Abstract
The genetically related ACI and Copenhagen (COP) rat strains display diametrically opposed susceptibilities to mammary cancer development when treated chronically with 17β-estradiol (E2). Here, we compare the actions of E2 on cell proliferation and lobuloalveolar development in the mammary glands of female ACI and COP rats. After 12 wk of E2 treatment, the mammary glands of ACI rats exhibited a significantly greater proliferative response to E2, compared with COP rats, as evidenced by quantification of S phase fraction and development of lobuloalveolar hyperplasia. Focal regions of atypical epithelial hyperplasia were observed in ACI, but not COP, rats. These strain differences were not because of differences in circulating E2, progesterone or, prolactin. Two-thirds of the induced mammary cancers in ACI rats exhibited aneuploidy. The E2-induced mammary cancers regressed when hormone treatment was discontinued, indicating that they were estrogen-dependent. Progesterone receptor was expressed by the great majority of epithelial cells within the E2-induced atypical hyperplastic foci and the mammary carcinomas, suggesting a link between these lesions. These data demonstrate a correlation between E2 action in the induction of mammary cell proliferation and atypical epithelial hyperplasia and genetically conferred susceptibility to E2-induced mammary cancers.
Epidemiologic studies suggest that estrogens are critical factors in breast cancer etiology (1–3). Supporting this link are the observations that early menarche, late menopause, late first full-term pregnancy, and nulliparity are each associated with increased risk of the disease, whereas oophorectomy prior to menopause significantly decreases breast cancer risk. A recent clinical trial demonstrated that the antiestrogen tamoxifen significantly reduces the incidence of breast cancer in women at high risk of developing the disease (4). One theory consistent with these data is that breast cancer risk is proportional to the cumulative number of ovulatory cycles and the repetitive stimulation of mammary cell proliferation by ovarian estrogens. At present, the molecular mechanisms through which estrogens contribute to the development of breast cancers are unknown.
The ACI rat appears unique among rat strains in that it is highly susceptible to estrogen-induced mammary cancers but rarely develops mammary cancers spontaneously (5–15). We have demonstrated that chronic treatment with 17β-estradiol (E2) induces mammary cancers in virtually 100% of ovary-intact ACI rats with a median latency of approximately 20 wk (15). In contrast, the Copenhagen (COP) rat is resistant to the development of estrogen-induced mammary cancers (5–8, 16). This difference between the ACI and COP rat strains is particularly striking because these strains are closely related genetically; the ACI strain was derived from a cross between the August and COP strains (17). Because the ACI and COP rat strains exhibit diametrically opposed susceptibilities to E2-induced mammary cancers (15, 16), they provide valuable models for studying the molecular mechanisms through which estrogens contribute to mammary cancer development.
In the present study, we have compared the ability of E2 to stimulate mammary cell proliferation and lobuloalveolar development in the mammary glands of female ACI and COP rats. Data presented herein reveal quantitative and qualitative differences in the manner in which the mammary epithelia of ACI and COP rats respond to E2, suggesting that genetic control of estrogen action in the mammary epithelium may contribute to the differing susceptibilities of these rat strains to E2-induced mammary cancers.
Materials and Methods
Care and Treatment of Animals.
All procedures involving live animals were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Female ACI and COP rats were obtained from Harlan–Sprague–Dawley and National Cancer Institute Breeding Program (Frederick, MD), respectively. The animals were housed in an accredited barrier facility under controlled temperature, humidity, and lighting conditions, and were fed a diet that was formulated in accordance with guidelines established by the American Society of Nutritional Science (18) and prepared as described previously (19, 20). Treatment with E2 was initiated at approximately 9 wk of age. Silastic tubing implants, empty or containing 27.5 mg of E2, were surgically inserted into the interscapular region as described previously (15, 16). Thereafter, each rat was examined twice weekly for palpable mammary cancers. Four hours preceding killing, each rat received an i.p. injection of BrdUrd (Sigma; 50 mg/kg body weight) solubilized in sterile PBS.
Collection, Processing, and Immunohistochemical Evaluation of Mammary Tissues.
Following treatment with E2 for the indicated period of time, each animal was killed by decapitation, trunk blood was collected, and serum was stored at −80°C. The anterior pituitary gland was removed and weighed. Serum E2 (15), prolactin (PRL) (15), and progesterone (21) were measured by radioimmunoassay as described previously. The location and size of each mammary tumor were recorded at necropsy. Mammary tissues, both grossly normal and tumors, were collected, fixed in Lillie's solution for 24 hr, processed, and embedded in paraffin. Portions of randomly selected mammary tumors were frozen in liquid nitrogen and stored at −80°C; spleens from these animals were also frozen and stored at −80°C. Sections from each mammary tissue specimen were stained with hematoxylin and eosin for histologic characterization. The density of the mammary parenchyma within each gland was estimated by computer-assisted (Optimas, Seattle, WA) quantification of the percentage of total cross-sectional area comprised of mammary parenchyma. Mammary glands from each animal were also prepared as whole mounts stained with alum carmine (Sigma) (22). Mammary cells incorporating BrdUrd during the 4-hr period preceding killing were quantified immunohistochemically as described previously (16, 19). A minimum of 2,000 mammary cells per animal were scored for incorporation of BrdUrd. To identify cells expressing progesterone receptor (PR), mammary sections were deparaffinized, rehydrated in water, and heated in citrate buffer (pH 6.0) for 20 min. Tissues were then incubated as follows: (i) 20 min at room temperature in 3% hydrogen peroxide; (ii) 20 min at room temperature in goat serum diluted 1:100 in PBS; (iii) 60 min at 37°C in rabbit anti-PR serum (C-19; Santa Cruz Biotechnology) diluted 1:100 in PBS. Bound primary antibody was detected by using biotinylated goat anti-rabbit IgG followed by incubation with avidin-biotin-peroxidase complex (Vector Laboratories) and visualized by using 3,3′-diaminobenzidine tetrahydrochloride (Dako). Sections were counterstained with Meyer's hematoxylin.
Analysis of Cellular DNA Content by Flow Cytometry.
Frozen mammary tumors and spleens from tumor-bearing animals were thawed on ice, minced, and mechanically dissociated in PBS using a Stomacher laboratory blender (Tekmar, Cincinnati). The dissociated cells were pelleted by centrifugation, resuspended in RPMI culture media (Life Technologies, Gaithersburg, MD), filtered through Spectra/Mesh polymer macrofiltration filters (Spectrum, Laguna Hills, CA), and counted. Following dilution to approximately 1 million cells per ml in Vendelov's reagent (23), the cells were analyzed by using a FACScalibur flow cytometer (Becton Dickinson). Cells prepared from normal spleen were used in parallel samples as an internal ploidy standard. The resulting data were analyzed by using modfit DNA analysis software (Becton Dickinson).
Statistical Analysis of Data.
Data are presented as the mean ± SEM. Differences between means were assessed by using two-way ANOVA and Newman–Keul's post hoc test. P values < 0.05 were considered significant.
Results
Responsiveness of Mammary Epithelium to E2 Is Rat Strain-Specific.
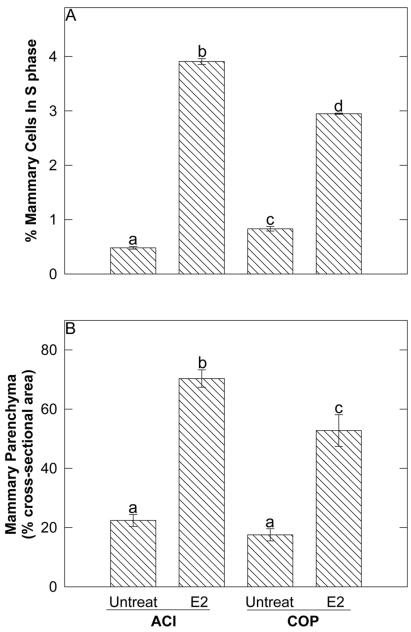
The mammary epithelium of the female ACI rat exhibited a more robust proliferative response to administered E2 than did that of the female COP rat. After 12 wk of treatment with E2 (a time point that precedes appearance of palpable mammary cancers), cell proliferation in the mammary glands of female ACI rats was increased approximately 8-fold relative to that observed in untreated controls (Fig. 1A). Whereas approximately 0.5% of mammary cells of control ACI rats stained positive for BrdUrd, 3.9% of mammary cells of E2-treated ACI rats were BrdUrd-positive (P < 0.01). By contrast, E2 treatment increased cell proliferation within the mammary glands of female COP rats 3.5-fold (P < 0.01). Approximately 0.8% and 3.0% of mammary cells of untreated and E2-treated COP rats, respectively, were BrdUrd-positive (Fig. 1A). The 30% difference in the S phase fraction between the E2-treated ACI and COP rats was significant (P < 0.01).
Figure 1.
The mammary epithelia of ACI and COP rats exhibited differing proliferative responses to E2. Ovary-intact ACI and COP rats were treated with E2 for 12 wk. (A) Mammary cells in S phase were identified by BrdUrd immunohistochemistry. Each data bar represents the mean ± SEM (n = 4–8) number of mammary cells incorporating BrdUrd, expressed as a percent of total mammary cells. Differing lower case letters above data bars indicate statistical significance (p ≤ 0.05) between group means as assessed by ANOVA. (B) The percentage of cross-sectional area comprised by mammary parenchyma was measured by computer-assisted image analysis. Each data bar represents the mean ± SEM (n = 7–8).
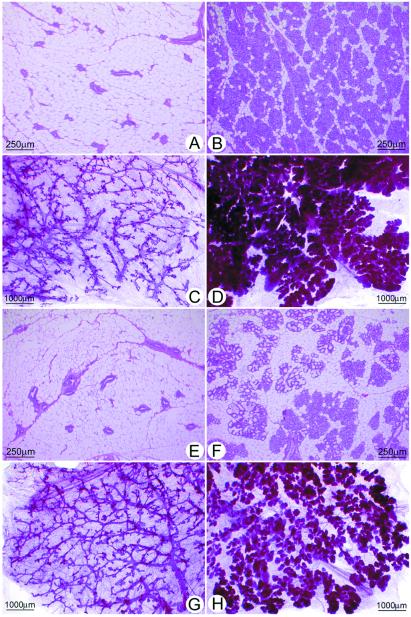
The difference in the proliferative response of the ACI and COP mammary epithelia to E2 was reflected histologically (Fig. 1B, and see Fig. 2). The mammary glands of untreated virgin ACI (Fig. 2 A and C) and COP (Fig. 2 E and G) females were similar in appearance and consisted of branched ducts extending throughout the mammary fat pad and terminating in alveolar buds. Treatment with E2 for 12 wk induced ductal branching and extensive lobuloalveolar hyperplasia in both strains. However, the induction of lobuloalveolar hyperplasia was clearly more pronounced in the ACI rats (Fig. 2 B and D) than in the COP rats (Fig. 2 F and H). Quantitative analysis of stained sections indicated that the mammary parenchyma comprised 70% of cross-sectional area in E2-treated ACI rats compared with 53% in the treated COP rats (Fig. 1B). This difference, reflecting the greater density of lobules in the ACI glands, was statistically significant (P < 0.01) and was also evident upon examination of mammary gland whole mounts (Fig. 2 D and H). Secretory activity was apparent in the mammary glands of treated ACI and COP rats and, although variable, appeared more pronounced in the COP strain compared with the ACI strain (Fig. 2 B and F).
Figure 2.
The differing proliferative responses of the ACI and COP mammary glands were reflected in mammary histology. (A and E) Thin sections from untreated ACI and COP rats, respectively. (C and G) Whole mounts from these strains. The mammary glands of untreated ACI and COP rats appeared similar and were comprised of branched ducts terminating in alveolar buds. (B and F) Thin sections from E2-treated ACI and COP rats, respectively. (D and H) Whole mounts. Although E2 treatment induced ductal branching and lobuloalveolar hyperplasia in both ACI and COP rats, the response in ACI rats was more pronounced than in COP rats.
Strain Differences in Lobuloalveolar Hyperplasia Are Independent of Circulating E2, Progesterone, and Pituitary Tumor-Associated Hyperprolactinemia.
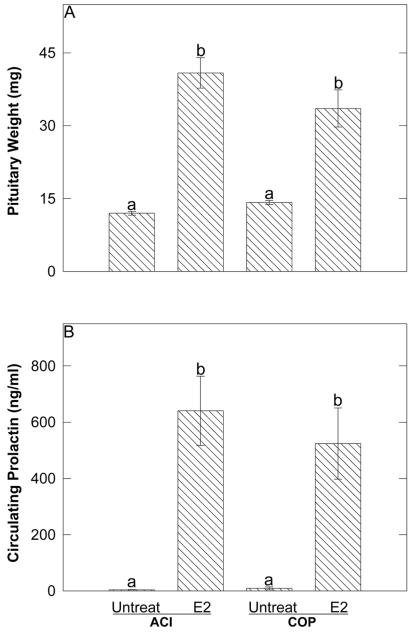
The levels of E2, progesterone, and PRL in trunk blood serum were measured to ascertain their relationships to the observed strain difference in E2-induced lobuloalveolar hyperplasia. Serum E2 levels in treated animals did not differ between the two strains, averaging 165 ± 24 pg/ml and 306 ± 119 pg/ml (mean ± SEM) in ACI and COP rats, respectively (data not shown). Progesterone levels were within the physiologic range and did not differ significantly as a consequence of E2 treatment or rat strain (data not shown). Treatment with E2 for 12 wk induced pituitary growth and hyperprolactinemia in both female ACI and COP rats. Pituitary weight was increased 3.4-fold (P < 0.01), from 12.0 ± 0.4 to 40.9 ± 2.9 mg, in female ACI rats, and 2.4-fold (P < 0.01), from 14.2 ± 0.4 to 33.5 ± 3.6 mg, in female COP rats (Fig. 3A). Similarly, treatment with E2 increased serum PRL from 4 ± 1 to 640 ± 114 ng/ml (P < 0.01) in female ACI rats, and from 9 ± 5 to 524 ± 118 ng/ml (P < 0.01) in female COP rats (Fig. 3B). The differences in pituitary weight (Fig. 3A) and serum PRL (Fig. 3B) between E2-treated ACI and COP rats were not significant. Therefore, the observed strain differences in E2-induced lobuloalveolar hyperplasia do not appear to result from differences in the levels of circulating E2, progesterone, or PRL in the E2-treated ACI and COP rats.
Figure 3.
E2 induced pituitary growth and hyperprolactinemia similarly in ACI and COP rats. (A) Each data bar represents the mean ± SEM (n = 7–8) pituitary wet weight, which corresponds to pituitary cell number (20). Differing lower case letters above data bars indicate statistical significance (p ≤ 0.05) between group means as assessed by ANOVA. (B) Each data bar represents the mean ± SEM (n = 7–8) level of circulating PRL.
Mammary Cancers Induced in ACI Rats by E2 Exhibit Genomic Instability.
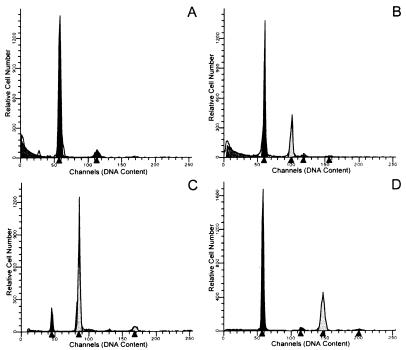
Flow cytometric analysis of cellular DNA content within mammary cancers induced in ACI rats by prolonged treatment with E2 indicated that the majority (67%) of these tumors exhibited aneuploidy (Fig. 4). Of 15 mammary cancers analyzed, 5 (33%) exhibited a normal DNA profile where the great majority of cells displayed a diploid DNA content (Fig. 4A); 7 (47%) cancers contained a significant fraction of cells that exhibited a hyperdiploid DNA content (Fig. 4B); 2 (13%) contained a large fraction of cells with tetraploid DNA content (Fig. 4C); and 1 (7%) contained a large fraction of cells that were hypertetraploid in their DNA content (Fig. 4D).
Figure 4.
Mammary cancers induced in ACI rats by E2 exhibited aneuploidy. Mammary cancers were induced in ovary-intact ACI rats by prolonged treatment with E2. The horizontal axis represents an arbitrary scale of relative fluorescence intensity, which is directly proportional to DNA content. The vertical axis indicates relative cell number. Cells from the spleen of each tumor-bearing animal were included in parallel samples to provide internal standards for diploid and tetraploid DNA content. (A) Mammary cancer from an ACI rat treated with E2 for 158 days. The majority of cells (≈96%) were diploid. (B) Mammary cancer from an ACI rat treated for 102 days. Approximately 68% of cells were diploid, whereas ≈32% of cells were aneuploid with hyperdiploid DNA content. (C) Mammary cancer from an ACI rat treated for 202 days. Only ≈12% of cells were diploid, whereas ≈88% exhibited a tetraploid DNA content. (D) Mammary cancer from an ACI rat treated for 268 days. The majority of cells (≈65%) were diploid, whereas ≈35% were aneuploid with a hypertetraploid DNA content.
Estrogen-Induced Mammary Cancers in ACI Rats Are Dependent on Exogenous E2.
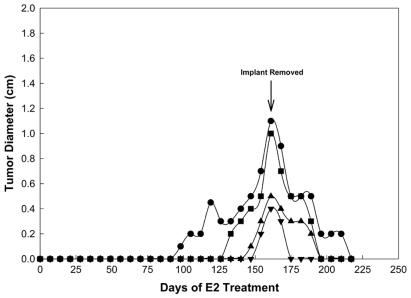
To determine whether continuous exposure to exogenous E2 is required for maintenance of established mammary cancers, the E2-containing implants were removed from a group of 4 tumor-bearing ACI rats harboring a total of 14 palpable mammary tumors. All of the mammary cancers regressed following implant removal. The regression of four tumors in one animal is illustrated in Fig. 5. In that animal, tumors 1, 2, 3, and 4 were detected following 98, 133, 147, and 154 days of E2 treatment, respectively. The E2-containing implant was removed on day 158 relative to initiation of estrogen treatment, when the tumors ranged from 0.4 to 1.1 cm in diameter. Within 38 days of implant removal, the largest of the four tumors had regressed to 0.2 cm in diameter and the three smaller tumors were no longer detectable by palpation. Regression of the largest tumor was complete within 59 days after removal of the E2-containing implant. These data, which are representative of those from three additional tumor-bearing animals, indicate that maintenance of elevated levels of circulating E2 are required for continued growth of E2-induced mammary cancers.
Figure 5.
Mammary cancers induced in ACI rats by E2 regressed upon removal of the hormone-containing implant. The four different symbols illustrate the development of four E2-induced mammary cancers in an individual rat and their subsequent regression following removal of the E2-containing implant 158 days after initiation of E2 treatment. Three of the tumors (■, ▴, ▾) regressed completely within 38 days of implant removal, whereas the largest tumor (●) regressed completely within 59 days of cessation of E2 treatment.
Estrogen-Induced Atypical Epithelial Hyperplasia and Mammary Cancers in ACI Rats Express PR.
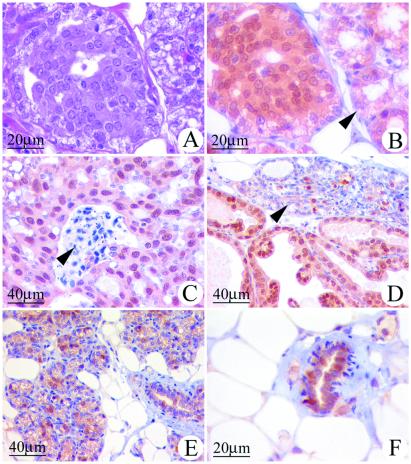
The mammary glands of ACI rats treated with E2 for as few as 12 wk contained focal regions of atypical epithelial hyperplasia in which the cells exhibited enlarged nuclear and cellular volumes and dense eosinophilic cytoplasmic staining (Fig. 6A). In contrast, no such foci were observed in the mammary glands of COP rats that were treated with E2 for 12 wk (data not shown). The majority of epithelial cells within the atypical foci observed in E2-treated ACI rats displayed strong immunoreactivity to an antibody to the PR (Fig. 6B). Similarly, the majority of epithelial cells within the mammary cancers that developed in response to prolonged E2 treatment also stained intensely for the presence of PR, regardless of whether they were of the comedo (Fig. 6C), papillary (Fig. 6D), or cribriform (data not shown) types. By contrast, only 20–30% of the epithelial cells within the hyperplastic lobules of ACI rats treated for 12 wk (Fig. 6E) or longer (Fig. 6D) with E2, or normal ductal epithelial cells of age-matched untreated controls (Fig. 6F) stained positive for PR. These data suggest that the atypical foci observed within 12 wk of initiation of E2 treatment may be precursors of the E2-induced carcinomas. The number of focal regions of atypical epithelial hyperplasia and the degree of cellular atypia within the foci were observed to increase in the mammary glands of female ACI rats as a function of the length of E2 treatment beyond the 12-wk time point. Consequently, atypical hyperplastic foci were numerous in ACI rats bearing E2-induced mammary cancers (data not shown).
Figure 6.
Atypical epithelial hyperplasias and mammary cancers induced in ACI rats by E2 express PR. Cells expressing PR were identified immunohistochemically as described in Materials and Methods. (A) Mammary gland, sectioned and stained with hematoxylin and eosin, from a 21-wk-old female ACI rat treated with E2 for 12 wk. Illustrated is a focal region of atypical epithelial hyperplasia and, to the right, adjacent acinar structures. The epithelial cells within the atypical hyperplasia exhibited enlarged nuclei and dense eosinophilic cytoplasmic staining. The illustrated atypical hyperplasia was minimally deviated relative to the surrounding lobules. The number of atypical hyperplastic foci and the degree of cellular atypia were observed to increase as a function of the duration of E2 treatment beyond 12 wk (data not illustrated). (B) A serial section to that in A that has been immunostained for PR. The majority of cells within the atypical epithelial hyperplasia exhibited immunoreactivity to PR, whereas fewer cells in the adjacent acinar structures stained positive for PR (arrow). (C) Mammary comedo carcinoma from an ACI rat treated with E2 for 193 days. The majority of the cancer cells exhibited immunoreactivity to PR. The arrow indicates necrotic debris characteristic of these cancers. (D) Mammary papillary carcinoma from an ACI rat treated with E2 for 216 days. The majority of the epithelial cells within the cancer exhibited immunoreactivity to PR. The arrow indicates adjacent acinar structures where a subset of the epithelial cells were immunoreactive to PR. (E) Mammary gland from an ACI rat treated with E2 for 12 wk exhibited lobuloalveolar hyperplasia. A subset of the epithelial cells stained positive for PR. (F) Mammary gland from an untreated, 21-wk-old, ovary-intact ACI rat; age-matched control for the E2-treated animals illustrated in A, B, and E. A subset of epithelial cells within the normal ductal structures exhibited immunoreactivity to PR.
Discussion
The genetically related ACI and COP rat strains exhibit diametrically opposed phenotypes with respect to susceptibility to E2-induced mammary cancers (15, 16). In the present study, we have demonstrated that the mammary gland of the highly susceptible ACI rat exhibits a greater proliferative response to administered E2 compared with the resistant COP rat. This difference was evidenced by quantification of the number of mammary cells incorporating BrdUrd as well as by histologic indicators of lobuloalveolar hyperplasia. The number of BrdUrd-positive cells was 30% greater in E2-treated ACI rats than in E2-treated COP rats; the difference (fold induction) between basal and E2-stimulated mammary cell proliferation in ACI rats was more than twice that observed in the COP strain; and the percentage of the mammary gland cross-sectional area comprised of parenchyma was 30% greater in E2-treated ACI rats than in treated COP rats. Moreover, focal regions of atypical epithelial hyperplasia were observed in the mammary glands of ACI, but not COP, rats that were treated with E2 for 12 wk. The associations between estrogens, cell proliferation, and breast cancer risk in human populations and mammary cancer development in experimental models have long been recognized (24–28). However, the data presented herein illustrate a genetically conferred difference in the ability of E2 to induce cell proliferation, lobuloalveolar hyperplasia, and atypical epithelial hyperplasia within the mammary glands of two genetically related inbred rat strains that differ in their susceptibility to E2-induced mammary cancers. Although the observed difference in the responsiveness of the ACI and COP mammary glands to administered E2 correlates with the differing susceptibilities of these strains to E2-induced mammary cancers, a cause and effect relationship cannot be concluded from the available data.
The quantitative and qualitative differences in the response of the mammary tissues of the ACI and COP rat strains to E2 did not appear to result from differences in circulating E2, progesterone, or PRL. Hyperprolactinemia has been shown to be sufficient to induce mammary cancer in certain strains of mouse (29–31) and rat (32, 33). Because rats treated chronically with estrogens to induce mammary cancers often develop PRL-producing pituitary tumors, it has been hypothesized or assumed that pituitary tumor-associated hyperprolactinemia is the proximal causative hormonal factor in estrogen-induced mammary carcinogenesis (12, 34–37). Data presented herein and published previously (16) illustrate that E2 induces pituitary tumors and associated hyperprolactinemia in female COP rats but does not induce a high incidence of mammary cancers. Moreover, ovariectomy (15) and dietary energy restriction (38) each inhibit development of E2-induced mammary cancers in female ACI rats without inhibiting development of pituitary tumors and associated hyperprolactinemia. Therefore, hyperprolactinemia is insufficient to induce a high incidence of mammary cancers in estrogen-treated rats. It is interesting to note that the genetic loci that confer and/or modulate susceptibility to E2-induced mammary cancers in F2 progeny from crosses between the highly susceptible ACI strain and two resistant rat strains, COP and Brown Norway, appear to be distinct from those that influence development of estrogen-induced pituitary tumors, indicating that these two estrogen-induced tumorigenic processes may be genetically separable (unpublished data).
In the present study, female ACI and COP rats exhibited similar increases in pituitary weight and circulating PRL in response to 12 wk of E2 treatment. By contrast, male ACI rats exhibit a significantly greater pituitary growth response to diethylstilbestrol (DES) than do male COP rats (39). The ACI rat appears not to display a significant sexual dimorphism in relation to the pituitary growth response to administered estrogen (our unpublished data). Consequently, the amount of E2-induced pituitary growth exhibited by female COP rats closely approached that exhibited by female ACI rats.
The majority of the E2-induced mammary cancers examined in this study exhibited aneuploidy. To our knowledge, this is the first report of genetic instability in mammary cancers induced in any rat strain by administered E2. Preliminary data suggest that loss of heterozygosity (LOH) is a common feature of E2-induced mammary cancers in ACI rats (unpublished data). The roles of aneuploidy and LOH in the development of E2-induced mammary cancers are not understood. Aneuploidy and LOH are commonly observed in human breast cancers (40).
Mammary cancers induced by E2 in ACI rats regressed upon removal of the E2-containing implants, indicating that these cancers are dependent upon exogenous estrogen for their maintenance. Shellabarger et al. (41) noted a similar observation for mammary cancers induced in ACI rats by DES, although data were not presented to indicate how rapidly regression occurred. Not established in these studies is whether and to what extent production of estrogens by the ovaries resumed after cessation of prolonged estrogen treatment.
The mammary glands of ACI rats treated with E2 for as few as 12 wk contained focal regions of atypical epithelial hyperplasia. The majority of cells comprising these foci were quite uniform in their expression of PR, relative to cells in the adjacent hyperplastic lobules. The epithelial cells within E2-induced mammary carcinomas were also uniform in expressing PR, suggesting a possible link between the atypical hyperplastic foci and the carcinomas. Atypical epithelial hyperplasia of this type has not been described previously in the mammary glands of estrogen-treated ACI rats. The functional significance of PR and its hormone ligands within the atypical hyperplastic foci and mammary cancers induced in ACI rats is unknown. It is interesting to note that cell proliferation in the human breast is greatest during the luteal phase of the menstrual cycle, when circulating progesterone is highest (42–43). Moreover, ovariectomy markedly inhibits development of mammary cancers in ACI rats treated with E2 (15) or DES (9). These data suggest that estrogens and progestins may interact in regulating mammary cell proliferation in the human and rat. However, Segaloff (9) demonstrated that treatment of ovariectomized ACI rats with DES and progesterone did not induce mammary cancer development to the extent observed in DES-treated, ovary-intact ACI rats. Because circulating progesterone was not measured by Segaloff (9), it should not be assumed that progesterone levels in the treated ovariectomized rats were restored to within the physiologic range of ovary-intact rats.
In summary, the mammary epithelia of ACI and COP rats exhibited quantitative and qualitative differences in their responsiveness to E2. These differences correlated with the differing susceptibilities of these two rat strains to E2-induced mammary cancers. Because the ACI rat appears unique in its high degree of susceptibility to E2-induced mammary cancers, whereas the genetically related COP rat is resistant to these cancers, these two rat strains provide useful models for studying the role of E2 in mammary cancer development. Ongoing genetic studies are addressing the mechanism underlying these strain differences in the belief that this information will prove relevant to our understanding of the etiology of human breast cancers.
Acknowledgments
We thank Dr. Karen Gould for her valuable comments during the preparation of this manuscript, Dr. Charles Kuszynski for assistance with flow cytometry, and David Heard, John Schoeman, Dondi Holland, and Connie Thomas for their assistance in animal care. This research was supported by Grants R01CA68529, R01CA77876, and P30CA36727 from the National Institutes of Health; DAMD17-98-1-8217 from the U.S. Army Breast Cancer Research Program; and 97A-146 from the American Institute for Cancer Research. D.M.E.H. and T.E.S. were supported by National Institutes of Health Grant T32CA09476.
Abbreviations
- COP
Copenhagen
- DES
diethylstilbestrol
- E2
17β-estradiol
- PR
progesterone receptor
- PRL
prolactin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050569097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050569097
References
- 1.Harris J R, Lippman M E, Veronesi U, Willet W. N Engl J Med. 1992;327:319–328. doi: 10.1056/NEJM199207303270505. [DOI] [PubMed] [Google Scholar]
- 2.Pike M C, Spicer D V, Dahmoush L, Press M F. Epidemiol Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein L, Ross R K. Epidemiol Rev. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino J P, Wickerham D L, Redmond C K, Kavanah M, Cronin W M, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 5.Dunning W F, Curtis M R, Segaloff A. Cancer Res. 1947;7:511–521. [Google Scholar]
- 6.Dunning W F, Curtis M R, Madsen M E. Acta Unio Int Contra Cancrum. 1951;7:238–244. [PubMed] [Google Scholar]
- 7.Dunning W F, Curtis M R. Cancer Res. 1952;12:702–706. [PubMed] [Google Scholar]
- 8.Dunning W F, Curtis M R, Segaloff A. Cancer Res. 1953;13:147–152. [PubMed] [Google Scholar]
- 9.Segaloff A. Cancer Res. 1974;34:2708–2710. [PubMed] [Google Scholar]
- 10.Shellabarger C J, Stone J P, Holtzman S. Cancer Res. 1976;36:1019–1022. [PubMed] [Google Scholar]
- 11.Shellabarger C J, Stone J P, Holtzman S. J Natl Cancer Inst. 1978;61:1505–1508. [PubMed] [Google Scholar]
- 12.Stone J P, Holtzman S, Shellabarger C J. Cancer Res. 1979;39:773–778. [PubMed] [Google Scholar]
- 13.Shellabarger C J. Banbury Rep. 1981;8:339–351. [Google Scholar]
- 14.Holtzman S. Carcinogenesis. 1988;9:305–307. doi: 10.1093/carcin/9.2.305. [DOI] [PubMed] [Google Scholar]
- 15.Shull J D, Spady T J, Snyder M C, Johansson S L, Pennington K L. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 16.Spady T J, Harvell D M E, Snyder M C, Pennington K L, McComb R D, Shull J D. Cancer Lett. 1998;124:95–103. doi: 10.1016/s0304-3835(97)00455-2. [DOI] [PubMed] [Google Scholar]
- 17.Festing M F W. In: The Laboratory Rat. Baker D E J, editor. Vol. 1. New York: Academic; 1979. pp. 55–72. [Google Scholar]
- 18.Reeves P G, Nielsen F H, Fahey G C., Jr J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Spady T J, Lemus-Wilson A M, Pennington K L, Blackwood D J, Paschall T M, Birt D F, McComb R D, Shull J D. Mol Carcinog. 1998;23:86–95. doi: 10.1002/(sici)1098-2744(199810)23:2<86::aid-mc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Shull J D, Birt D F, McComb R D, Spady T J, Pennington K L, Shaw-Bruha C M. Mol Carcinog. 1998;23:96–105. [PubMed] [Google Scholar]
- 21.Roy S K, Greenwald G S. Biol Reprod. 1987;37:39–46. doi: 10.1095/biolreprod37.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Delp C R, Treves J S, Banerjee M R. Cancer Lett. 1990;55:31–37. doi: 10.1016/0304-3835(90)90062-3. [DOI] [PubMed] [Google Scholar]
- 23.Vindelov L L. Virchows Arch B Cell Pathol. 1977;24:227–242. [PubMed] [Google Scholar]
- 24.Mühlbock O. J Natl Cancer Inst. 1972;48:1213–1216. [PubMed] [Google Scholar]
- 25.Miller A B, Bulbrook R D. N Engl J Med. 1980;303:1246–1248. doi: 10.1056/NEJM198011203032130. [DOI] [PubMed] [Google Scholar]
- 26.Preston-Martin S, Pike M C, Ross R K, Jones P A, Henderson B E. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- 27.Nandi S, Guzman R C, Yang J. Proc Natl Acad Sci USA. 1995;92:3650–3657. doi: 10.1073/pnas.92.9.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo J, Russo I H. Endocrine-Related Cancer. 1997;4:7–21. [Google Scholar]
- 29.Van Nie R, Dux A. J Natl Cancer Inst. 1971;46:885–897. [PubMed] [Google Scholar]
- 30.Briand P. Anticancer Res. 1983;3:273–282. [PubMed] [Google Scholar]
- 31.Nagasawa H, Morii S, Furuichi R, Iwai Y, Iwai M, Mori T, Goto Y. Lab Anim. 1993;27:358–363. doi: 10.1258/002367793780745570. [DOI] [PubMed] [Google Scholar]
- 32.Meites J. J Natl Cancer Inst. 1972;48:1217–1224. [PubMed] [Google Scholar]
- 33.Welsch C W. Banbury Rep. 1981;8:299–315. [Google Scholar]
- 34.Kim U, Furth J. Vitam Horm. 1976;34:107–136. doi: 10.1016/s0083-6729(08)60074-6. [DOI] [PubMed] [Google Scholar]
- 35.Welsch C W, Nagasawa H. Cancer Res. 1977;37:951–963. [PubMed] [Google Scholar]
- 36.Blankenstein M A, Broerse J J, van Zwieten M J, van cer Molen H J. Breast Cancer Res Treat. 1984;4:137–141. doi: 10.1007/BF01806396. [DOI] [PubMed] [Google Scholar]
- 37.Russo I H, Russo J. J Mammary Gland Biol Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 38.Spady T J, Harvell D M E, Lemus-Wilson A, Strecker T E, Pennington K L, Vander Woude E A, Birt D F, McComb R D, Shull J D. J Nutr. 1999;129:587S–590S. doi: 10.1093/jn/129.2.587S. [DOI] [PubMed] [Google Scholar]
- 39.Spady T J, Pennington K L, McComb R D, Shull J D. Endocrinology. 1999;140:2828–2835. doi: 10.1210/endo.140.6.6757. [DOI] [PubMed] [Google Scholar]
- 40.Wenger C R, Beardslee S, Owens M A, Pounds G, Oldaker T, Vendely P, Pandian M R, Harrington D, Clark G M, McGuire W L. Breast Cancer Res Treat. 1993;28:9–20. doi: 10.1007/BF00666351. [DOI] [PubMed] [Google Scholar]
- 41.Stone J P, Holtzman S, Shellabarger C J. Cancer Res. 1980;40:3966–3972. [PubMed] [Google Scholar]
- 42.Meyer J S. Hum Pathol. 1977;8:67–81. doi: 10.1016/s0046-8177(77)80066-x. [DOI] [PubMed] [Google Scholar]
- 43.Masters R W, Drife J O, Scarisbrick J J. J Natl Cancer Inst. 1977;58:1263–1265. doi: 10.1093/jnci/58.5.1263. [DOI] [PubMed] [Google Scholar]