector (pcDNA3) or GFP, Gγ2, and either wild-type Gβ2 or Gβ1 by repolarization to −70 mV after repetitive (50 times for 6 sec) test pulses to +30 mV (6 ms), from −90 mV. Shown are variance versus current plots fitted to the function σ2 = i I(t) − I(t)2/N (solid line). Superimposed is the mean number of active channels in the patch (N/pF). Notably, the number of active channels (N/pF) was increased with Gβ1γ2 expression but reduced with Gβ2γ2 expression compared with control (each n = 4, P < 0.05). Values for unitary current (i) and Pomax were unchanged in each condition: control (i = 0.73 ± 0.06 pA, Pomax = 0.57 ± 0.03, n = 4), Gβ2γ2 (i = 0.81 ± 0.04 pA, Pomax = 0.60 ± 0.03, n = 4), Gβ1 (i = 0.82 ± 0.06 pA, Pomax = 0.62 ± 0.05, n = 4). (D) Quantification of the expressed levels of HA-tagged α1H channels by luminometry. Chemiluminescence from fixed TSA201 cells coexpressing HA-tagged α1H channels, Gγ2, and either empty vector Gβ1 or Gβ2 subunits. Intensity of photon emission is expressed as relative light units (RLU). The histogram plots values for α1H surface expression. Numbers indicate individual cells studied for each condition. ∗, P < 0.05 compares control with treatment groups by ANOVA.
Because cell-surface biotinylation experiments (9) suggested that Gβ2γ2 did not reduce the surface expression of α1H channels, we interpreted the Gβ2γ2-elicited decrease in active channel number (N) to be the result of reduced channel activity. To verify this interpretation, we used an α1H channel construct that incorporates a hemagglutinin (HA) epitope on the large extracellular loop of domain I (S5-H5). We evaluated surface expression of channels, using luminometry to monitor the levels of chemiluminescence (22). α1H-HA channels are fully functional and, like wild-type channels, are modulated by Gβ2γ2 (58.3 ± 7.4% inhibition at −20 mV, n = 12). Although the expression of the Gγ2 subunit alone did not affect the membrane expression of α1H-HA channels, coexpression with either Gβ1 or Gβ2 significantly increased the membrane expression of channels [45 ± 3.2% of control (Gβ1γ2, n = 54) and 59 ± 4.8% of control (Gβ2γ2, n = 53); Fig. 2D]. These data likely explain the Gβ1γ2-elicited increase in active channel number revealed with noise analysis but cannot account for the reduction in functional channel number mediated by Gβ2γ2. Thus, we conclude that Gβ2γ2 markedly decreases α1H channel NPo solely by diminishing the stochastic behavior of α1H channels.
The Gβ subunit is a member of a large family of proteins that share a highly conserved WD40 repeating motif. In the Gβ subunit, the seven repeating motifs define a circular propeller-like structure, with each “blade” formed by four antiparallel β-strands designated a–d (13). Extensive mutational analysis has identified the top and side of the β-torus as surfaces for interaction with specific effectors, such as adenylyl cyclase, phospholipase C-β, and several ion channels (13). Interestingly, these same surfaces also mediate the inactivation of the Gα subunit as it reassembles into the α:βγ heterotrimer (13, 23).
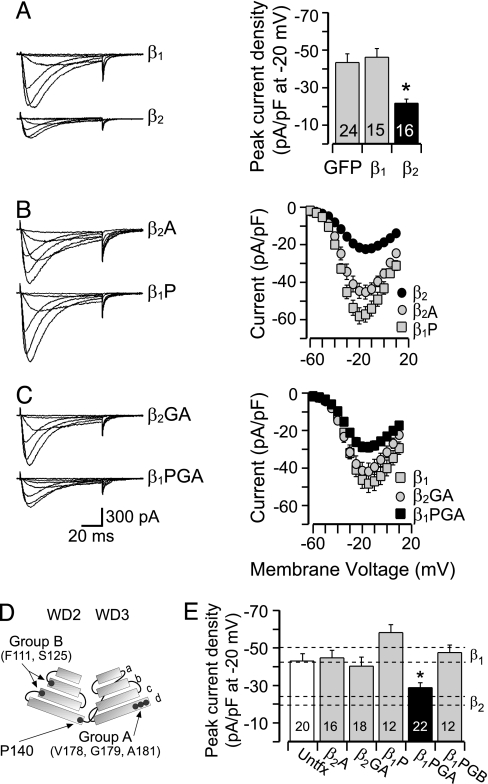
To identify a nonhomologous set of residues in Gβ2 that could determine the observed specificity of Gβγ action, we mutated candidate residues that were unique to Gβ2 and predicted to be surface-exposed based on the crystal structure of bovine Gβ1. We selected P140 (human β2 sequence), positioned at the end of the outermost d-strand of blade 2 in Gβ2, and residues sited on the d-strand of blade 3 (Group A: V178, G179, A181) or on the b- and c-strands of blade 2 (Group B: F111, S125). Together, these solvent-exposed amino acids cluster to form distinct domains on the surface of the Gβ protein (Fig. 3D and Fig. 6, which is published as supporting information on the PNAS web site) and, thus, could form interaction sites that functionally distinguish Gβ1 from Gβ2.
Fig. 3.
Four residues are critical for Gβ2-mediated inhibition of α1H channels. Shown are records from HEK-293 α1H-stable cells expressing various Gβxγ2 dimers and analysis of ICa whole-cell currents. (A–C Left) Family of sample currents recorded at Vm = −60, −50, −40, −20, −5, and +10 mV (from Vhold −90 mV) from cells coexpressing GFP and Gγ2 and either wild-type Gβx (A) or mutant Gβx proteins β2A(P140A), β1P(A140P) (B); β2GA(V178S, G179S, A181S), β1PGA(A140P, S178V, S179G, S181A) (C). (A–C Right) (A) Histogram plots mean ± SEM of peak current density (pA/pF) obtained from full current–voltage relationships (I–V). (B and C) Maximal current at each test depolarization plotted to construct I–V relationships for cells expressing wild-type or mutant Gβγ dimers. Current amplitude is expressed as pA/pF to correct for differences in cell size. (D) Schematic model of WD2 and WD3, each showing β-strands (a–d) from Gβ2. Locations of amino acids selected for mutation are indicated: Group A (GA), Group B (GB), and residue 140. (E) Mean current density (±SEM) at −20 mV (I–V peak) is plotted for each Gβxγ2 construct. Dashed lines denote I-V peak (mean ± SEM) for wild-type Gβ2- or Gβ1-expressing cells for comparison. Numbers indicate cells recorded for each condition. ∗, P < 0.05 compares untransfected (untfx) peak current density with that of Gβxγ2-expressing cells by ANOVA.
Consistent with observations (9), the selective overexpression of Gβ2-containing dimers in HEK-293 cells expressing α1H channels decreased whole-cell current at all voltages, reducing peak current density to 50.1 ± 12.4% of that recorded from cells expressing GFP alone (Fig. 3A). Mutation of P140 in Gβ2 to the corresponding residue in Gβ1 (P140A) restored current density to levels that were indistinguishable from cells expressing Gβ1 dimers (Fig. 3 B and E). However, the reciprocal mutation in Gβ1 (A140P) did not confer current inhibition. We hypothesized that a spatially contiguous region on Gβ2, the d-strand of blade 3, may also be required for channel-modulatory activity. Indeed, mutagenesis of the selected Group A residues on Gβ2 to the corresponding residues in Gβ1 (V178T, G179T, and A181T) restored current density to levels recorded in GFP-transfected or untransfected cells (Gβ2GA; Fig. 3 C and E). Because the independent mutagenesis of P140 and Group A residues removed inhibition, we reasoned that, together, these residues might define a region critical for α1H channel interaction. In combination with the mutagenesis of A140P, the reciprocal mutation of Group A residues in Gβ1 conferred channel-inhibitory activity to Gβ1 (Gβ1PGA; Fig. 3 C and E) that notably was identical to that of wild-type Gβ2. Moreover, this set of residues (P140, V178, G179, and A181) produced a channel-inhibitory activity in Gβ1 that was not mimicked by the Gβ1PGB mutant (A140P, Y111F, and N125S), highlighting the specificity of the identified region for channel-inhibitory interactions (Fig. 3E).
Because all Gβ(1–4)γx subunits stimulate GIRK1,4 channel activity (13, 14), we tested each mutant in a GIRK1,4 whole-cell channel assay to confirm activity. As expected, both Gβ1- and Gβ2-containing dimers increased the conductance of GIRK channels equivalently, eliciting an ≈4-fold stimulation that was mimicked by Gβ mutants that either lacked or possessed α1H channel-inhibitory activity (Fig. 7, which is published as supporting information on the PNAS web site). Thus, residue swapping on blades 2 and 3 of Gβγ did not disrupt GIRK-effector contact sites that map to other regions of the Gβγ structure.
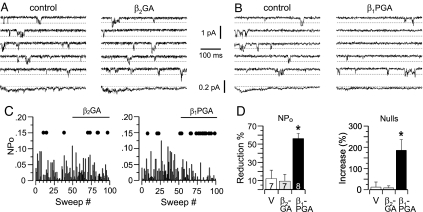
Based on our whole-cell studies, we anticipated that the Gβ2GA mutations would remove Gβ2 activity, and the Gβ1PGA mutations would confer activity to recombinant proteins tested for direct channel-inhibitory activity. We prepared recombinant Gβγ protein encoding these mutant sequences. Unlike wild-type Gβ2, Gβ2GA failed to decrease single-channel activity (Fig. 4A). Neither the NPo nor the number of null sweeps was changed within the 5-min recording period by Gβ2GA added to each of seven patches (Fig. 4 A, C, and D). By contrast, Gβ1PGA consistently and significantly reduced the single-channel open probability, decreasing NPo by 56 ± 6% and concomitantly increased the number of silent sweeps by 186 ± 50% (Fig. 4 B–D). These changes in single-channel activity fully replicate the inhibitory activity of wild-type Gβ2. Thus, candidate sites identified in our whole-cell studies provide the molecular basis for Gβ2 inhibitory activity.
Fig. 4.
After residue swapping, recombinant Gβ1PGAγ2, but not Gβ2GAγ2, inhibits α1H channel activity in the excised patch. Shown are records and analysis of single-channel ICa currents elicited by repeated (6-sec) test pulses to −35 mV from −90 mV (Methods). (A and B) Five consecutive sweeps and the ensemble average of 105 sweeps concatenated from three cells (bottom trace) recorded before (Left) and after (Right) reconstitution of 1 nM recombinant Gβ2GAγ2 (A) or Gβ1PGAγ2 (B). (C) Time course of NPo from two representative patches. Sequential exposure to vehicle (sweeps 1–50) and in sweeps 51–100 Gβxγ2 dimer: Gβ2GAγ2 (Left) or Gβ1PGAγ2 (Right). Blank/inactive sweeps (nulls) are indicated by filled circles arbitrarily drawn at 0.15 (NPo) for clarity. (D) Bar graphs plot mean ± SEM of the percent reduction in NPo (Left) or percent increase in nulls (Right) elicited by Gβ2GAγ2 or Gβ1PGAγ2 relative to vehicle exposure for each patch. Numbers indicate patches recorded for each condition. ∗, P < 0.05 compares vehicle with treatment groups by ANOVA.
Discussion
It is now well established that ion channels are direct downstream targets of Gβγ dimers. Gβγ dimers increase GIRK K+ currents (13, 14) and persistent Na+ currents (24) and inhibit high-voltage-activated Ca2+ currents carried by N-, P/Q- and R-type Ca2+ channels (19, 25). We show here that the inhibition of α1H channels also depends on a direct Gβγ–channel interaction. Only the dimeric isoform that binds to the II-III loop of α1H channels, Gβ2γ2, inhibits α1H unitary current in the excised patch, and α1H(GII-III) chimeric channels, to which Gβ2γ2 cannot bind, are not inhibited by Gβ2γ2. However, even though this inhibition is membrane-delimited, Gβ2γ2 neither slows the kinetics of channel opening to prolong the latency to first opening nor shifts the voltage-dependence of channel activation. Moreover, inhibition cannot be temporarily relieved by a strong depolarizing prepulse. Thus, the inhibition of α1H channels does not possess the hallmarks of voltage-dependent inhibition of N- and P/Q-type channels transduced by Gβγ dimers (15, 25).
On the other hand, voltage-independent (VI) inhibition of high-voltage-activated (HVA) channels manifests as a scaled reduction in steady-state current (26) that is not reversed by prepulse (27, 28). VI inhibition does not rely on a direct Gβγ-channel interaction but, rather, may be the result of either second-messenger-induced phosphorylation and internalization of the channel pore protein (28–30) or the depletion of membrane acidic phosphoinositides (PtdIns(4,5)P2) (26, 31) that destabilizes channel activity. Here, we show that, although Gβ2γ2 effects a scaled reduction in current and reduces the number of functional channels, α1H channels are not removed from the plasma membrane, and channel activity is not destabilized in the excised patch configuration, even with Ca2+ chelation. Thus, from our experiments, we conclude that the inhibition of α1H channels by Gβ2γ2 defines a unique inhibitory behavior.
Our experiments also identify a cluster of 4 aa (P140, V178, G179, and A181) that can account for the specificity of Gβ2γ2 action on α1H channels. The loss of function upon removal of these residues from Gβ2 and the complementary gain of function upon their transfer to Gβ1 argues strongly that these residues, located on the outermost strand of blades 2 and 3, are essential for the interaction of Gβ2-containing dimers with α1H channels. By contrast, multiple effector-binding regions on the β-torus have been found to be important for regulating HVA channel activity. Early mutagenesis studies identified residues that cluster on the top surface of the β-torus and along the side of blade 1 within a binding surface shared by the GDP-bound form of the α-subunit (32). However, other regions that lie opposite to the Gα-interaction surface, containing critical residues Y111 and S189, also have been identified for their importance in Gβγ subtype-selective potency (33, 34). Nonetheless, these regions are not sufficient to confer inhibitory activity to inactive rat Gβ5, suggesting additional critical N-type Ca2+ channel-interacting sites remain to be identified (33). On the other hand, the C-terminal 25 aa of the β-torus can confer full Gβγ regulation to both P/Q-type Ca2+ channels and GIRK K+ channels, suggesting that blades 6 and 7 may form a common binding surface on the β-torus for these effectors (35).
Specificity in Gβγ signaling typically is achieved by coupling specific heterotrimeric G proteins to distinct types of receptors (36) and by unique responses among effector isotypes (36, 37). Our data indicate that specificity can also be determined by unique effector contact sites on the surface of the β-torus. We have identified a set of residues on Gβ2 that can account for its differential regulation of α1H channel activity. These residues may not be the only sites of effector contact with α1H channels, because other residues conserved across all Gβγ subtypes may also be important structural determinants. Nevertheless, our studies show that Gβγ subtype-selective activity can be determined by a few residues. This mode of selectivity allows Gβγ the choice of effector for the transmission of its signal and raises the possibility that, in the dorsal root ganglion, LVA Ca2+ channels are chosen for inhibition by Gβ2.
Methods
Cell Culture and Transfection.
HEK-293 cells stably expressing α1H channels were cultured (9) and transiently cotransfected with plasmids for GFP/Gγ2/Gβx in a ratio of 1:6:6 μg by using CaPO4 as reported (9). Cells were cultured 2 days before overnight plating for electrophysiological recording made 72 h after transfection (9).
Molecular Biology.
The residue 140, Group A, and Group B mutations were introduced by using the QuikChange XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) (see Supporting Methods, which is published as supporting information on the PNAS web site, for primer sequences). All mutations were confirmed by sequencing (University of Virginia Sequencing Facility), and coding regions were excised (BamH1/XhoI) and subcloned into the mammalian expression vector pcDNA3.1.
Generation of Recombinant Gβxγ2.
The coding regions from all wild-type and mutant Gβ1 or Gβ2 constructs (Guthrie Research Institute, Sayre, PA) were excised from pcDNA3.1 by BamH1/XbaI restriction digest and subcloned into pVL1393 baculovirus transfer vector (BD Biosciences/Pharmingen, San Diego, CA). Constructs were validated by restriction enzyme digest, fragment analysis, and sequencing. Recombinant Gβx baculoviruses were generated in Sf9 insect cells by using the BaculoGold system (BD Biosciences/Pharmingen), plaque purified, and a high-titer stock prepared (36). Sf9 cells were infected at a MOI of 3 with high-titer virus encoding for a [His]6-tagged Gαi1 and the desired Gβx and Gγ2 subunits, cell membranes prepared, and the extracts applied to a Ni2+-NTA column. Gβxγ2 subunits were eluted from the Ni2+-bound αi subunit with AlF, ensuring the recovery of correctly folded Gβγ dimers (36). The activity of pure Gβγ dimers was verified by testing for PLC-β activation in synthetic lipid vesicles (37).
Electrophysiology.
HEK-293 cells were recorded as described by using an Axopatch 200A amplifier, and data were collected with PCLAMP 9.2 software.
Single-channel.
Currents from inside-out excised patches were elicited by a test pulse to −35 or −40 mV from −90 mV (200 msec, 6-sec interpulse) (38). Currents were filtered at 2 kHz and sampled at 100 kHz. The external (pipette) solution was 75 mM CsCl, 60 mM CaCl2, and 10 mM Hepes, pH 7.4 (with CsOH). The bath solution was 140 mM potassium-aspartate, 5 mM MgCl2, 10 mM EGTA, 20 mM Hepes, pH 7.4 (with KOH), 0.162 mM CHAPS (0.01%), and 0.04 mM DTT. Purified Gβγ subunits were diluted in bath solution. At the end of each recording, the exact Gβγ concentration (1–20 nM) was determined from the measured bath volume. Analysis of single-channel records was performed by using Clampfit 9.2 (Axon Instruments/Molecular Devices, Sunnyvale, CA) using the 50% threshold crossing criterion for event detection (further details in Supporting Methods).
Whole-cell.
Currents were elicited as described (9). The internal (pipette) solution was 115 mM CsCl, 1 mM TBACl, 1 mM MgCl2, 5 mM Mg-ATP, 1 mM Li-GTP, 20 mM Hepes, pH 7.2 (with CsOH), and 11 mM BAPTA; added CaCl2 fixed free Ca2+ at 27 nM (0.9 mM). The bath solution was 127 mM TEACl, 10 mM CaCl2, 0.5 mM MgCl2, 10 mM Hepes, 5 mM dextrose, and 32 mM sucrose, pH 7.4 (with CsOH). Currents were filtered at 2 kHz and sampled at 12.5 kHz, and leak subtraction was performed on line by using scaled hyperpolarizing steps of one-fourth amplitude (P/N4). ANOVA was used for statistical examination with post hoc Dunnett’s test, where significance was taken as P < 0.05. Data are given as mean ± SEM.
Noise analysis.
Tail currents were elicited by −70 mV after repetitive (6-sec) test pulses (6 msec, +30 mV, from −90 mV) from HEK-293 cells stably expressing α1H channels. The internal (pipette) solution was identical to that described above for whole-cell recording. Bath solution replaced Ca with Ba to reduce charge screening: 117 mM TEACl, 20 mM BaCl2, 0.5 mM MgCl2, 10 mM Hepes, 5 mM dextrose, and 32 mM sucrose, pH 7.4 (adjusted with CsOH). Currents were filtered at 5 kHz and sampled at 25 kHz; leak subtraction was performed on line by using scaled hyperpolarizing steps of one-fourth amplitude (P/N4). ANOVA was used for statistical examination with post hoc Dunnett’s test, where significance was taken as P < 0.05.
Nonstationary Noise (Variance) Analysis.
Briefly, [I(t)] for each isochrone was used to compute the experimental nonstationary ensemble variance. To correct for basal noise, the average variance at −90 mV was subtracted from that obtained during the test pulse. The subtracted variance (σ2) of the tail current (−70 mV) was plotted versus the mean current and the data fitted to (20) σ2 = iI(t) − I(t)2/N, where i = single-channel current amplitude, and N = number of active channels. i was obtained from the initial slope and N from nonlinear curve-fitting analysis performed using Origin (OriginLab, Northampton, MA). The maximum open probability, Pomax, was obtained from the relationship: Pomax = Imax/iN, where Imax is the maximum mean current measured in the experiment.
Surface Expression Measurements.
TSA201 cells cultured in 24-well plates were transiently transfected by using Fugene 6 (Roche, Basel, Switzerland) with plasmids for α1H-HA, (Cav3.2-HA-GFP-pEGFP-C), Gγ2, and either the Gβ1 or Gβ2 at a 1:1:1 ratio (0.5 μg of DNA per well). The culture medium (Invitrogen) was changed 24 h after transfection and the luminometric assay performed as described (22) on 4% paraformaldehyde-fixed cells. Primary and secondary antibodies used for α1H channel detection were a monoclonal rat anti-HA (clone 3F10; Roche Applied Science) and a horseradish peroxidase (The Jackson Laboratory, Bar Harbor, ME), respectively (further details in Supporting Methods). Eleven independent sets of transfections were performed for each condition and results presented as mean ± SEM (P < 0.05).
Supplementary Material
Acknowledgments
We thank Robert Clay for technical assistance. This work was supported by National Institutes of Health Grants HL36977 (to P.Q.B.) and DK19952 (to J.C.G.). S.D.D. was supported by a predoctoral fellowship from the American Heart Association Mid-Atlantic Affiliate.
Abbreviations
- GIRK
G protein-activated inwardly rectifying K
- HA
hemagglutinin
- LVA
low-voltage-activated
- NPo
channel open probability.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Perez-Reyes E. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 2.Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Pain. 2004;109:328–339. doi: 10.1016/j.pain.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. EMBO J. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, et al. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 5.Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. J Neurosci. 2003;23:10116–10121. doi: 10.1523/JNEUROSCI.23-31-10116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon IM, Randall AD, Perez-Reyes E, Peers C. Pflugers Arch Eur J Physiol. 2000;441:181–188. doi: 10.1007/s004240000424. [DOI] [PubMed] [Google Scholar]
- 7.Marchetti C, Carbone E, Lux HD. Pflugers Arch Eur J Physiol. 1986;406:104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- 8.Lledo PM, Homburger V, Bockaert J, Vincent JD. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. Nature. 2003;424:209–213. doi: 10.1038/nature01772. [DOI] [PubMed] [Google Scholar]
- 10.Scott RH, Wootton JF, Dolphin AC. Neuroscience. 1990;38:285–294. doi: 10.1016/0306-4522(90)90028-3. [DOI] [PubMed] [Google Scholar]
- 11.Scott RH, Dolphin AC. Topics and Perspectives in Adenosine Research, Proceedings of the Third International Adenosine Symposium; Munich: Springer; 1987. pp. 549–558. [Google Scholar]
- 12.Hildebrandt JD. Biochem Pharmacol. 1997;54:325–339. doi: 10.1016/s0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 13.Clapham DE, Neer EJ. Ann Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 14.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 15.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Velasco V, Ikeda SR. J Neurosci. 2000;20:2183–2191. doi: 10.1523/JNEUROSCI.20-06-02183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnot MI, Stotz SC, Jarvis SE, Zamponi GW. J Physiol (London) 2000;527(Pt 2):203–212. doi: 10.1111/j.1469-7793.2000.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia DE, Li B, Garcia-Ferreiro RE, Hernandez-Ochoa EO, Yan K, Gautam N, Catterall WA, Mackie K, Hille B. J Neurosci. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolphin AC. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- 20.Sigworth FJ. J Physiol (London) 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez O, Gonzalez C, Latorre R. Adv Physiol Educ. 2002;26:327–341. doi: 10.1152/advan.00006.2002. [DOI] [PubMed] [Google Scholar]
- 22.Dubel SJ, Altier C, Chaumont S, Lory P, Bourinet E, Nargeot J. J Biol Chem. 2004;279:29263–29269. doi: 10.1074/jbc.M313450200. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 24.Ma JY, Catterall WA, Scheuer T. Neuron. 1997;19:443–452. doi: 10.1016/s0896-6273(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 25.Zamponi GW, Snutch TP. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 27.Diverse-Pierluissi M, Dunlap K. Neuron. 1993;10:753–760. doi: 10.1016/0896-6273(93)90175-q. [DOI] [PubMed] [Google Scholar]
- 28.Diverse-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tombler E, Cabanilla NJ, Carman P, Permaul N, Hall JJ, Richman RW, Lee J, Rodriguez J, Felsenfeld DP, Hennigan RF, Diverse-Pierluissi MA. J Biol Chem. 2006;281:1827–1839. doi: 10.1074/jbc.M508829200. [DOI] [PubMed] [Google Scholar]
- 30.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, et al. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 31.Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. J Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, et al. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 33.Doering CJ, Kisilevsky AE, Feng ZP, Arnot MI, Peloquin J, Hamid J, Barr W, Nirdosh A, Simms B, Winkfein RJ, Zamponi GW. J Biol Chem. 2004;279:29709–20717. doi: 10.1074/jbc.M308693200. [DOI] [PubMed] [Google Scholar]
- 34.Tedford HW, Kisilevsky AE, Peloquin JB, Zamponi GW. J Neurophysiol. 2006;96:465–470. doi: 10.1152/jn.00216.2006. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Hummer A, Han J, Xie M, Melnik-Martinez K, Moreno RL, Buck M, Mark MD, Herlitze S. J Biol Chem. 2005;280:23945–23959. doi: 10.1074/jbc.M414078200. [DOI] [PubMed] [Google Scholar]
- 36.McIntire WE, MacCleery G, Garrison JC. J Biol Chem. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- 37.Myung CS, Yasuda H, Liu WW, Harden TK, Garrison JC. J Biol Chem. 1999;274:16595–16603. doi: 10.1074/jbc.274.23.16595. [DOI] [PubMed] [Google Scholar]
- 38.Barrett PQ, Lu HK, Colbran R, Czernik A, Pancrazio JJ. Am J Physiol. 2000;279:C1694–C1703. doi: 10.1152/ajpcell.2000.279.6.C1694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.