Abstract
Allopregnanolone (ALLO) and tetrahydrodeoxycorticosterone (THDOC) are potent positive allosteric modulators of GABA action at GABAA receptors. ALLO and THDOC are synthesized in the brain from progesterone or deoxycorticosterone, respectively, by the sequential action of two enzymes: 5α-reductase (5α-R) type I and 3α-hydroxysteroid dehydrogenase (3α-HSD). This study evaluates 5α-R type I and 3α-HSD mRNA expression level in mouse brain by using in situ hybridization combined with glutamic acid decarboxylase 67/65, vesicular glutamate transporter 2, glial fibrillary acidic protein, and S100β immunohistochemistry. We demonstrate that 5α-R type I and 3α-HSD colocalize in cortical, hippocampal, and olfactory bulb glutamatergic principal neurons and in some output neurons of the amygdala and thalamus. Neither 5α-R type I nor 3α-HSD mRNAs are expressed in S100β- or glial fibrillary acidic protein-positive glial cells. Using glutamic acid decarboxylase 67/65 antibodies to mark GABAergic neurons, we failed to detect 5α-R type I and 3α-HSD in cortical and hippocampal GABAergic interneurons. However, 5α-R type I and 3α-HSD are significantly expressed in principal GABAergic output neurons, such as striatal medium spiny, reticular thalamic nucleus, and cerebellar Purkinje neurons. A similar distribution and cellular location of neurosteroidogenic enzymes was observed in rat brain. Taken together, these data suggest that ALLO and THDOC, which can be synthesized in principal output neurons, modulate GABA action at GABAA receptors, either with an autocrine or a paracrine mechanism or by reaching GABAA receptor intracellular sites through lateral membrane diffusion.
Keywords: 3α-hydroxysteroid dehydrogenase, 5α-reductase (type I), GABAergic neurons, glutamatergic neurons
The neurosteroids 3α-hydroxy-5α-pregnan-20-one [allopregnanolone (ALLO)] and 3α,21-dihydroxy-5α-pregnan-20-one [tetrahydrodeoxycorticosterone (THDOC)] are potent positive allosteric modulators of GABA action at GABAA receptors (1–6). These neurosteroids can be synthesized in the brain from progesterone (7) or deoxycorticosterone (8, 9), respectively, by the sequential action of two enzymes, 5α-reductase (5α-R) type I and 3α-hydroxysteroid dehydrogenase (3α-HSD) (10).
Two types (I and II) of 5α-Rs, which convert progesterone into 5α-dihydroprogesterone (5α-DHP) or convert deoxycorticosterone into 5α-dihydrodeoxycorticosterone (5α-DHDOC), have been identified in tissues of rodents and humans (11). Whereas 5α-R type I and II are abundantly expressed in several peripheral tissues, 5α-R type I is the most abundant 5α-R molecular form detected in the adult brains of rats, mice, and humans (11–17). The human brain expresses four types of 3α-HSD, which, under different optimal conditions, either catalyze the reduction of 5α-DHP into ALLO or reverse this reaction (18). So far, only one 3α-HSD isoform has been identified in the rat or mouse brain (19–22). The mRNA sequences of 5α-R type I (≈88%) and 3α-HSD (≈89%) are highly homologous in mouse (5α-R type I GeneBank accession number (GBAN) NM_175283.3; 3α-HSD, GBAN AY730283.1) and rats (5α-R type I, GBAN NM_017070.3; 3α-HSD, GBAN NM_138547.1).
In various mouse and rat brain regions, the rank order of 5α-R type I and 3α-HSD expression matches the nonuniform distribution of ALLO and 5α-DHP (7, 14). A down-regulation of brain 5α-DHP and ALLO can be induced by environmental factors (i.e., social isolation) (23–26) and may be mediated by a decrease of brain 5α-R type I expression (14). Taken together, this information suggests that 5α-R type I and 3α-HSD may act in concert to control ALLO or THDOC and 5α-DHP or 5α-DHDOC biosynthesis.
Neuronal cultures from neonatal or embryonic rats synthesize ALLO and express 5α-R type I (27–29). However, in the adult rat brain, 5α-R type I immunoreactivity is expressed primarily by glial cells that also express S100β (30, 31). Hence, it is not clear whether 5α-R type I is selectively expressed in the neurons of the adult rodent brain and whether it coexists with 3α-HSD.
Because GABAA receptors are expressed in neuronal populations of several brain regions, it is important to establish whether ALLO, which positively modulates GABAergic signal transduction, is released from GABAergic axon terminals directly on GABAA receptors or if it reaches GABAA receptors by diffusion from contiguous synapses, perhaps via a local paracrine mechanism. This article deals with our attempts to clarify these issues by using mouse brain sections and in situ histochemistry technologies to compare the subcellular expression of 5α-R type I and 3α-HSD in various brain regions. We also have combined in situ antisense hybridization and immunohistochemistry labeling with specific markers to verify the brain-region distribution of neurons coexpressing 5α-R type I and 3α-HSD with confocal fluorescence microscopy.
Results
Cerebral Cortex.
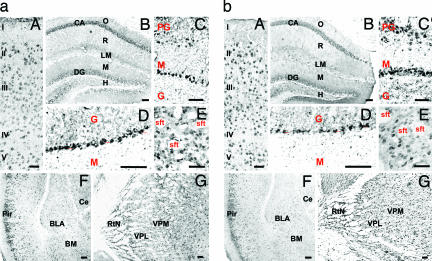
5α-R type I (Fig. 1a) and 3α-HSD (Fig. 1b) mRNAs are expressed in somatosensory (Fig. 1aA and bA) and piriform (Fig. 1aF and bF) cortices. In the somatosensory cortex, 5α-R type I and 3α-HSD mRNAs are primarily expressed in layer II, III, and V pyramidal neurons. The expression of these transcripts is below detection limits in layer I neurons, which are mostly GABAergic (32, 33). The hybridization signals for 5α-R type I and 3α-HSD are mostly similar, but there are some differences. For example, 5α-R type I mRNA expression is primarily cytosolic (Fig. 1 aA), whereas 3α-HSD mRNA expression appears to be cytosolic and nuclear (Fig. 1bA).
Fig. 1.
Mouse brain distribution of 5α-R type I (a) and 3α-HSD (b) mRNAs. (aA and bA) Somatosensory cortex, coronal section at bregma +1.10 mm (49). I to V identify the cortical layers. (Fig. 1 aB and bB) Hippocampus, coronal section at bregma −2.46 mm (49). CA, cornus ammonis; DG, dentate gyrus; O, stratum oriens; R, stratum radiatum; LM, layer lacunosum moleculare; M, molecular layer dentate gyrus; H, hilus. (aC and bC) Olfactory bulb, coronal section at bregma +3.92 mm (49). M, mitral; PG, periglomerular; G, granular cell layers. (aD and bD) Cerebellum. The Purkinje cell layer is indicated by arrowheads (▴). G, granule cell layer; M, molecular layer. (aE and bE) Striatal medium spiny neurons, coronal section as in A. Shown is unstained striatal fiber tract (stf). (aF and bF) Piriform cortex and amygdala, coronal section at bregma −1.58 mm (49). BLA, basolateral anterior amygdaloid nucleus; BM, basomedial amygdaloid nucleus; Ce, central amygdaloid nucleus; Pir, piriform cortex. (aG and bG) Thalamic nuclei, coronal section as in F. VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteromedial thalamic nucleus. [Scale bars, 50 μm (aA and bA) and 100 μm (aB and bB–aG and bG).]
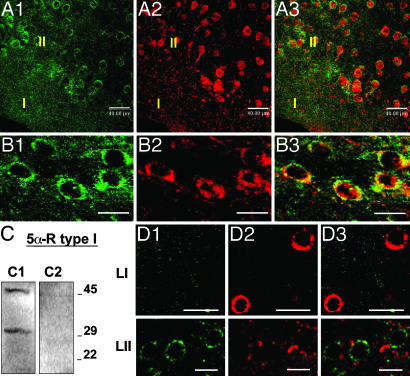
In Fig. 2A, the confocal fluorescence images of 3α-HSD mRNA and 5α-R type I protein show that, very frequently (>95%), both enzymes are coexpressed in the same neurons.
Fig. 2.
5α-R type I and 3α-HSD colocalize in glutamatergic (pyramidal) neurons but not in GABAergic interneurons of somatosensory cortex. (A) 5α-R type I protein colocalizes with 3α-HSD mRNA in somatosensory cortical layer II. 3α-HSD mRNA, green. (A2) 5α-R type I protein, red. (A3) Merge of A1 and A2. Note that 3α-HSD mRNA and 5α-R type I protein cellular staining is absent in cortical layer I. (B) 5α-R type I colocalizes with VGLUT1 mRNA. (B1) VGLUT1 mRNA, green. (B2) 5α-R type I protein, red. (B3) Merge of B1 and B2. (C) 5α-R type I antiserum specificity. Western blot of mouse brain extract incubated with 5α-R type I antibody (C1), and 5α-R type I antibody preabsorbed with the 5α-R type I immunizing antigen (see Materials and Methods). Molecular markers are indicated at the right. (D) The 3α-HSD mRNA is not expressed with GAD67/65 in somatosensory layer I (LI) and layer II (LII) cortical interneurons. (D1) 3α-HSD mRNA, green. (D2) GAD67/65 protein, red. (D3) Merge of D1 and D2. [Scale bars, 40 μm (A) and 20 μm (B–D).]
Fig. 2B shows that vesicular glutamate transporter (VGLUT)1 mRNA, a glutamatergic neuronal marker, and 5α-R type I protein colocalize. In contrast, we could not detect a colocalization of 5α-R type I or 3α-HSD with glutamic acid decarboxylase (GAD)67/65 (Fig. 2D), a marker for GABAergic neurons. Finally, 5α-R type I mRNA fails to colocalize with S100β, a specific glial cell marker (Table 1).
Table 1.
Expression intensity of 5α-R type I and 3α-HSD mRNAs
| Brain areas | 5α-R type I mRNA | 3α-HSD mRNA | S100β/GFAP proteins |
|---|---|---|---|
| Cortex | |||
| Glutamatergic (pyramidal) neurons | +++ | +++ | |
| GABAergic interneurons | − | − | |
| Glial cells | − | − | +++ |
| Hippocampus | |||
| Glutamatergic (pyramidal) neurons (CA1–3) | +++ | +++ | |
| Glutamatergic dentate gyrus granular cells | ++ | +++ | |
| Hilus cells* | +/− | ++ | |
| GABAergic interneurons | − | − | |
| Glial cells | − | − | +++ |
| Olfactory bulb | |||
| Glutamatergic mitral cells | +++ | +++ | |
| Periglomerular cells* | ++ | ++ | |
| GABAergic granule cells | +/− | +/− | |
| Glial cells | − | − | +++ |
| Striatum | |||
| GABAergic (medium spiny) neurons | ++ | ++ | |
| Thalamus | |||
| Glutamatergic dorsomedial nuclei neurons | +++ | +++ | |
| GABAergic reticular thalamic nuclei neurons | ++ | ++ | |
| Amygdala | |||
| Glutamatergic basolateral nucleus neurons | ++ | ++ | |
| Cerebellum | |||
| Glutamatergic granular neurons | +/− | +/− | |
| GABAergic Purkinje neurons | +++ | +++ | |
| Corpus callosum | |||
| Glial cells | − | − | +++ |
The degree of immunostaining intensity is indicated by (+++), (++), (+/−), and (−).
*The neurotransmitters expressed by these cells were not characterized.
Hippocampus.
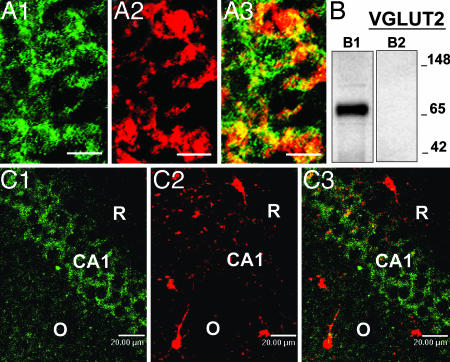
The detection signals for 5α-R type I and 3α-HSD mRNAs are reported in Fig. 1 aB and bB, respectively. The hybridization signal of both expressed transcripts in CA1 and CA2 is very intense, but, in CA3, the signal is faint. The cells of dentate gyrus and hilus also show a less-intense staining than that detected in CA1 and CA2, especially in the case of 5α-R type I (Fig. 1 aB). The stratum oriens, stratum radiatum, and layer lacunosum moleculare, and the molecular layers of the dentate gyrus show faint hybridization signal. However, a few cells are labeled for 5α-R type I mRNA in the stratum radiatum and, very likely, these are interneurons. A coexistence of 5α-R type I or 3α-HSD mRNAs with GAD67/65 immunoreactivity was not detected (Table 1). In the hippocampus, as in the cortex, the 5α-R type I signal fails to colocalize with S100β (Fig. 3C). As in the cortex, in CA1 pyramidal neurons, the 5α-R type I in situ hybridization signal consistently colocalizes with the VGLUT2 immunostaining (Fig. 3A).
Fig. 3.
5α-R type I is expressed in pyramidal neurons of the hippocampus. (A) 5α-R type I mRNA colocalizes with VGLUT2 in CA1 pyramidal layer. (A1) 5α-R type I mRNA, green. (A2) VGLUT2 protein, red. (A3) Merge of A1 and A2. (B) VGLUT2 antiserum specificity. Western blot of mouse brain extract incubated with VGLUT2 antibody (B1) and VGLUT2 antibody preabsorbed with VGLUT2 immunizing antigen (B2) (see Materials and Methods). Molecular markers are indicated at the right. (C) 5α-R type I mRNA does not colocalize with S100β in the CA1 area of the hippocampus. (C1) 5α-R type I mRNA, green. (C2) S-100β protein, red. (C3) Merge of C1 and C2. O, stratum oriens; R, stratum radiatum. (Scale bars, 20 μm.)
The VGLUT2 antibodies predominantly label glutamatergic neuron axon terminals (34). However, under the conditions of double in situ hybridization and immunohistochemistry shown in Fig. 3A, the cell bodies are also labeled. This labeling is eliminated when the VGLUT2 antiserum is preabsorbed with the immunizing peptide (Fig. 3B).
Olfactory Bulb.
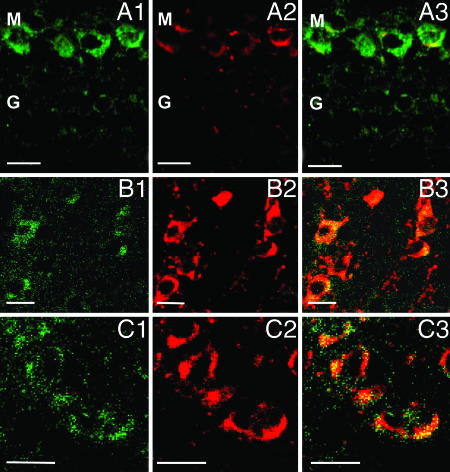
The olfactory bulb is characterized by the expression of intense 5α-R type I (Fig. 1 aC) and 3α-HSD (Fig. 1bC) hybridization signals, primarily expressed in the mitral and periglomerular cells. The granule GABAergic neurons, express faint staining for both 5α-R type I and 3α-HSD (Fig. 4A). In the mitral cell layer, 5α-R type I mRNA colocalizes with VGLUT1 mRNA (data not shown) or with VGLUT2 proteins (Fig. 4A). However, we failed to observe the colocalization of 5α-R type I mRNA with glial fibrillary acidic protein, a specific glial cell marker (Table 1).
Fig. 4.
Neuronal expression of 5α-R type I mRNA in olfactory bulb (OB), striatum, and RtN. (A) In OB, 5α-R type I mRNA colocalizes with VGLUT2 protein in mitral (M) cells. (A1) 5α-R type I mRNA, green. (A2) VGLUT2 protein, red. (A3) Merge of A1 and A2. Note the intense 5α-R type I mRNA staining in M cells (glutamatergic) and the weak 5α-R type I mRNA staining in granule (G) cells (GABAergic). (B) In the striatum, 5α-R type I mRNA colocalizes with GAD67/65. (B1) 5α-R type I mRNA, green. (B2) GAD67/65 protein, red. (B3) Merge of B1 and B2. (C) In RtN, 5α-R type I mRNA colocalizes with GAD67/65. (C1) 5α-R type I mRNA, green. (C2) GAD67/65 protein, red. (C3) Merge of (C1) and (C2). (Scale bars, 20 μm.)
Striatum.
The striatum includes numerous neurons that express strong 5α-R type I (Fig. 1aE) and 3α-HSD (Fig. 1bE) staining. Confocal fluorescence images show that 5α-R type I mRNA colocalizes with GAD67/65 in striatal neurons (Fig. 4B).
Thalamus.
Sparse 5α-R type I (Fig. 1 aG) and 3α-HSD mRNA (Fig. 1 bG) positive neurons are expressed in various thalamic nuclei. An intense hybridization signal is detected in neurons of the reticular thalamic nucleus (RtN), where 5α-R type I is colocalized with GAD67/65 (Fig. 4C). In contrast, in the ventromedial thalamic nucleus, 5α-R type I colocalizes with VGLUT1 mRNA and VGLUT2 proteins (Table 1).
Amygdala.
The central and basolateral amygdaloid nuclei are characterized by sparse neurons exhibiting a faint staining for either 5α-R type I (Fig. 1 aF) or 3α-HSD mRNAs (Fig. 1 bF). Strong staining is expressed in the basolateral anterior amygdaloid nucleus (BLA), whereas, in the central amygdaloid nucleus, staining is weak. The somata of glutamatergic principal neuron expressed in the BLA show a colocalization of 5α-R type I mRNA with VGLUT2 (Table 1).
Cerebellum.
The staining of 5α-R type I and 3α-HSD mRNA is selectively localized in the somata of Purkinje neurons (Fig. 1 aD and bD). A fainter staining is also present in the granule cell layer but virtually absent in the molecular layer. As expected, 5α-R type I mRNA is colocalized with GAD67/65 proteins in Purkinje cells (Table 1).
Overview of 5α-R Type I and 3α-HSD mRNA Expression in the Mouse Brain.
Table 1 shows that, in the mouse brain, 5α-R type I and 3α-HSD are expressed in principal output neurons, for instance, by the glutamatergic principal output neurons in the cortex, hippocampus, olfactory bulb, thalamus, and amygdala and also by GABAergic principal output neurons from the striatum, RtN, and cerebellum. However, 5α-R type I and 3α-HSD have not been detected in cortical and hippocampal GABAergic interneurons. The expression of 5α-R type I and 3α-HSD could not be detected in S100β- or glial fibrillary acidic protein-positive cells in various brain areas, including the corpus callosum.
Discussion
Brain Regional and Cellular Distribution of Enzymes Regulating Neurosteroid Biosynthesis.
The expression of 5α-R type I and 3α-HSD transcripts was greater in the mitral cell layer of the olfactory bulb than in layer II, III, or V of the neocortex or CA1–3 and dentate gyrus of the hippocampus. In these structures, the transcript expression was stronger than in the striatum, thalamus, amygdala, or cerebellum. This brain-region distribution of 5α-R type I and 3α-HSD is consistent with that of the distribution of their respective mRNAs quantified with RT-PCR in homogenates of various mouse brain regions (14).
The identification of cell types expressing 5α-R type I and 3α-HSD was carried out by using specific neuronal or glial markers, such as S100β and glial fibrillary acidic protein (for glial cells), VGLUT1 and VGLUT2 (for glutamatergic neurons), and GAD67/65 (for GABAergic neurons).
Regardless of the brain region studied, 5α-R type I and 3α-HSD appear to colocalize in the same neuronal population. A systematic cellular mapping of the transcripts for these two specific neurosteroidogenic enzymes indicates that 5α-R type I and 3α-HSD expression occurs in neurons but not in glial cells. In neurons, this expression is independent of the chemical structure of the neurotransmitter phenotype (glutamatergic or GABAergic). The neurosteroidogenic enzymes are highly expressed in “principal” output neurons (i.e., glutamatergic pyramidal, GABAergic reticulothalamic, striatal, and Purkinje neurons) and are virtually absent in GABAergic interneurons expressed in both the telencephalon or hippocampus (Table 1). The only interneurons that express weak staining for both 5α-R type I and 3α-HSD are the GABAergic granule cells of the olfactory bulb and the glutamatergic granular neurons of the cerebellum (Table 1).
The evidence that the 5α-R type I mRNA and protein and the 3α-HSD mRNA are highly expressed in neurons of the adult mouse brain confirms the Melcangi et al. (27, 28) finding on the expression of 5α-R type I activity in primary cultures of neurons obtained from various brain regions of rat embryos. Our studies, however, differ from the preceding immunohistochemical studies conducted with 5α-R type I antibodies (30, 31, 35) in rat brains. In fact, using a specific 5α-R type I antibody (see Fig. 2C for specificity) we could not confirm, in mouse brain, the studies in rats (30, 31, 35) showing that 5α-R type I immunoreactivity is abundantly expressed in glial cells. In preliminary experiments, we observed that, in the rat brain, the distribution and cellular location of 5α-R type I mRNA is virtually identical to that of mouse. This apparent discrepancy in 5α-R type I cellular distribution patterns between our in situ hybridization and the above-mentioned immunohistochemical studies (30, 31, 35) may be resolved by considering the differences in mRNA and protein expression: the former is highly localized in cell bodies, and the latter is expressed in axon terminals and dendrites where only a weak staining could be detected.
Significance of 5α-R Type I and 3α-HSD Expression Heterogeneity in Specific Neuronal Populations.
GABAA receptor modulation by neurosteroids expressed in GABAergic neurons.
Belelli and Lambert (5) have hypothesized that neurosteroids (i.e., ALLO or THDOC) secreted from axon terminals of GABAergic neurons will impinge on GABAA receptors and modulate GABA action at postsynaptically or extrasynaptically expressed GABAA receptors located on dendrites or cell bodies of glutamatergic or GABAergic neurons. The GABAergic neurons of the RtN express high levels of 5α-R type I and 3α-HSD, and their nerve endings may secrete neurosteroids and release GABA in the proximity of GABAA receptors located postsynaptically on somata of glutamatergic thalamocortical output neurons located in the mediodorsal thalamus (36). Similar properties may also apply to medium spiny GABAergic neurons of the caudate or putamen. These neurons express 5α-R type I and 3α-HSD and could potentially release GABA and neurosteroids on GABAA receptors expressed in cell bodies or dendrites of nigrostriatal dopaminergic neurons in the substantia nigra (37) or on GABAA receptors located on glutamatergic neurons somata projecting to the thalamus. This finding suggests that, in the basal ganglia, neurosteroids are operative in the modulation of circuits regulating sensory, motor, cognitive, and motivational functions. Finally, a positive allosteric modulatory action of neurosteroids very likely also applies to the Purkinje cells that modulate GABAA receptors located postsynaptically on the cell bodies or the dendrites of deep cerebellar nuclei (dentate, emboliform, globose, and fastigial) neurons.
GABAA receptor modulation by neurosteroids expressed in glutamatergic neurons.
The above-described classic circuitry scheme may not explain the positive allosteric modulatory action of ALLO or THDOC on GABAA receptors located in the cell bodies or dendritic membranes of pyramidal neurons in the cortex and hippocampus or on mitral cells in the olfactory bulb. This supposition is inferred from the notion (Figs. 2, 3, and 4A and Table 1) that 5α-R type I and 3α-HSD are not expressed in GABAergic interneurons but, rather, are expressed in the same glutamatergic principal neurons that also express GABAA receptors.
These data may support the view that neurosteroids synthesized in principal corticolimbic glutamatergic output neurons [including axon terminals of neighboring pyramidal neurons or thalamocortical (36), or amygdalocortical glutamatergic neurons (38)] probably act in a paracrine fashion at GABAA receptors located on cell bodies or dendrites of distal cortical pyramidal neurons. We can also propose that neurosteroids act at GABAA receptors located on dendrites or cell bodies of cortical pyramidal neurons after being secreted in an autocrine fashion from dendrites or cell bodies of the same neurons.
Furthermore, one could propose that ALLO or THDOC, synthesized in either cortical and hippocampal pyramidal neurons or in mitral cells might not be released but may access GABAA receptors located on the cell bodies or dendritic membranes of glutamatergic neurons by acting at the intracellular sites of the GABAA receptors by lateral diffusion into the plasma membrane without being secreted into the extracellular milieu (39).
Neurosteroid effects distinct from an allosteric direct action on GABAA receptors.
In independent studies, it was found that, in addition to 5α-R type I and 3α-HSD, cortical and hippocampal neurons express 20α-HSD, the enzyme that converts progesterone into the inactive 20α-hydroxyprogesterone or converts ALLO into 20α-hydroxyallopregnanolone (40). Cortical and hippocampal neurons also express 3β-HSD, the enzyme that converts pregnanolone into progesterone (41). Thus, glutamatergic cortical and hippocampal neurons and, also, very likely, the olfactory mitral neurons, may synthesize both progesterone and 5α-DHP, which may be involved in the regulation of intracellular progesterone-receptor function.
In this context, it is noteworthy to mention that neurosteroids have been reported to (i) influence prefrontal cortex structure and thalamocortical connectivity (42), (ii) increase cerebellar granule-cell neurogenesis (43), (iii) increase the proliferation of neuroprogenitor cells expressed in the rat hippocampus (44), and (iv) increase the proliferation of human embryonic neural stem cells expressed in the cerebral cortex (44). It is probable that a receptor mechanism different from a direct modulation of GABAA receptor signal transduction may be operative in these actions. We can speculate that the microtubular-associated protein 2 (MAP2) is a possible putative intracellular receptor for neurosteroids (i.e., ALLO) (45).
Conclusion
A decrease of brain neurosteroid availability has been associated with psychiatric conditions, including anxiety, aggression, premenstrual dysphoria, and cognitive and mood disorders. Antidepressants (fluoxetine and other selective serotonin-reuptake inhibitors, “SSRIs”) and antipsychotics (clozapine) may exert their beneficial effects, at least in part, by increasing the brain levels of neurosteroids (26, 46–48).
Although the neurosteroids that act as positive allosteric modulators of GABA action at GABAA receptors were initially considered endocrine messengers that are indiscriminately synthesized and secreted from glial cells in all brain regions, the present histochemical data suggest that there is a cellular and molecular basis to imply that neurosteroids are synthesized in neurons and act locally at GABAA receptors expressed on specific corticolimbic circuitries. Understanding the contribution of local brain neurosteroid biosynthesis to the pathogenesis of neurological and psychiatric disorders may become a stimulus to develop new psychoactive drugs that act selectively by normalizing neurosteroid action on GABAergic neurotransmission.
Materials and Methods
Animals and Tissue Preparation.
Adult male Swiss–Webster mice (Harlan, Indianapolis, IN), 25–30 g in body weight, were perfused with 0.9% NaCl and 4% paraformaldehyde. The brains were (i) postfixed for 72h in 4% paraformaldehyde and (ii) embedded in 30% sucrose in 0.15 M PBS, pH 7.4, at 4°C. All experiments were conducted in groups of three to five animals. For each condition, (antisense probes or antibodies) four to six sections were processed for each animal. All animal procedures were approved by the University of Illinois Animal Care Committee.
mRNA in Situ Hybridization.
Antisense probe design.
To visualize 5α-R type I mRNA, free-floating 16- to 20-μm coronal sections (49) were incubated for 72 h at 42°C with a mixture of 50 pmol/ml of three antisense oligonucleotide probes: R1 (nt 910–933), R2 (nt 989–1,012), and R3 (nt 1180–1203) (GBAN NM_175283). To visualize 3α-HSD mRNA, adjacent sections were incubated with two antisense oligonucleotide probes, H1 (nt 526–549) and H2 (nt 804–827) (GBAN AY730283.1). To visualize VGLUT1 mRNA, we used a hybridization technique with antisense oligonucleotide probes complementary to bases 626–649 (V1) and 1499–1522 (V2) of the mouse VGLUT1 cDNA (GBAN NM_182993). The oligonucleotide 3′ terminals were labeled with digoxigenin by using the Oligonucleotide Digoxigenin Tailing kit (Roche Diagnostics, Indianapolis, IN). The in situ hybridization protocol followed a variation of the procedure described by Rodriguez et al. (32) for the avidin–biotin–peroxidase complex (ABC; Vector Laboratories, Burlingame, CA) method and by Pesold et al. (50, 51) and Veldic et al. (52) for confocal immunofluorescence.
Antisense probes: specificity tests.
The antisense probe sequences did not match any other known mRNA sequences, as determined by multiple genome-wide BLAST comparisons. However, because genomic sequences that have not been reported as part of the intron/exon structures of transcribed genes can be transcribed (53), we performed separate in situ hybridization studies with the three 5α-R type I antisense probes, the two 3α-HSD antisense probes, and the two VGLUT1 antisense probes to establish specificity. In coronal prefrontal cortex slices, the distribution of neurons stained with the 5α-R type 1 probe R1 is virtually identical to the distribution of neurons stained with probes R2 and R3. The distribution of neurons stained with the 3α-HSD probe H1 is also virtually identical to the distribution of neurons detected with H2. The same virtually identical distribution was also found with the VGLUT1 probes V1 and V2. Oligoprobe specificity was tested by using digoxigenin-labeled scrambler oligonucleotides for 5α-R type I, 3α-HSD, and VGLUT1. As expected, specific neuronal staining was not detected.
Double in Situ Hybridization and Immunohistochemistry.
Double in situ hybridization and immunohistochemistry were performed by following a variation of the procedure described by Pesold et al. (50, 51) and Veldic et al. (52). After the in situ hybridization procedure was terminated, the following antibodies were used: (i) rabbit anti-GAD67/65 (diluted 1:2,000; Chemicon, Temecula, CA), (ii) rabbit anti-VGLUT2 (diluted 1:500; Synaptic Systems, Gottingen, Germany), (iii) rabbit anti-GFAP (diluted 1:250; Chemicon), (iv) rabbit anti-S-100β (diluted 1:5,000; Swant, Bellinzona, Switzerland), and (iv) rabbit anti-rat 5α-R type I (diluted 1:100; Acris Antibodies, Hiddenhausen, Germany).
After the double in situ hybridization and immunohistochemistry procedures, the slices were incubated with Cy5-labeled goat anti-rabbit IgG (diluted 1:1,000; Amersham Biosciences, Piscataway, NJ) or Cy5-labeled goat anti-guinea pig IgG (diluted 1:1,000; Abcam, Cambridge, MA) to produce red fluorescent staining or Cy2-labeled streptavidin (diluted 1:1,000; Amersham Biosciences) to produce green fluorescent staining, as indicated in the figure legends. The number of cells in which green and red fluorescence colocalize compared with the number of cells that express only green or only red fluorescence was quantified with confocal microscopy (Leica, Bannockburn, IL) at a magnification of ×40 in a counting box of 100 × 100 × 20 μm.
Western Blot Analysis to Assess 5α-R Type I and VGLUT2 Antibody Specificity.
Mouse brain extracts (10 mg per 200 μl of SDS loading buffer) were electrophorized on 10–20% SDS/PAGE gel and blotted onto a nitrocellulose membrane (Amersham Biosciences). For the study of 5α-R type I antiserum specificity, the membranes were incubated with either anti-5α-R type I (diluted 1:2,500; Acris Antibodies) or anti-5α-R type I (diluted 1:2,500) preabsorbed for 12 h at 4°C with a 1 mM solution of the immunizing peptide (V-V-F-A-L-F-T-L-S-T-L-T-R-A-K-Q-H-H-Q-W-Y) in 0.005 M NaPHO4 buffer (pH 7.2), 0.2 M NaCl, and 5% BSA. Two immunoreactive bands, one of ≈45 kDa and one of ≈29 kDa, are recognized by the nonpreabsorbed antiserum (Fig. 2C1). The immunoreactive bands disappear when the preabsorbed antiserum is used (Fig. 2C2). The size of 5α-R type I in mice is ≈29 kDa, according to the GenBank accession no. AAH94503; however, as indicated by Russell and Wilson (11), the hydrophobic amino acid content of 5α-R type I (≈37%) may explain the aberrant electrophoretic mobilities in SDD/PAGE that have been reported for the 5α-R isoenzymes. The preabsorbed antiserum failed to immunoreact with mouse brain slices.
The membranes were also incubated with anti-VGLUT2 antiserum (diluted 1:4,000; Synaptic Systems) or anti-VGLUT2 antiserum (diluted 1:4,000) preabsorbed for 12 h at 4°C with 20 μl of solution, 1 mg per 1 ml of the immunizing control peptide (amino acids 510–582 of rat VGLUT2/DNPI; Synaptic Systems) in blotting buffer (3% nonfat dry milk/0.1% Tween-20). Only one immunoreactive band of 65 kDa is recognized by the nonpreabsorbed antiserum (Fig. 3B1). This band completely disappears in the preabsorbed antiserum (Fig. 3B2). The preabsorbed antiserum failed to immunoreact with mouse brain slices.
Digital Photomicrography.
DAB (3-3′-diaminobenzidine tetrahydrochloride) (Sigma, St. Louis, MO) staining images were captured by Axiovision 3.1 (Zeiss) and confocal immunofluorescence by a confocal microscope (Leica Microsystems, Bannockburn, IL). The final composites were processed by using Photoshop (Adobe Systems, Mountain View, CA) and Powerpoint (Microsoft, Redmond, WA).
Acknowledgments
We thank Dr. A. L. Morrow (University of North Carolina, Chapel Hill, NC) and Dr. H. Möhler (University of Zurich, Switzerland) for the constructive criticism and suggestions in the preparation of the manuscript. This work was supported by National Institute of Mental Health Grant R01-M4 56890 (to A.G.).
Abbreviations
- 3α-HSD
3α-hyroxysteroid dehydrogenase
- 5α-DHP
5α-dihydroprogesterone
- 5α-R
5α-reductase
- ALLO
allopregnanolone
- GAD
glutamic acid decarboxylase
- GBAN
GenBank accession no.
- RtN
reticular thalamic nucleus
- THDOC
tetrahydrodeoxycorticosterone
- VGLUT
vesicular glutamate transporter.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mienville JM, Vicini S. Brain Res. 1989;489:190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- 2.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu EE. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 4.Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- 5.Belelli D, Lambert JJ. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 6.Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Cheney DL, Uzunov D, Costa E, Guidotti A. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khisti RT, Boyd KN, Kumar S, Morrow AL. Brain Res. 2005;1049:104–111. doi: 10.1016/j.brainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Karavolas HJ, Hodges DR. In: Neurosteroids and Brain Function. Costa E, Paul SM, editors. New York: Thieme; 1991. pp. 135–145. [Google Scholar]
- 11.Russell DW, Wilson JD. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 12.Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. J Steroid Biochem Molec Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 13.Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. J Clin Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steckelbroeck S, Watzka M, Reichelt R, Hans VH, Stoffel-Wagner B, Heidrich DD, Schramm J, Bidlingmaier F, Klingmuller D. J Clin Endocrinol Metab. 2001;86:1324–1331. doi: 10.1210/jcem.86.3.7325. [DOI] [PubMed] [Google Scholar]
- 16.Stoffel-Wagner B. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 17.Torres JM, Ortega E. FASEB J. 2003:1428–1433. doi: 10.1096/fj.02-1119com. [DOI] [PubMed] [Google Scholar]
- 18.Penning TM, Jin Y, Steckelbroeck S, Lanišnik Rizner T, Lewis M. Mol Cell Endocrinol. 2004;215:63–72. doi: 10.1016/j.mce.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Hara A, Inoue Y, Nakagawa M, Naganeo F, Sawada H. J Biochem (Tokyo) 1988;103:1027–1034. doi: 10.1093/oxfordjournals.jbchem.a122374. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski JE, Huizinga M, Penning TM. J Biol Chem. 1991;266:8820–8825. [PubMed] [Google Scholar]
- 21.Hoog SS, Pawlowski JE, Alzari PM, Penning TM, Lewis M. Proc Natl Acad Sci USA. 1994;91:2517–2521. doi: 10.1073/pnas.91.7.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penning TM, Jin Y, Heredia VV, Lewis M. J Steroid Biochem Mol Biol. 2003;85:247–255. doi: 10.1016/s0960-0760(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienville JM, Guidotti A, Costa E. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 24.Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinna G, Costa E, Guidotti A. Psychopharmacology (Berlin) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 27.Melcangi RC, Celotti F, Castano P, Martini L. Endocrinology. 1993;132:1252–1259. doi: 10.1210/endo.132.3.8440186. [DOI] [PubMed] [Google Scholar]
- 28.Melcangi RC, Celotti F, Martini L. Brain Res. 1994;639:202–206. doi: 10.1016/0006-8993(94)91731-0. [DOI] [PubMed] [Google Scholar]
- 29.Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- 30.Tsuruo Y, Miyamoto T, Yokoi H, Kitagawa K, Futaki S, Ishimura K. Brain Res. 1996;722:207–211. doi: 10.1016/0006-8993(96)00188-6. [DOI] [PubMed] [Google Scholar]
- 31.Kiyokage E, Toida K, Suzuki-Yamamoto T, Ishimura K. J Comp Neurol. 2005;493:381–395. doi: 10.1002/cne.20760. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A. J Comp Neurol. 2002;451:279–288. doi: 10.1002/cne.10341. [DOI] [PubMed] [Google Scholar]
- 33.Gabbott PL, Somogyi P. Exp Brain Res. 1986;61:323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- 34.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier G, Luu-The V, Labrie F. Mol Cell Neurosci. 1994;5:394–399. doi: 10.1006/mcne.1994.1049. [DOI] [PubMed] [Google Scholar]
- 36.Pinault D. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Gale K, Guidotti A, Costa E. Science. 1977;95:503–505. doi: 10.1126/science.13499. [DOI] [PubMed] [Google Scholar]
- 38.Gisabella B, Bolshakov VY, Benes FM. Proc Natl Acad Sci USA. 2005;102:13301–13306. doi: 10.1073/pnas.0506034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelletier G, Luu-The V, Li S, Labrie F. Brain Res Mol Brain Res. 2004;125:143–146. doi: 10.1016/j.molbrainres.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 42.Grobin AC, Gizerian S, Lieberman JA, Morrow AL. Neuroscience. 2006;138:809–819. doi: 10.1016/j.neuroscience.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 43.Keller EA, Zamparini A, Borodinsky LN, Gravielle MC, Fiszman ML. Brain Res Dev Brain Res. 2004;153:13–17. doi: 10.1016/j.devbrainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang JM, Johnston PB, Ball BG, Brinton RD. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu EE, Robel P. Proc Natl Acad Sci USA. 2006;103:4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guidotti A, Costa E. Biol Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 47.Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- 48.Barbaccia ML. Crit Rev Neurobiol. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- 49.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 50.Pesold C, Pisu MG, Impagnatiello F, Uzunov DP, Caruncho HJ. Brain Res Brain Res Prot. 1998;3:155–160. doi: 10.1016/s1385-299x(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 51.Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. Proc Natl Acad Sci USA. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapranov P, Cawley SE, Drenkow J, Bekiranov S, Strausberg RL, Fodor SP, Gingeras TR. Science. 2002;296:916–919. doi: 10.1126/science.1068597. [DOI] [PubMed] [Google Scholar]