Abstract
Estrogen receptor (ER)-β is the predominant ER subtype in prostate cancer (PCa). We previously demonstrated that ICI 182,780 (ICI), but not estrogens, exerted dose-dependent growth inhibition on DU145 PCa cells by an ER-β-mediated pathway. Transcriptional profiling detected a greater than three-fold upregulation of seven genes after a 12-hour exposure to 1 µM ICI. Semi-quantitative reverse transcriptase polymerase chain reaction confirmed the upregulation of four genes by ICI: interleukin-12α chain, interleukin-8, embryonic growth/differentiation factor, and RYK tyrosine kinase. Treatment with an ER-β antisense oligonucleotide reduced cellular ER-β mRNA and induced loss of expression of these genes. Sequence analysis revealed the presence of consensus NFκB sites, but not estrogen-responsive elements, in promoters of all four genes. Reporter assay and chromatin immunoprecipitation experiments demonstrated that ICI-induced gene expression could be mediated by crosstalk between ER-α and the NFκB signaling pathway, denoting a novel mechanism of ER-β-mediated ICI action. Therefore, combined therapies targeting ER-β and NFκB signaling may be synergistic as treatment for PCa.
Keywords: cDNA microarray, Sp1 transcription factor, interleukins, embryonic growth/differentiation factor (GDF-1), RYK tyrosine kinase
Introduction
In the United States, prostate cancer (PCa) is the second leading cause of cancer deaths in men. Approximately 50% of men with PCa have locally advanced or metastatic disease [1], and 30% of patients with apparent localized disease have biochemical relapse after the first line of treatment [2]. Androgen ablation therapy is the mainstay treatment for metastatic PCa [3], although most neoplasms ultimately become androgen-refractory, at which time virtually no effective therapies are available. Therefore, there is a strong demand for alternatives to the treatment of androgeninsensitive PCa.
The pioneering work of Huggins and Hodges [4,5] established the use of diethylstilbestrol (DES) as a low-cost and effective treatment of metastatic PCa. However, because of serious adverse side effects associated with DES treatment, including exacerbation of heart failure, vascular complications, and gynecomastia [6], this therapy has gone out of fashion during the past two decades. Recently, interest in using estrogenic therapies for advanced PCa has reemerged, primarily in response to the following developments [7]. First, lower doses of DES [DES in conjunction with antiandrogen therapies, an estrogenic herbal therapy (PC-SPES), and 2-methoxy-estradiol therapy] have proven effective in subpopulations of patients with advanced PCa in phase 2 trials [8–10]. Second, administration of estrogens parenterally, which avoids hepatic firstpass metabolism, appears to lower the riskofthromboembolism [9]. Last, a second estrogen receptor (ER), the β subtype, has been cloned [11] and shown to be expressed at high levels in normal and malignant prostate epithelial cells [11–17], offering a new molecular target for devising novel therapies.
We now know that the biologic effects of estrogens/antiestrogens are mediated by two ER subtypes ER-α and ER-β, which are ligand-dependent transcription factors belonging to the steroid/thyroid nuclear receptor superfamily [18]. Although the DNA-binding domains of ER-α and ER-β share a high level (86%) of amino acid homology, their N-termini, C-termini, and ligand-binding domains (LBDs) have 23%, 17%, and 58% homology, respectively. Because of the divergence in their LBDs, the two ER subtypes bind ligands (agonists or antagonists) with different affinities [19,20]. After the ligands have bound to these receptors, the complexes interact with specific DNA sequences, known as estrogen response elements (EREs), on the promoter regions of target genes, recruit coregulators and components of the transcriptional machinery to the transcriptional start site, and initiate transcription. Recent studies have shown, however, that ERs may transactivate target genes by interacting with other transcriptional factors (TFs) such as AP-1, instead of binding directly to EREs [21,22]. The utilization of other TF-binding sequences is specific for both ligand and receptor subtype [22]. This latter scenario adds complexity to the mode of action of estrogens and/or antiestrogens and has presented new challenges in our attempts to fully understand their action.
Using a highly specific ER-β antibody, we recently demonstrated that ER-β is the predominant ER subtype expressed in normal basal epithelial cells of the prostate, in local PCa, and in PCa metastasized to the lymph nodes and bones [14]. We also showed that ER-β is expressed in abundance in most established PCa cell lines, including DU145, which we found to express only the β subtype of ER [23,24]. Collectively, these data suggest that ER-β may confer survival advantages to PCa cells [7]. Thus, targeted activation or blockade of ER-β action with selective ligands may present an attractive strategy for the therapeutic intervention of PCa.
Our previous study [23] reported the inhibition of DU145 cell growth by the antiestrogen ICI182,780 (ICI), but not 17-β estradiol (E2), by an ER-β-dependent mechanism. Our primary goal for this study was to discover putative ER-β-regulated genes using transcriptional profiling to identify ICI-regulated genes in DU145 cells. The proximal promoters of four confirmed gene candidates all harbor NFκB cis-acting elements, but not EREs. We further demonstrated the tethering of ER-β with NFκB components. Collectively, these data suggested that ER-β-NFκB crosstalk could be a new mechanism of ER-β signaling with ligand or tissue specificity.
Materials and Methods
Cell Culture
The human PCa cell line DU145 and the human embryonic kidney cell line HEK293 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were routinely maintained in ATCC-recommended conditions.
Plasmids
The pSp13 luciferase reporter construct was kindly provided by Dr. Stephen Safe (Texas A&M University, College Station, TX); the pNFκB luciferase reporter construct was kindly provided by Dr. Francis Chan (University of Massachusetts Medical School, Worcester, MA); the luciferase reporter plasmid (pt109-ERE3-Luc) carrying x3 vitellogenin ERE was kindly provided by Dr. Criag Jordan (Fox Chase Cancer Center, Philadelphia, PA); the ER-β expression plasmid was provided by Dr. Leigh C. Murphy (University of Manitoba, Winnipeg, Canada); and the ERβΔ8 expression vector was prepared by removing exon 8 of full-length ER-β transcript, which we found to have no intrinsic transactivation activity (unpublished data).
Chemicals and Reagents
The steroid E2 was purchased from Sigma (St. Louis, MO), and ICI was a generous gift from Zeneca Pharmaceuticals (Macclesfield, UK). E2 and ICI were dissolved in absolute ethanol (Pharmco, Brookfield, CT). LipofectAMINE PLUS reagent was purchased from Invitrogen (Carlsbad, CA), whereas the luciferase assay and β-galactosidase enzyme assay systems were purchased from Promega (Madison, WI).
cDNA Microarray Analysis
The Atlas Human cDNA Expression Array (Clontech, Palo Alto, CA), which includes 588 known genes, was used as previously described [25]. Briefly, cells maintained in culture medium containing 5% charcoal-stripped heat-inactivated fetal bovine serum (FBS) were treated for 12 hours in the absence or presence of 1 µM ICI. Cellular RNA was extracted and treated with DNase I, whereas polyadenylated RNA was isolated with two rounds of affinity chromatography. [α-32P]dCTP was used for cDNA probe synthesis from polyA-RNA samples obtained from untreated and ICI-treated cells. Approximately 0.6 x 106 cpm of each probe was hybridized overnight to the membrane microarray with continuous agitation at 68°C. After washing, hybridization signals on the screens were read by phosphorimager Storm 830 (General Electric Healthcare, Piscataway, NJ). The signal intensities of spots were quantified with Kodak Image Analysis Software (Eastman Kodak, Rochester, NY) and normalized against the intensities of control cDNA on the arrays. Hybridization signals from two separate experiments were used to identify ICI-induced changes in gene expression profile.
Treatment of DU145 Cells with ICI, E2, or ER-β Antisense Oligodeoxynucleotide (ODN)
DU145 cells were seeded in six-well plates at a density of 3 x 105 cells/well in a final volume of 2 ml of culture medium with 5% charcoal-stripped FBS. The effective concentration of ICI to trigger apoptosis was determined previously [23]. Twenty-four hours after seeding, triplicate wells of cells were treated with ICI (1 or 10 µM), E2 (10 or 100 nM), or 2.5 µM ER-β antisense ODN for 4 days. The sequence of ER-β antisense ODN has been described previously [23].
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated with TRI reagent (Sigma) according to the manufacturer's protocol. After isolation, 1 µg of total RNA was reverse-transcribed for 65 minutes at 42°C in 60 µl of reaction mixture, including 5 mM MgCl2, 1 x GeneAmp PCR buffer II (50 mM KCl, 10 mM Tris-HCl, pH 8.3), 1 mM of deoxynucleoside triphosphate, 5 nmol of random hexamers, 60 U of ribonuclease inhibitor, and 150 U of MuLV reverse transcriptase. All reagents were purchased from Perkin-Elmer (Shelton, CT). RT reaction was terminated by heating to 95°C for 5 minutes, and 1 µl of the resulting cDNA was used in each PCR.
Relative expression levels of interleukin-12α chain (IL-12), interleukin-8 (IL-8), embryonic growth/differentiation factor (GDF-1), RYK tyrosine kinase (RYK), BFL-1, ER-α, ER-β, and GAPDH were determined by semiquantitative RT-PCR [23]. The sequences of primers used in this study are listed in Table 1. Hot-start PCR using AmpliTaq Gold DNA polymerase (Perkin-Elmer) was used in all amplification reactions. The PCR programs were 24 cycles for GAPDH; 34 cycles for ER-α and ER-β; and 30 cycles of 30 seconds at 95°C, 45 seconds at 60°C, and 45 seconds at 72°C for other genes. PCR products were subjected to electrophoresis in 2% agarose gel with ethidium bromide, and fluorographs under ultraviolet irradiation were captured by a Kodak DC290 digital camera (Eastman Kodak). The signals of the PCR product were quantified with Kodak 1D Image Analysis software (Eastman Kodak). Levels of GAPDH cDNA served as loading control. Relative levels of mRNA for the genes were calculated following normalization against the signal intensity for GAPDH mRNA.
Table 1.
Primer Sequence for RT-PCR Analysis.
| Target Gene | Primer Sequence | Location (nt) | Expected Size (bp) | |
| RYK | ||||
| RYK-F | 5′-ATTTCCTGCACTTCACCTGG-3′ | 414–433 | 633 | |
| RYK-R | 5′-CTTTGGCCTCCAAAAGAGTG-3′ | 1046–1065 | ||
| GDF-1 | ||||
| GDF-1-F | 5′-CTCATCGTCTCCTCCTACGC-3′ | 637–656 | 491 | |
| GDF-1-R | 5′-GTTCAGAAGCGCTTGTCCTT-3′ | 1127–1146 | ||
| IL-12 | ||||
| IL-12-F | 5′-ACTCCAGACCCAGGAATGT-3′ | 402–421 | 590 | |
| IL-12-R | 5′-AGGGACCTCGCTTTTTAGGA-3′ | 991–1010 | ||
| IL-8 | ||||
| IL-8-F | 5′-GTCTGTCAGCCAGGATCCAC-3′ | 845–864 | 499 | |
| IL-8-R | 5′-ACACAGCTGGCAATGACAAG-3′ | 1343–1362 | ||
| BFL-1 | ||||
| BFL-1-F | 5′-TCTCAGCACATTGCCTCAAC-3′ | 116–135 | 482 | |
| BFL-1-R | 5′-TACAAAGCCATTTTCCCAGC-3′ | 599–618 | ||
| ER-β | ||||
| ER-β-F | 5′-TGAAAAGGAAGGTTAGTGGGAACC-3′ | 230–253 | 528 | |
| ER-β-R | 5′-TGGTCAGGGACATCATCATGG-3′ | 737–757 | ||
| ER-α | ||||
| ER-α-F | 5′-TACTGCATCAGATCCAAGGG-3′ | 41–60 | 650 | |
| ER-α-R | 5′-ATCAATGGTGCACTGGTTGG-3′ | 671–690 | ||
| GAPDH | ||||
| GAPDH-F 5′-CCACCCATGGCAAATTCCATGGCA-3′ | 152–175 | 598 | ||
| GAPDH-R | 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ | 726–749 |
Transient Transfection and Luciferase Assay
DU145 or HEK293 cells (4 x 104 cells/well) were plated in 24-well plates (Corning, Corning, NY) in a final volume of 0.5 ml of culture medium with 5% charcoal-stripped FBS. Cells were seeded for 24 hours before transfection so that they could reach 70% confluence on the day of transfection. All experiments were performed in triplicate. After 24 hours, cells were transfected with LipofectAMINE PLUS reagent (Invitrogen). Cells were transfected with 0.2 µg of luciferase reporter plasmid. Human ER-β expression plasmid (0.05 µg) was applied to HEK293 cells during transfection, and ERβΔ8 expression vector was used in the same experiments to serve as control. Transfection efficiency was normalized by cotransfecting 0.02 µg of pSV-β-galactosidase control vector (Promega) in each case. After 24 hours, the medium was changed, and cells were treated with vehicle (dimethyl sulfoxide), E2, ICI, or a combination of E2 and ICI for 48 hours. Luciferase assays were performed with the Luciferase Assay System (Promega). β-Galactosidase activity was measured with the β-Galactosidase Enzyme Assay System (Promega).
Chromatin Immunoprecipitation (ChIP) Assay
DU145 cells were cultured in charcoal-stripped medium for 3 days before treatment with E2 or ICI at a final concentration of 10 nM and 1 µM, respectively, for another 24 hours. Cells were incubated in a cross-linking buffer (1% formaldehyde) at 37°C for 10 minutes. Cells were harvested, washed once with ice-cold 1 x phosphate-buffered saline, and resuspended in 500 µl of lysis buffer [1% sodium dodecyl sulfate (SDS), 5 mM EDTA, 50 mM Tris-HCl (pH 8.1), and 1 x Calbiochem protease inhibitor cocktail]. Cells were incubated on ice for 10 minutes and sonicated thrice for 10 seconds. The resulting lysate was precleared by centrifugation for 10 minutes at 4°C and incubated with 45 µl of protein G agarose beads (Upstate Biotechnology, Charlottesville, VA), 2 µg of sheared salmon sperm DNA, and 40 µl of normal rabbit serum (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour. After a brief centrifugation, the supernatant was incubated with 8 µg of either p65- or ERβ-specific antibody (Santa Cruz Biotechnology) for 16 hours at 4°C. Immune complexes were mixed with 40 µl of a protein G agarose suspension followed by incubation for 1 hour at 4°C with rotation. Bead pellets were sequentially washed for 10 minutes with 1 ml each of the following buffers: low-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-Cl (pH 8.1), and 150 mM NaCl], high-salt wash buffer [0.1% SDS, 1 % Triton X-100,2 mM EDTA, 20 mM Tris-Cl (pH 8.1), and 500 mM NaCl], and LiCl wash buffer [0.25 mM LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris-Cl (pH 8.1)]. The beads were then washed thrice with 1 ml of TE buffer [Tris-HCl (pH 8.0) and 1 mM EDTA (pH 8.0)]. The immune complexes were eluted with 100 µl of freshly prepared elution buffer (1 % SDS and 100 mM NaHCO3). Cross-linking reaction was reversed by overnight incubation of DNA at 65°C, and samples were recovered by DNA purification spin columns (Promega). Approximately 5% of the bound DNA fraction was used for PCR to detect the proximal promoter region of the IL-8 locus, which has been reported to contain a functional NFκB element [26]. As a control experiment, we have used a pair of primers to amplify a DNA sequence containing an AP-2 site.
Statistical Analysis
Data are expressed as the mean of three experiments, each with triplicate samples for individual treatments or dosage regimens. Statistical analysis was carried out with two-tailed Student's t tests. Values are presented as mean ± 95% confidence intervals. All statistical tests were two-sided and considered statistically significant at P < .02. One-way ANOVA with Tukey B post hoc analysis was applied to determine significance among different treatment groups in DU145 transfection experiments.
Results
Investigation of Differential Gene Expression through cDNA Microarray Analysis
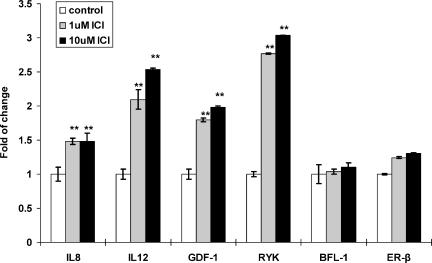
To identify novel molecular targets of ICI in DU145 cells, the Atlas Human cDNA Expression Array (Clontech) was used for transcriptional profiling. The DU145 cells were used for these experiments because this cell line exclusively expresses ER-β [24]. Two independent transcriptional profiling experiments were performed to identify ICI-induced changes in DU145 cells. A three-fold increase in intensity was arbitrarily used as a significant increase in gene expression (data not shown). Seven genes (IL-12, IL-8, RYK, GDF-1, BFL-1, IGF-1, and HTR-1A) were upregulated by antiestrogen treatment in both array experiments. Among them, four genes (IL-12, IL-8, GDF-1, and RYK) were confirmed by semiquantitative RT-PCR to show upregulation in ICI-treated DU145 cells (Figure 1). The expression of other genes, which are observed to be upregulated in the arrays, was found unaltered by ICI treatment in confirmation experiments.
Figure 1.
Confirmation of ICI-induced gene expression in a dose-dependent study. DU145 cells (3 x 105 cells/well) were plated in triplicate on a six-well plate. After 24 hours of cell attachment, the cells were treated for 4 days with two concentrations of ICI: 1 µM (grey bars) and 10 µM (black bars). Cells treated with vehicle (white bars) were used as controls. Three individual experiments were performed. Data are presented as mean (columns) and standard deviation (bars); Student's t test was applied to compare gene expression levels of ICI-treated cells (1 or 10 µM) versus their respective controls. **P < .02 compared with control.
Upregulation of Gene Expression by ICI, But Not E2
Four genes were confirmed to be upregulated following the treatment of DU145 cell cultures with ICI (Figure 1). A three-fold increase in RYK and 2.0- to 2.5-fold increases in GDF-1, IL-8, and IL-12 expression were detected when the DU145 cells were treated with 1 or 10 µM ICI. It appears that treatment with 1 µM ICI achieved almost the maximal level of stimulation. In contrast, BFL-1 and ER-β gene expression did not change significantly under the same experimental conditions (Figure 1). Similarly, IGF-1 and HTR-1A expression remained unchanged following ICI treatment (unpublished data). Parallel experiments using 10 and 100 nM E2 as treatment regimens did not induce expression changes in ICI-regulated genes (unpublished data).
Effect of ER-β Antisense ODN on Gene Expression in DU145 Cells
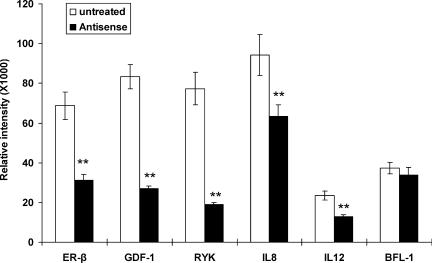
To determine whether upregulation of target gene expression was mediated by an ER-β pathway, we used an ER-β antisense ODN [23] to reduce ER-β mRNA levels in DU145 cells (Figure 2). A 60% reduction in the ER-β mRNA level was achieved following transfection of PCa cells with 2.5 µM ER-β antisense ODN. Concomitantly, the transfection reduced the expression of RYK, GDF-1, IL-12, and IL-8, but not BFL-1 (Figure 2). Reduction in the levels of RYK, GDF-1, IL-12, and IL-8 mRNA in the transfected cells ranged from 30% to 80%. These results indicated that downregulation of ER-β expression in DU145 cells was associated with reduced expression of newly identified ICI-regulated genes.
Figure 2.
Downregulation of ER-β expression is associated with reduction in the expression of putative ICI-regulated genes. DU145 cells (3 x 105 cells/well) were plated in triplicate on a six-well plate. Cells were treated with ER-β antisense ODN (black bar). After 4 days of treatment, total RNA was extracted and subjected to RT-PCR analyses. Three individual experiments were performed. Student's t test was applied to compare gene expression in antisense ODN-treated cells versus their untreated controls. Data are presented as mean (columns) and standard deviation (bars). **P < .02 compared with control.
Analysis of Cis-Acting Elements in the Gene Promoter Region
To further elucidate the mechanisms underlying the regulation of gene expression in prostate by ICI, we used MatInspector (www.genomatix.de) to identify common cisacting elements in the proximal promoter regions of these ICI-regulated genes (Table 2). Interestingly, all of the genes have the NFκB-binding element, whereas the Sp1 site was found in three of four genes. However, only one of the gene promoters contained an ERE. These results suggested that transcriptional activities of ICI-regulated genes were related to these cis-acting elements.
Table 2.
Promoter Region Analyses in ICI-Regulated Genes.
| NFκB | Sp1 | AHRARNT | ERE | AP-1 | E2F | ||
| IL-8 | + | + | |||||
| IL-12 | + | + | + | + | |||
| RYK | + | + | + | + | + | ||
| GDF-1 | + | + | + | + |
Transcriptional Activation of ER-β by ICI or E2 by an NFκB/Sp1-Binding Sequence
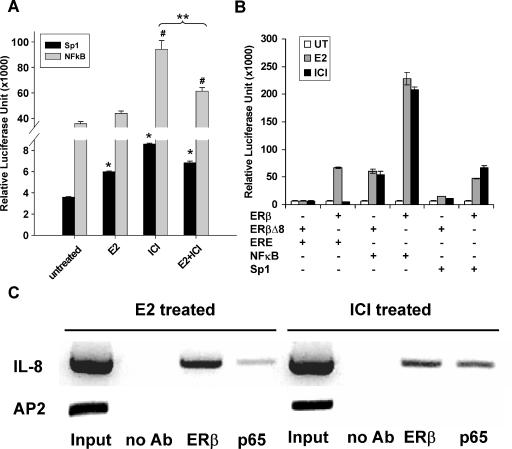
To provide experimental evidence that the transcriptional activation of ER-β by ICI or E2 is mediated by an NFκB-B- or an Sp1-binding element, we transiently transfected DU145 cells, which we demonstrated to express only the b subtype of ER [23,24], with a luciferase reporter gene driven by a promoter carrying either a tandem NFκB- or an Sp1-binding sequence. Relative luciferase activity data showed that transactivation at the NFκB element was about 10 times more robust than that at the Sp1 element in untreated DU145 cells (background activities). We then evaluated the ligand dependency of transactivation by these two cis-acting elements by treating the cells with ICI and/or E2 (Figure 3A). ICI was found to be more potent than E2 in the transactivation of the reporter gene. Cotreatment of DU145 cells with ICI and E2 markedly reduced reporter activity to a level lower than that induced by ICI, suggesting that the two ligands competed for the same endogenous ER-β pool.
Figure 3.
(A) Effects of E2 and ICI on the induction of Sp1- and NFκB-driven reporter activities in DU145 cells. Cells were transiently transfected with pSp13 or PNFκB luciferase reporter plasmid. After 24 hours of transfection, cells were treated with vehicle only (untreated), 10 nM estradiol (E2), 10 µM ICI (ICI), or 10 nM E2 + 10 µM ICI (E2 + ICI). After 48 hours, cells were harvested and assayed for luciferase activity. Luciferase values were corrected for transfection efficiency by measuring the β-galactosidase activity of cotransfected pSV-β-galactosidase construct. Three individual experiments were performed. Data are presented as mean (columns) and standard deviation (bars). One-way ANOVA with Tukey B post hoc analysis was applied to determine significance among different treatment groups in this experiment. *P < .01 compared with untreated Sp1 control; #P < .01 compared with untreated NFκB control. (B) Comparison of ER-β transactivation efficiency on various DNA-binding elements on E2 and ICI treatment in ER-β-overexpressed HEK293 cells. Transfection experiments were performed as described in Materials and Methods section. After 24 hours of transfection, cells were treated with vehicle (dimethyl sulfoxide), 10 nM E2, or 1 µM ICI. ER-β and ERβΔA 8 are the expression vectors expressing wild-type ER-β and inactive mutant, respectively, whereas ERE, NFκB, and Sp1 are the reporter vectors used in this study. Three individual experiments were performed. Data are expressed as mean (columns) and standard deviation (bars). (C) ChIP analysis of ER-β and p65 on IL-8 promoter. DU145 cells were treated with either 10 nM E2 (left panel) or 1 µM ICI (right panel). ChIP assay was performed as described in Materials and Methods section. After reverse cross-linking and DNA purification, PCR was performed to confirm the involvement of the NFκB element on the IL-8 promoter. Asa control for sequence specificity, PCR was performed using primers for a sequence containing an AP-2 element. No signal was detected in any of the immunoprecipitated pulldown products, except for the total DNA input.
To demonstrate that ICI preferentially used NFκB/Sp1 over the classic ERE, we then conducted transfection experiments in HEK293 cells, which have no detectable expression of ER (-α and -β, data not shown). HEK293 was transiently transfected with expression vectors carrying wild-type ER-β or an inactive mutant (ERβΔ8). In the absence of ligand, no transactivation activities were detected with all combinations of receptors and cis-regulatory elements. As expected, at the classic ERE, E2 exerted agonistic action, whereas ICI was antagonistic. In contrast, at the NFκB element, both E2 and ICI were potent inducers of luciferase expression, with a three-fold induction. Similarly, two ligands both served as agonists at the Sp1 site, inducing an approximately 2.5-fold increase in reporter activity. These data confirmed the finding that ER-β could tether on Sp1 for transactivation [27], but revealed for the first time that ER-β also crosstalked with the NFκB signaling. To definitely demonstrate direct interaction between ER-β and the subunit of the NFκB complex in DU145 cells, we performed a ChIP assay with a well-characterized promoter (IL-8) that contains a single NFκB-binding element in a specific promoter region [26]. Our data clearly demonstrated that ER-β was recruited to an NFκB-binding site on the IL-8 promoter possibly by interaction with p65. To demonstrate sequence specificity, we have conducted control experiments using primers to amplify an unrelated DNA sequence (AP-2 site), and we found no amplification products (Figure 3C).
Discussion
We have successfully used transcriptional profiling to identify ICI-regulated genes in the DU145 PCa cell line. Among the genes identified by array analysis, four genes (IL-12, IL-8, GDF-1, and RYK) were confirmed by semiquantitative RT-PCR to be upregulated by ICI, but not E2. Reduction of cellular ER-β mRNA levels achieved with antisense ODN transfection in DU145 cells resulted in downregulation of these genes, suggesting that ER-β plays an essential role in their maintenance. In silico analysis revealed the presence of a consensus NFβB cis-acting element, but not ERE, in the promoters of all four genes and of an Sp1 site in the promoters of three genes. In DU145 cells that expressed only ER-β, ICI, but not E2, acted as a potent agonist in activating reporter expression bythe NFκB, whereas both ligands could transactivate gene transcription by an Sp1-responsive element. In contrast, in an ER-null cell line (HEK293) expressing transgenic ER-β, E2 and ICI both acted as agonists at both NFβ and Sp1 sites but served as an agonist and an antagonist, respectively, at the classic ERE. ChIP analysis using chromatin isolated from DU145 cells revealed a direct interaction between the ER-β and a short region of the IL-8 promoter containing an NFκB-binding site. Collectively, these data suggested that ER-β may use multiple and diverse mechanisms in gene regulation.
Estrogens and antiestrogens, including several selective estrogen receptor modulators (SERMs), have been implicated as potential therapeutic agents for androgen-independent PCa [7]. However, their mechanisms of action in the genesis and progression of PCa are still unclear. It seems controversial that both estrogen agonists and antagonists can induce the inhibition of cell growth or apoptosis in androgenindependent human PCa cell lines [28–30]. This observation is due, in part, to the complexity of the interactions between ER and the coactivators/corepressors within the promoter regions of target genes [14]. The discovery of the ER-β subtype in human prostates [31] opened up a new chapter in the understanding of estrogen signaling pathways and provided important clues into the role of estrogen/ER in prostate carcinogenesis. In this study, we chose DU145 cells, which expressed only the ER-β subtype, to study the mechanism of cell growth inhibition by the pure antiestrogen ICI [11]. The induction of G0-G1 cell cycle arrest by ICI was mediated by the ER-β subtype because this biologic effect could be reversed by exposure of the cells to an ER-β antisense ODN. Recently, another SERM, raloxifene, which is a mixed estrogen agonist/antagonist, has been shown to induce apoptosis in DU145 cells, mostly likely mediated by ER-β [29]. In contrast, the antiprostatic and antitumor responses in the PAIII rat PCa cells elicited by trioxifene (LY133314) are mediated by ER-α, but not by ER-β, and are considered to be an SERM with selective ER-α antagonist activity in prostate malignancy [30]. The different responses of ER-α and ER-β to different SERMs may be attributed to structural dissimilarities in their N-terminal A/B and LBDs, as well as to their interactions with different coregulators in target tissues. In addition, homodimerization or heterodimerization among ER subtypes also plays a significant role in the determination of ligand-binding affinity [32].
Gene regulation mediated by ER uses classic and nonclassic pathways [33]. In a classic pathway, ligand-bound ER directly binds to a palindromic ERE to turn on gene transcription. Ligands such as E2 are classified as estrogens if they are agonistic at the ERE, and as antiestrogens (ICI or tamoxifen) if they exert antagonistic action at the same site. However, in noncanonic pathways, ER initiates transcription by interacting with third-party proteins, such as Jun/Fos, that transactivate at an AP-1 site [34] or with an Sp1 protein binding to an Sp1 element [35]. Using this mechanism, antiestrogens may behave like agonists [22]. In the presence of E2 or classic estrogens, ER-α tends to use the classic pathway. In contrast, when ER-β binds to SERMs or antiestrogens such as ICI, it prefers to activate target genes by molecular tethering to AP-1 [34] or Sp1 sites [27,36]. We observed here that both ICI and E2 were agonistic at an Sp1 site through interaction with endogenous ER-βs in DU145 cells or with transgenic ER-βs in HEK293 cells. These findings suggested that, in DU145 cells, ER-β activated by ICI employs nonclassic pathways for signaling. Recognizing the small number of probes in our cDNA array, it is logical to predict that if high-density arrays were to be used in future experiments, many ICI-regulated molecular targets could be identified. It will be interesting to determine which subsets of these targets would use these nonclassic ER-β signaling pathways.
Another molecular tether for ER-β in DU145 cells that we found was NFκB. Although NFκB has been reported to interact with ER-α to mediate gene transcription [37], before our study, no direct evidence has demonstrated its interaction with this ER subtype. In DU145 cells, only ICI, but not E2, caused significant enhancement of reporter activity. Unexpectedly, in HEK293 cells expressing transgenic ER-β, we found that both E2 and ICI can transactivate the reporter through the NFκB element. Taken together, these data suggested that ligand-dependent activation of ER-β on the NFκB cis-acting element is dependent on cell context, which may include the absence/presence of different nuclear receptor coactivators, as well as posttranslational activation of the receptor. Additionally, ChIP assays using chromatin extracted from DU145 cells treated with either E2 or ICI showed direct interactions between ER-β and p65 targeting at the NFκB site, yet only ICI caused significant transactivation in this cancer cell line. One explanation for this discrepancy is that only ICI-liganded ER-β could recruit a functional set of coregulators to trigger transactivation, whereas the E2-liganded ER-β could not. Further investigations are needed to dissect the complexity of TF crosstalk in various cell types, such as those exemplified in our model.
NFκB-regulated genes are often shown to be antiapoptotic and to enhance survival in specific cell types [38]. Inhibition of NFκB by salicylate has been shown to sensitize the cells to programmed cell death by proapoptotic drugs [39]. We previously reported that 4 days of treatment of DU145 cells with ICI induced the inhibition of cell growth [23], which we now discovered to be caused by apoptosis (unpublished data). In this study, we identified several early-response (12 hours) genes that are upregulated by an ICI-induced ER-β/NFκB tethering mechanism. This finding appears to be paradoxical to the general belief that NFκB-regulated genes protect against apoptosis. A possible explanation is that upregulation of the NFκB gene family is the first line of cellular response when DU145 cells are challenged with ICI. With prolonged treatment, other ICI-stimulated ER-β signaling pathways are turned on and finally trigger apoptosis. The involvement of ER-β in ICI-induced apoptosis is further supported by the inability of ICI to initiate programmed cell death in ER-β-null glioma cells [39]. This finding raises the possibility that modulation of NFκB signaling may significantly alter the apoptotic sensitivity of PCa cell toward ICI-induced ER-β-mediated cell death.
In summary, we have identified several ICI-induced early response genes in DU145 cells whose expression may be regulated by a novel mechanism involving ER-β tethering on an NFκB complex. Because both primary and metastatic PCa express primarily the ER-β subtype, therapies targeting both TFs may give rise to novel modalities for the treatment of PCa.
Acknowledgements
We thank Paul Mak for critical review of this manuscript.
Footnotes
This research was supported, in part, by National Institutes of Health grants DK61084, CA94221, and CA15776 and Department of Defense grant DAMD-W81XWH-04-1-0165.
Kin-Mang Lau is currently at the Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong, The Prince of Wales Hospital, Shatin, New Territories, Hong Kong SAR, China.
References
- 1.Bott SR. Management of recurrent disease after radical prostatectomy. Prostate Cancer Prostatic Dis. 2004;7:211–216. doi: 10.1038/sj.pcan.4500732. [DOI] [PubMed] [Google Scholar]
- 2.Loberg RD, Fielhauer JR, Pienta BA, Dresden S, Christmas P, Kalikin LM, Olson KB, Pienta KJ. Prostate-specific antigen doubling time and survival in patients with advanced metastatic prostate cancer. Urology. 2003;62(Suppl 1):128–133. doi: 10.1016/j.urology.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Taplin ME, Ho SM. Clinical review 134: the endocrinology of prostate cancer. J Clin Endocrinol Metab. 2001;86:3467–3477. doi: 10.1210/jcem.86.8.7782. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 5.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;167:948–951. [PubMed] [Google Scholar]
- 6.Malkowicz SB, McKenna WG, Vaughn DJ, Wan XS, Propert KJ, Rockwell K, Marks SH, Wein AJ, Kennedy AR. Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate. 2001;48:16–28. doi: 10.1002/pros.1077. [DOI] [PubMed] [Google Scholar]
- 7.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 8.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 9.Oh WK. The evolving role of estrogen therapy in prostate cancer. Clin Prostate Cancer. 2002;1:81–89. doi: 10.3816/cgc.2002.n.009. [DOI] [PubMed] [Google Scholar]
- 10.Scherr DS, Pitts WR., Jr The nonsteroidal effects of diethylstilbestrol: the rationale for androgen deprivation therapy without estrogen deprivation in the treatment of prostate cancer. J Urol. 2003;170:1703–1708. doi: 10.1097/01.ju.0000077558.48257.3d. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54:79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 13.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 14.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sar M, Welsch F. Oestrogen receptor alpha and beta in rat prostate and epididymis. Andrologia. 2000;32:295–301. doi: 10.1046/j.1439-0272.2000.00396.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, Koji T. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab. 2003;88:1333–1340. doi: 10.1210/jc.2002-021015. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson JA. Novel aspects of estrogen action. J Soc Gynecol Invest. 2000;7:S8–S9. doi: 10.1016/s1071-5576(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 21.DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogeninduced/activator protein-1-dependent genes. Mol Endocrinol. 2005;19:362–378. doi: 10.1210/me.2004-0267. [DOI] [PubMed] [Google Scholar]
- 22.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 23.Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182. [PubMed] [Google Scholar]
- 24.Mobley JA, L'Esperance JO, Wu M, Friel CJ, Hanson RH, Ho SM. The novel estrogen 17alpha-20Z-21-[(4-amino)phenyl]-19-norpregna-1,3,5(10),20-tetraene-3,17beta-diol induces apoptosis in prostate cancer cell lines at nanomolar concentrations in vitro. Mol Cancer Ther. 2004;3:587–595. [PubMed] [Google Scholar]
- 25.Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143:2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie MH, Fillmore RA, Lausch RN, Oakes JE. A role for NF-kappa B binding motifs in the differential induction of chemokine gene expression in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:2299–2305. doi: 10.1167/iovs.03-0367. [DOI] [PubMed] [Google Scholar]
- 27.Zou A, Marschke KB, Arnold KE, Berger EM, Fitzgerald P, Mais DE, Allegretto EA. Estrogen receptor beta activates the human retinoic acid receptor alpha-1 promoter in response to tamoxifen and other estrogen receptor antagonists, but not in response to estrogen. Mol Endocrinol. 1999;13:418–430. doi: 10.1210/mend.13.3.0253. [DOI] [PubMed] [Google Scholar]
- 28.Corey E, Quinn JE, Emond MJ, Buhler KR, Brown LG, Vessella RL. Inhibition of androgen-independent growth of prostate cancer xenografts by 17beta-estradiol. Clin Cancer Res. 2002;8:1003–1007. [PubMed] [Google Scholar]
- 29.Kim IY, Kim BC, Seong DH, Lee DK, Seo JM, Hong YJ, Kim HT, Morton RA, Kim SJ. Raloxifene, a mixed estrogen agonist/antagonist, induces apoptosis in androgen-independent human prostate cancer cell lines. Cancer Res. 2002;62:5365–5369. [PubMed] [Google Scholar]
- 30.Neubauer BL, McNulty AM, Chedid M, Chen K, Goode RL, Johnson MA, Jones CD, Krishnan V, Lynch R, Osborne HE, et al. The selective estrogen receptor modulator trioxifene ( LY133314) inhibits metastasis and extends survival in the PAII I rat prostatic carcinoma model. Cancer Res. 2003;63:6056–6062. [PubMed] [Google Scholar]
- 31.Robertson CN, Roberson KM, Padilla GM, O'Brien ET, Cook JM, Kim CS, Fine RL. Induction of apoptosis by diethylstilbestrol in hormone-insensitive prostate cancer cells. J Natl Cancer Inst. 1996;88:908–917. doi: 10.1093/jnci/88.13.908. [DOI] [PubMed] [Google Scholar]
- 32.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 33.Pearce ST, Jordan VC. The biological role of estrogen receptors alpha and beta in cancer. Crit Rev Oncol Hematol. 2004;50:3–22. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan V, Wang X, Safe S. Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J Biol Chem. 1994;269:15912–15917. [PubMed] [Google Scholar]
- 36.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem. 2005;280:347–354. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- 37.Cerillo G, Rees A, Manchanda N, Reilly C, Brogan I, White A, Needham M. The oestrogen receptor regulates NFkappaB and AP-1 activity in a cell-specific manner. J Steroid Biochem Mol Biol. 1998;67:79–88. doi: 10.1016/s0960-0760(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 39.Hui AM, Zhang W, Chen W, Xi D, Purow B, Friedman GC, Fine HA. Agents with selective estrogen receptor (ER) modulator activity induce apoptosis in vitro and in vivo in ER-negative glioma cells. Cancer Res. 2004;64:9115–9123. doi: 10.1158/0008-5472.CAN-04-2740. [DOI] [PubMed] [Google Scholar]