Abstract
The MEK-ERK growth signaling pathway is important in human hepatocellular carcinoma (HCC). To evaluate the targeting of this pathway in HCC, we characterized a novel, orally-active MEK inhibitor, PD184161, using human HCC cells (HepG2, Hep3B, PLC, and SKHep) and in vivo human tumor xenografts. PD184161 inhibited MEK activity (IC50 = 10–100 nM) in a time- and concentration-dependent manner more effectively than PD098059 or U0126. PD184161 inhibited cell proliferation and induced apoptosis at concentrations of > 1.0 µM in a time- and concentration-dependent manner. in vivo, tumor xenograft P-ERK levels were significantly reduced 3 to 12 hours after an oral dose of PD184161 (P < .05). Contrarily, tumor xenograft P-ERK levels following long-term (24 days) daily dosing of PD184161 were refractory to this signaling effect. PD184161 significantly suppressed tumor engraftment and initial growth (P < .0001); however, established tumors were not significantly affected. In conclusion, PD184161 has antitumor effects in HCC in vitro and in vivo that appear to correlate with suppression of MEK activity. These studies demonstrate that PD184161 is unable to suppress MEK activity in HCC xenografts in the long term. Thus, we speculate that the degree of success of MEK-targeted treatment in HCC and other cancers may, in part, depend on the discovery of mechanisms governing MEK inhibitor signaling resistance.
Keywords: Hepatocellular carcinoma, liver cancer, MEK, ERK, PD184161
Introduction
One of the most common causes of solid organ cancer mortality worldwide is hepatocellular carcinoma (HCC) [1,2]. Although HCC is historically an uncommon malignancy in the United States, its incidence has been rapidly increasing in the last decade [1]. At present, the most successful treatment is surgical resection and/or transplantation, which can only be performed in a very select group of patients [3]. Current nonsurgical treatment strategies that have demonstrated some effectiveness in selected patients include chemoembolization, radiofrequency ablation, percutaneous ethanol injection, and, occasionally, systemic chemotherapeutics [4]. For most patients, there are no successful treatments available. Treatment advances have been stalled, in part, by a poor understanding of the molecular pathogenesis of HCC. Multiple etiologies of HCC, including aflatoxin B1 hepatitis B virus, hepatitis C virus, and cirrhosis, also make the study of molecular pathogenesis challenging [5]. Nonetheless, most HCCs are linked to a common pathway of hepatocyte necrosis followed by rapid proliferation. This process results in genetic or epigenetic changes, which can lead to the disinhibition and/or overexpression of growth and survival signaling pathways [6,7].
The extracellular signal-regulated mitogen-activated protein kinase (p42/p44 MAPK) pathway plays an integral role in coordinating growth and survival signaling and may play an important role in the development and progression of HCC. Extracellular signal-related kinase (ERK) is the terminal kinase in this pathway. ERK has two isoforms, ERK1 and ERK2 (or ERK1,2), which are proline-directed serine/threonine protein kinases activated by dual phosphorylation on both tyrosine and threonine residues [8]. During phosphorylation cascade, ERK1,2 are activated by MAPK kinases (i.e., MEK1,2), which are in turn activated through the phosphorylation of two serine residues by serine/threonine kinases such as MEK kinase or Raf [9,10]. Mitogen-stimulated cell growth, through survival/apoptosis and cell cycle control, is frequently linked to phosphorylated ERK's translocation from the cytoplasm to the nucleus and to the initiation of transcriptional activation of cell regulatory genes [11–13]. The activation of p42/p44 MAPK pathway intermediates is sufficient to transform cells, such as NIH3T3 cells[14]. Similarly, Rasmutations resulting in the constitutive activation of the p42/p44 MAPK pathway also cause oncogenic transformation [15].
An observed increase in the expression and activity of many p42/p44 MAPK signaling intermediates ex vivo in human HCC tissues has been demonstrated in our laboratory as well as in others [16–20]. Additionally, the functional pathways of p42/p44 MAPK signaling and the importance of the p42/p44 MAPK pathway signaling in experimental HCC have also been documented [18,19,21–25]. The activation and overexpression of members of the p42/p44 MAPK cascade in HCC do not appear to be dependent on Ras or B-Raf mutations, as these genes are not typically mutated in HCC [26–28]. However, activated Raf-1 has been shown to be overexpressed in human HCC [29]. Importantly, EGFR and transforming growth factor-α are upregulated in HCC, both of which lead to downstream activation of p42/p44 MAPK members [30–32]. Finally, alterations in guanine nucleotide regulatory proteins [25]—and, more recently, viral proteins from the two most common etiologies of HCC, hepatitis B and hepatitis C—have been shown to activate the p42/p44 MAPK pathway [33,34].
These data have fueled significant research aimed at members of the p42/p44 MAPK cascade as potential pharmacotherapeutic targets [35–37]. To that end, small molecule inhibitors that target MEK (e.g., PD098059 and U0126) have been identified in drug discovery programs and have provided researchers with the means of elucidating of the role of p42/p44 MAPK signaling in cellular function [38]. The following study is an investigation of the efficacy of a novel, orally active MEK inhibitor PD184161 on human HCC in vitro and in vivo for the purpose of characterizing its therapeutic potential in experimental HCC and, ultimately, in human disease.
Materials and Methods
Cell Culture
HepG2, Hep3B, PLC, and SKHep cells were obtained from the American Type Culture Collection (Bethesda, MD). Adherent cells underwent media changes three times per week and were maintained in 5% CO2 at 37°C in modified Eagle's medium-alpha (10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin). Commercially available MEK inhibitors PD098059 and U0126 (CalBiochem, San Diego, CA) as well as PD184161 were used (Pfizer, Ann Arbor, MI). These MEK inhibitors were administered 24 hours after HCC cells were plated for indicated time periods.
Proliferation Assays and Cell Counts
Cellular proliferation rates were determined using the colorimetric assay CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI), in which a tetrazolium compound is bioreduced by cells into a colored formazan product that is soluble in tissue culture medium. The assay was performed according to manufacturer's protocol and using methods previously described [39]. Relative cellular growth (expressed as a percentage) was determined by the ratio of the average absorbance of treatment wells to the average absorbance of control wells. Results were confirmed using trypan blue-excluded cell counts. Statistical analyses were performed using t-test and one-way analysis of variance (ANOVA) with Tukey post hoc test.
Apoptosis Assay
Apoptosis was measured using Cell Death Detection DNA Fragmentation ELISA Kit (Roche Diagnostics, Indianapolis, IN), which allows for quantitative determination of the amount of cytoplasmic histone-associated DNA fragments induced by cells undergoing apoptosis. Cells were plated in 96-well plates (1 x 104 cells) and then treated for 48 hours with PD184161 (1–20 µM). The assay was conducted according to the manufacturer's protocol and using methods previously described [39].
Western Blot Analysis
Cells were lysed in radioimmune precipitation buffer [phosphate-buffered saline (PBS), 1 % Nonidet P-40,0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate(SDS), 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, and 1 mM sodium vanadate (Na3VO4)]. Cell lysates were centrifuged at 10,000 rpm for 10 minutes. Supernatants (10 µg of total protein) were then resolved by SDS polyacrylamide gel electrophoresis on 4% to 20% gradient gels (Invitrogen, Carlsbad, CA). Separated proteins were transferred to Immobilon P membranes (Millipore, Bedford, MA) then incubated for 1 hour in blocking solution [Tris-buffered saline (TBS), 0.05% Tween, and 5% nonfat dry milk]. Membranes were washed in TBS Tween and incubated with primary antibody according to the manufacturer's recommendations. After washing with TBS Tween, they were incubated with appropriate secondary antibody solution (horseradish peroxidase-conjugated IgG) for 60 minutes at room temperature. Membranes were washed again with TBS Tween prior to detection using enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Primary antibodies employed included specific phospho-p42/44 MAP kinase (Thr202/Tyr204) antibody (Cell Signaling, Beverly, MA) and total ERK1/2 (K-23) antibody (Santa Cruz Biotech, Santa Cruz, CA). All Western blot analyses displayed in this manuscript were stripped of their antibodies and reprobed with actin (c-11) (Santa Cruz Biotech) to confirm equal protein loading (data not shown). Densitometric analysis, when applied, was performed using a Hewlett-Packard (Palo Alto, CA) scanner and analyzed with Totallab software (Nonlinear Dynamics; Biodynamics, Durham, NC). Statistical methods (t-test; one-way ANOVA, and Tukey post hoc test) were applied to densitometric data.
Tumor Xenograft Model
BALB/c athymic nude mice were inoculated with 3 to 5 million freshly isolated Hep3B, SKHep, or PLC cells. Prior to inoculation, the cells were washed with PBS, trypsinized, collected, and washed three times with PBS after being pelleted (500 rpm). Cells were resuspended in sterile saline and injected subcutaneously into the right flank of athymic nude mice. Inoculated animals were then subjected to one of three protocols. The inoculated animals in protocol 1 were monitored until the tumors had reached 100 mm3, after which the animals were treated (by orogastric gavage) with PD184161 or vehicle (10% ethanol, 10% cremophor EL, and 80% H2O) and sacrificed 3 hours after treatment. In protocol 2, PD184161 (300 mg/kg) or vehicle was administered twice daily by orogastric gavage for a total of 6 weeks, beginning 12 hours after HCC cell inoculation. Inoculated animals in protocol 3 were monitored until the tumors had reached 100 mm3, after which treatment with PD184161 (300 mg/kg, twice daily, by orogastric gavage) or vehicle was begun and continued for 24 days, during which time tumors were measured every 3 days. The animals were sacrificed, and the tumors were removed 3 hours after the last treatment with PD184161. Necrotic portions of tumors, when present, were not used to create tumor homogenates for the analysis of MAPK signaling end points. Statistical analyses, including t-test and one-way ANOVA, were applied to tumor growth results from each protocol (P < .05). All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Results
Specificity of PD184161
As reflected by 50% inhibitory concentration (IC50) values >10 µM, PD184161 (structure shown in Figure 1) did not inhibit the enzymatic activity of a diverse array of kinases, comprising a panel of 27 various tyrosine and serine/threonine kinases (Table 1). Experiments showed that the compound directly inhibits MEK with an IC50 = 10 to 100 nM. Kinetic experiments performed indicate that this compound is not competitive with ATP or the MAPKsite on MEK (C. Omer, personal communication).
Figure 1.
Chemical structure of PD184161 and PD184352.
Table 1.
PD184161 Has an IC50 of > 10µM against the following Kinases.
| AMPK | GSK3b | PDK1 | S6K1 |
| CDK2/cyclinA | JNK/SAPK1c | Phos. kinase | SAPK2a/p38 |
| CHK1 | Lck | PKA | SAPK2b/p38b2 |
| CK1 | MAPKAP-K1a | PKBph | SAPK3/p38d |
| CK2 | MAPKAP-K2 | PKCa | SAPK4/p38d |
| CKS | MAPK2/ERK2 | PP2a | SGK |
| DYRK1a | MSK1 | ROCK-II |
Effects of PD098059, U0126, and PD184161 on ERK Phosphorylation in HCC Cells
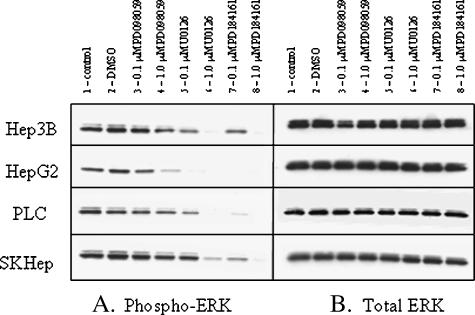
For purposes of comparison, the effects of the selective MEK inhibitors U0126 [40] and PD098059 [41] and the novel MEK inhibitor PD184161 on MEK activity were examined in four human HCC cell lines (Hep3B, HepG2, PLC, and SKHep). Phosphorylated-ERK level was evaluated by examining the phosphorylation status of ERK1,2, which are substrates of MEK. Immunoblots in Figure 2 demonstrate the level of ERK1,2 phosphorylation after 1 hour of treatment with the indicated MEK inhibitors (PD098059, U0126, and PD184161) at concentrations of 0.1 and 1.0 µM. Of the three inhibitors, PD184161 proved to be the most effective inhibitor of ERK1,2 phosphorylation in all four cell lines, as evidenced by complete inhibition of phosphorylation at 1.0 µM. No changes were found in total ERK1,2 expression as a result of any treatment. HepG2 cells were most sensitive to all MEK inhibitor treatments showing the absence of MEK activity, as demonstrated by phospho-ERK1,2 immunoblot, whereas Hep3B and SKHep cells were relatively more resistant.
Figure 2.
Representative Western immunoblots of phosphorylated ERK1,2 and total ERK1,2 proteins from lysates of human HCC cell lines following treatment with PD098059, U0126, and PD184161. Actively dividing cells (Hep3B, HepG2, PLC, and SKHep) were treated for 1 hour with one of the following mitogen-activated protein kinase kinase (MEK) inhibitors: PD098059, U0126, or PD184161 (0.1 and 1.0 µM). Following treatment, the cells were lysed, and Western immunoblots were performed using antibodies to phosphorylated ERK1,2 (A) and total ERK1,2 (B). Similar results were obtained in at least two independent experiments.
Effect of MEK Inhibition on HCC Cellular Proliferation
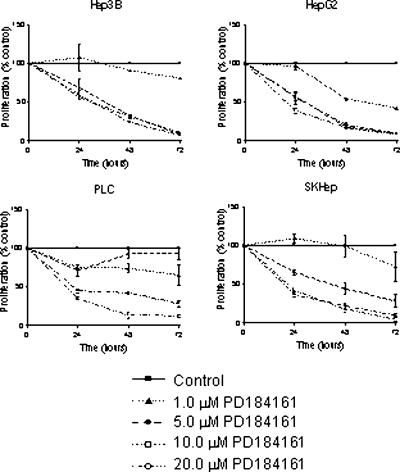
To determine the effects of PD184161 on HCC growth, four human HCC cell lines were treated with varying concentrations of PD184161 (1.0–20 µM) for 24, 48, or 72 hours. Figure 3 depicts the results of a colorimetric proliferation assay following PD184161 treatment of Hep3B, HepG2, PLC, and SKHep cells. All cell lines examined exhibited a time and concentration-dependent reduction in proliferation after treatment with PD184161. As was the case with suppression of phosphorylated ERK1,2 levels, HepG2 cells proved to be most sensitive to antiproliferative treatment with this compound. Effects were observed at all concentrations of PD184161 and at all time points examined in HepG2 cells, whereas Hep3B, PLC, and SKHep cells exhibited significant growth reduction at higher concentrations.
Figure 3.
The effect of the MEK inhibitor PD184161 on the proliferation of four human HCC cell lines (Hep3B, HepG2, PLC, and SKHep). Actively dividing cells were treated for 24, 48, or 72 hours with concentrations of PD184161 varying from 1.0 to 20 µM. A colorimetric proliferation assay was used to determine proliferation relative to control dimethyl sulfoxide-treated cells. Data are expressed as percentage of control. Each point on the graph represents the mean of triplicate samples, and error bars represent SEM. Similar results were obtained in at least two independent experiments.
Effect of MEK Inhibition on Apoptosis in Human HCC Cells
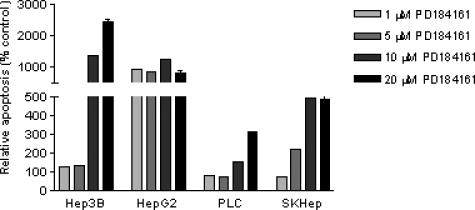
To determine if the antiproliferative effects of PD184161 involve apoptosis as a mechanism, we used DNA fragmentation ELISA to quantify apoptosis. Previous MEK inhibitor studies have demonstrated that significant effects in apoptosis may be observed as early as 48 hours into treatment [39]. Figure 4 shows the effects of PD184161 (1–20 µM) on the degree of apoptosis in Hep3B, HepG2, PLC, and SKHep cells 48 hours into treatment. All cell lines showed increases in apoptosis as a result of PD184161 treatment. HepG2 cells were most sensitive to PD184161, with >1000% increase in relative apoptosis at low-dose PD184161 (1 µM). Interestingly, Hep3B cells were more sensitive to higher-dose PD184161 (10–20 µM) than HepG2 cells, increasing their apoptosis to >2400%. In contrast, SKHep and PLC cells may have less apoptosis at low concentrations of PD184161 and less increases (100–500%) at higher doses (5–20 µM).
Figure 4.
The effect of the MEK inhibitor PD184161 on apoptosis in four human HCC cell lines (Hep3B, HepG2, PLC and SKHep). Actively dividing cells were treated for 48 hours with concentrations of PD184161 varying from 1.0 to 20 µM. A DNA fragmentation ELISA assay was used to determine apoptosis relative to control dimethyl sulfoxide-treated cells. Data are expressed as the percentage of control. Each point on the graph represents the mean of triplicate samples, and error bars represent SD. Similar results were obtained in at least two independent experiments.
Effect of MEK Inhibition on Human HCC Engraftment and Early Tumor Growth
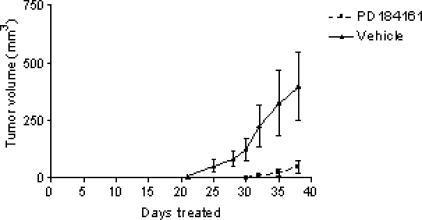
To determine the effect of PD184161 on human HCC engraftment and early tumor growth, we employed the MEK inhibitor PD184161 in an in vivo human tumor xenograft model. Fourteen athymic nude mice were inoculated with Hep3B cells (randomly assigned to two groups of seven) and, 12 hours later, were dosed by orogastric gavage twice daily with either 300 mg/kg PD184161 or vehicle for 38 days. Twice-daily dosing was based on previous studies demonstrating significant P-ERK reduction 1 to 12 hours after dosing (data not shown). Tumor growth was monitored for the duration of the study, and measurements were taken every 3 days. As shown in Figure 5, treatment with PD184161 resulted in a significant delay in tumor formation (9 days) relative to the controls. In addition, early tumor growth was significantly decreased in treated animals. The time required for tumors to reach 50 mg in size was 38 vs 25 days, in the treated and control groups, respectively. Using the same protocol, a separate evaluation of PD184161 treatment in human C26 colon adenocarcinomaxenografts also resulted in a significant delay in tumor formation (6 days) and early tumor growth rate (data not shown). Metastatic colon cancer is relevant insofar as it is the most common secondary tumor of the liver. There were no significant differences in weight gain or loss as a result of any treatment (data not shown). No treatment-related deaths or toxicity were associated with PD184161 treatment.
Figure 5.
The effect of twice-daily oral dosing with the MEK inhibitor PD184161 on the growth of newly inoculated Hep3B tumor xenografts in nude mice (n = 7). Mice were treated with PD184161 (300 mg/kg) beginning 12 hours after Hep3B inoculation and continued for 38 days. Tumors were measured every 3 days, and volumes are represented in the figure (▲ vehicle; ■ PD184161). Tumor measurements in PD184161-treated animals were found to be statistically significant from control at all time points shown (t-test; P < .05).
Effect of MEK Inhibition on Established Human HCC Tumor Growth
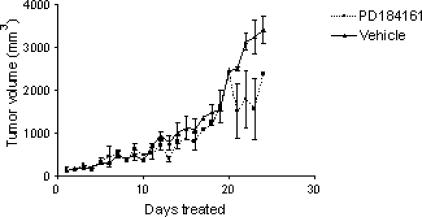
Having established an effect of MEK inhibition on tumor engraftment and early tumor growth, we examined the effects of PD184161 on established tumors. Fourteen athymic nude mice were inoculated with Hep3B cells, mice were randomly assigned to two groups of seven, and treatment with PD184161 (300 mg/kg, twice daily) or vehicle was initiated when the tumors had reached 100 mm3 and continued for 24 days. No significant difference was detected in tumor growth in animals treated with PD184161 (P = .17) (Figure 6). There were no significant differences in weight gain or loss as a result of any treatment (data not shown). Using the same protocol, we examined the effects of PD184161 on established SKHep and PLC tumors. As in Hep3B tumors, PD184161 did not significantly decrease tumor growth (data not shown).
Figure 6.
The effect of twice-daily oral dosing with the MEK inhibitor PD184161 on the growth of established Hep3B tumor xenografts in nude mice (n = 7). Nude mice were inoculated in the flank with 3 to 5 million Hep3B cells. Once the tumors had reached 100 mm3, the mice were dosed orally with 300 mg/kg twice daily for 24 days. Tumor measurements were taken every 3 days, and tumor volume is represented in the figure (▲ vehicle; ■ PD184161). There was no statistically significant difference between treatment groups (P = .17).
Effect of PD184161 on MEK Activity in Human Tumor Xenografts
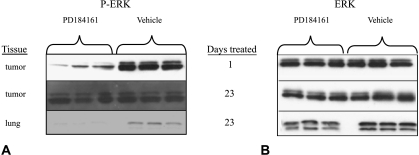
The early effects of PD184161 in inhibiting tumor engraftment and early growth contrast sharply with the effects of PD184161 in established tumors. To evaluate the effects of PD184161 treatment on the phosphorylation status of ERK in HCC tumors in vivo, Hep3B cells were inoculated into the flanks of 28 nude mice and the tumors were allowed to grow up to 100 mm3. At this point, half of the mice were dosed orally with 300 mg/kg PD184161 or vehicle. Fourteen animals were sacrificed 3 hours after the first dose, whereas 14 others were treated with 300 mg/kg PD184161 or vehicle twice daily for 24 days and then sacrificed 3 hours after the last treatment. MEK activity, as measured by phosphorylated ERK1,2, was significantly decreased in tumors in animals treated with a single dose of PD184161 compared to controls (Figure 7). Densitometric analysis revealed a statistically significant 80% decrease in phosphorylated ERK1,2 levels in tumors from treated animals (P < .05). Similar results were demonstrated in C26 human colon adenocarcinoma tumor xenografts (data not shown). In contrast, phosphorylated ERK1,2 levels were no longer decreased in HCC tumor xenografts from animals that underwent treatment for 24 days, suggesting that tumors become refractory to the effects of PD184161 on MEK activity (i.e., P-ERK level). To exclude a generalized effect of the drug or its bioavailability, phosphorylated ERK1,2 levels were assessed in lung tissues from these same animals (baseline levels of phosphorylated ERK1,2 in normal liver were barely detectable, making comparisons of treated group to control group not feasible). Analysis of lung tissues from treated animals showed suppression of phosphorylated ERK1,2 levels, indicating that the drug was still active in vivo. These data suggest that signaling resistance to MEK inhibition with PD184161 occurs in the long-term treatment of established tumors. No gross or histologic lung pathology was observed as a result of PD184161 treatment.
Figure 7.
The effect of single and multiple oral dosing with the MEK inhibitor PD184161 on the expression of phosphorylated ERK1,2 (A) and total ERK1,2 (B) in nude mouse Hep3B tumor xenografts and lung. Homogenates from Hep3B tumors were prepared from treated and control mice and assessed by Western blot analysis for phosphorylated ERK1,2 and total ERK1,2 levels. Representative Western blot analyses are shown here. Results were reproducible in multiple assays. Densitometric analysis was performed, and results were analyzed by t-test (P < .05).
Discussion
The effects of a number of growth and survival signals are mediated by p42/p44 MAPK signaling [37,42–45]. There is increasing evidence that this pathway is abnormally regulated in HCC and other cancers and plays a central role in tumorigenesis and maintenance of tumor growth [17,19,46]. Treatment strategies targeting p42/p44 MAPK signaling intermediates such as MEK, therefore, have significant merit [37,43,47,48]. This study examined the effects of PD184161, a novel, high-affinity, orally active MEK inhibitor, on p42/p44 MAPK signaling, proliferation, apoptosis, tumor engraftment, and early and established tumor growth in human HCC.
PD184161, which is structurally distinct from PD098059 and U0126, joins this mechanistic class of agents that inhibit the downstream phosphorylation of ERK through their direct effects on MEK [40,47,49]. PD098059 prevents the activation of MEK1, whereas U0126 appears to inhibit the activation of MEK through Raf, but also the downstream phosphorylation of ERK by activated MEK [40,50]. Here we report that PD184161 significantly inhibits p42/p44 MAPK signaling in human HCC cell lines, as evidenced by the inhibition of downstream ERK1,2 phosphorylation. PD184161, compared to PD098059, resulted in greater suppression of signaling and was superior to or equally effective as U0126. The effectiveness of U0126 is likely linked to its significantly higher affinity for MEK than PD098059 [40]. PD184161 is believed to share this enhanced affinity for MEK, but unlike PD098059 and U0126, PD184161 has the obvious advantages of solubility and oral bioavailability. The pharmacological potential of targeting MEK capitalizes on the overexpression and dependence of tumor cells on the p42/p44 MAPK activation for cell survival. A normal liver has almost undetectable levels of p42/p44 MAPK activity compared to high levels of p42/p44 MAPK activity in human HCC [19]. This significant difference in activity makes the regional delivery of p42/p44 MAPK-targeted treatments seem very feasible. Because this pathway has important effects in normal cellular functions, the systemic delivery of MEK-targeted agents may have untoward effects. Clinical trials with CI1040, a structurally related analog of PD184161, in breast cancer, colon cancer, non-small cell lung cancer, and pancreatic cancer did not show sufficient antitumor activity to continue the trials; however, significant systemic toxicity was not found to be an issue with this drug [51]. A second-generation MEK inhibitor PD0325901 has recently shown promise in clinical trials for the treatment of melanoma [52].
By examining the growth of human HCC cell lines following treatment with PD184161, we found this compound to inhibit the growth of these cells in a time- and concentration- dependent manner, which appears to correlate with suppression of p42/p44 MAPK signaling. In a related study, Huynh et al. [17] recently reported that overexpression of MEK1 in transfected HepG2 cells resulted in resistance to apoptosis caused by U0126 treatment, demonstrating the connection between MEK activity and MEK-targeted treatments. Although the efficacy of PD098059 and U0126 as inhibitors of in vitro cell growth and promoters of apoptosis has been documented in our laboratory and others [17,53], the experiments reported here indicate that PD184161 is more effective in vitro and has the distinct advantage of also being effective in vivo.
MEK-targeted treatment has been studied in colon and breast tumors [54–56], but the use of small molecule MEK inhibitors in human HCC tumors, to our knowledge, has not been examined. Targeting MEK with PD184161 in vivo resulted in a significant delay in HCC tumor engraftment and inhibition of early tumor growth. MEK-transfected HepG2 cells have been shown to grow more rapidly in vivo than mock-transfected HepG2 cells, linking HCC growth to MEK activity [17].
In contrast, PD184161 was ineffective at suppressing established HCC tumor growth. In addition, MEK suppression by PD184161 was not achieved in tumors “conditioned” by multiple doses of the drug, but only in “naïve” tumors that had received a single drug dose. The systemic efficacy of PD184161 is unlikely to be responsible for the lack of drug effectiveness because the lung, an organ expressing high MEK activity, was effectively suppressed with PD184161 treatment after repeated dosing. It is possible that organspecific bioavailability (lung versus tumor), tumor vascularity, or P-glycoprotein expression could be involved in our observations, and these issues may be the subject of future study. Although Hep3B cells do not harbor Ras or Raf mutation, the growth of this cell line responds to MEK inhibition in vitro; importantly, early Hep3B tumor growth in vivo is delayed by PD184161 treatment. Although we have not established a causal link between the lack of P-ERK signal suppression in the long-term dosing of PD184161 and the lack of effect on established tumor growth, we cannot exclude the possibility that effective MEK inhibition, if achieved, would reduce HCC tumor growth. In this investigation, the lack of growth inhibition appears to correlate with the lack of suppression of P-ERK level, which was the target of PD184161. However, other signaling pathways could be involved in the growth of these tumors. Not all tumor types behave in this fashion. Our coauthor reported tumor growth reduction and concomitant reduction in MEK activity in established colon tumor xenografts after treatment with CI-1040, a structurally related analog of PD184161 [55]. Melanoma and lung cancer also appear to be similarly susceptible [55,57,58]. Thus, HCC appears to be unique in its ability to develop resistance to PD184161. The study of chemoresistance of HCC tumors to small molecule inhibitor treatment is an extremely important but relatively untapped field of investigation. Understanding the novel resistance mechanism of HCC to MEK-targeted treatment may have broader implications and may shed light on mechanisms of small molecule inhibitor resistance in other systems.
In summary, we have shown that a novel, highly specific MEK inhibitor PD184161 more effectively suppresses MEK activity in multiple HCC cell lines when compared to MEK inhibitors PD098059 and U0126. Its efficacy as an inhibitor of HCC cell proliferation and promoter of apoptosis correlates with its observed effects on MEK activity. Importantly, in vivo, PD184161 successfully inhibits tumor engraftment and early growth of human HCC xenografts. Growth of established tumors, however, was not significantly affected by PD184161. Correspondingly, MEK activity in human tumors was suppressed with PD184161 initially, but not with longerterm administration. MEK remains an intriguing chemotherapeutic target for the treatment of human HCC; however, the degree of success of MEK-targeted treatment in HCC and other cancers may depend, in part, on the discovery of mechanisms governing MEK inhibitor signaling resistance.
Abbreviations
- p42/p44 MAPK
extracellular signal-regulated mitogen-activated protein kinase
- ERK
extracellular signal-related kinase
- HCC
hepatocellular carcinoma
- MEK
MAPK kinase
- MEKK
MEK kinase
Footnotes
This work was supported by a Clarian Health Values grant (VFR-21).
Patrick J. Klein and C. Max Schmidt share first authorship.
References
- 1.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Olthoff KM. Surgical options for hepatocellular carcinoma: resection and transplantation. Liver Transplant Surg. 1998;4:S98–S104. [PubMed] [Google Scholar]
- 4.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 5.Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573–588. doi: 10.2174/1566524033479546. [DOI] [PubMed] [Google Scholar]
- 6.Romeo R, Colombo M. The natural history of hepatocellular carcinoma. Toxicology. 2002;181–182:39–42. doi: 10.1016/s0300-483x(02)00252-4. [DOI] [PubMed] [Google Scholar]
- 7.Feo F, Pascale RM, Simile MM, De Miglio MR, Muroni MR, Calvisi D. Genetic alterations in liver carcinogenesis: implications for new preventive and therapeutic strategies. Crit Rev Oncog. 2000;11:19–62. [PubMed] [Google Scholar]
- 8.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 9.Rossomando AJ, Sanghera JS, Marsden LA, Weber MJ, Pelech SL, Sturgill TW. Biochemical characterization of a family of serine/threonine protein kinases regulated by tyrosine and serine/threonine phosphorylations. J Biol Chem. 1991;266:20270–20275. [PubMed] [Google Scholar]
- 10.Mitra G, Weber M, Stacey D. Multiple pathways for activation of MAP kinases. Cell Mol Biol Res. 1993;39:517–523. [PubMed] [Google Scholar]
- 11.Lenormand P, Pages G, Sardet C, L'Allemain G, Meloche S, Pouyssegur J. MAP kinases: activation, subcellular localization and role in the control of cell proliferation. Adv Second Messenger Phosphoprot Res. 1993;28:237–244. [PubMed] [Google Scholar]
- 12.Lenormand P, Brondello JM, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 14.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 15.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi Y, Ichida T, Sugitani S, Genda T, Inayoshi J, Takamura M, Matsuda Y, Nomoto M, Aoyagi Y. Overexpression of extracellular signal-regulated protein kinase and its correlation with proliferation in human hepatocellular carcinoma. Liver Int. 2004;24:432–436. doi: 10.1111/j.1478-3231.2004.0940.x. [DOI] [PubMed] [Google Scholar]
- 17.Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. doi: 10.1186/1471-230X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. The role of cAMP-MAPK signalling in the regulation of human hepatocellular carcinoma growth in vitro. Eur J Gastroenterol Hepatol. 1999;11:1393–1399. doi: 10.1097/00042737-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997;236:54–58. doi: 10.1006/bbrc.1997.6840. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 21.McKillop IH, Schmidt CM, Cahill PA, Sitzmann JV. Inhibitory guanine nucleotide regulatory protein activation of mitogen-activated protein kinase in experimental hepatocellular carcinoma in vitro. Eur J Gastroenterol Hepatol. 1999;11:761–768. doi: 10.1097/00042737-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 22.McKillop IH, Vyas N, Schmidt CM, Cahill PA, Sitzmann JV. Enhanced Gi-protein-mediated mitogenesis following chronic ethanol exposure in a rat model of experimental hepatocellular carcinoma. Hepatology. 1999;29:412–420. doi: 10.1002/hep.510290218. [DOI] [PubMed] [Google Scholar]
- 23.McKillop IH, Schmidt CM, Cahill PA, Sitzmann JV. Altered Gq/G11 guanine nucleotide regulatory protein expression in a rat model of hepatocellular carcinoma: role in mitogenesis. Hepatology. 1999;29:371–378. doi: 10.1002/hep.510290201. [DOI] [PubMed] [Google Scholar]
- 24.McKillop IH, Schmidt CM, Cahill PA, Sitzmann JV. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;26:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. Alterations in guanine nucleotide regulatory protein expression and activity in human hepatocellular carcinoma. Hepatology. 1997;26:1189–1194. doi: 10.1002/hep.510260516. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz M, Buchmann A, Bock KW. Role of cell proliferation at early stages of hepatocarcinogenesis. Toxicol Lett. 1995;82–83:27–32. doi: 10.1016/0378-4274(95)03465-x. [DOI] [PubMed] [Google Scholar]
- 27.Stahl S, Ittrich C, Marx-Stoelting P, Kohle C, Altug-Teber O, Riess O, Bonin M, Jobst J, Kaiser S, Buchmann A, et al. Genotypephenotype relationships in hepatocellular tumors from mice and man. Hepatology. 2005;42:353–361. doi: 10.1002/hep.20768. [DOI] [PubMed] [Google Scholar]
- 28.Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang YH, Choi JY, Kim S, Chung ES, Kim T, Koh SS, Lee B, Bae SH, Kim J, Park YM. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol Res. 2004;29:113–121. doi: 10.1016/j.hepres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Hopfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherubl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008–1016. doi: 10.1016/j.jhep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Harada K, Shiota G, Kawasaki H. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver. 1999;19:318–325. doi: 10.1111/j.1478-3231.1999.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 32.Moon WS, Chang KJ, Majumdar AP, Tarnawski AS. Reduced expression of epidermal growth factor receptor-related protein in hepatocellular carcinoma: implications for cancer growth. Digestion. 2004;69:219–224. doi: 10.1159/000079151. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi T, Suzuki T, Moriya K, Shintani Y, Fujie H, Miyoshi H, Matsuura Y, Koike K, Miyamura T. Hepatitis C virus core protein activates ERK and p38 MAPK in cooperation with ethanol in transgenic mice. Hepatology. 2003;38:820–828. doi: 10.1053/jhep.2003.50399. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda K, Tsuchihara K, Hijikata M, Nishiguchi S, Kuroki T, Shimotohno K. Hepatitis C virus core protein enhances the activation of the transcription factor, Elk1, in response to mitogenic stimuli. Hepatology. 2001;33:159–165. doi: 10.1053/jhep.2001.20794. [DOI] [PubMed] [Google Scholar]
- 35.Duesbery NS, Webb CP, Vande Woude GF. MEK wars, a new front in the battle against cancer. Nat Med. 1999;5:736–737. doi: 10.1038/10457. [DOI] [PubMed] [Google Scholar]
- 36.Milella M, Kornblau SM, Andreeff M. The mitogen-activated protein kinase signaling module as a therapeutic target in hematologic malignancies. Rev Clin Exp Hematol. 2003;7:160–190. [PubMed] [Google Scholar]
- 37.Kohno M, Pouyssegur J. Pharmacological inhibitors of the ERK signaling pathway: application as anticancer drugs. Prog Cell Cycle Res. 2003;5:219–224. [PubMed] [Google Scholar]
- 38.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- 39.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg. 2004;198:410–421. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 41.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 42.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 43.Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002;25:511–518. doi: 10.1159/000068621. [DOI] [PubMed] [Google Scholar]
- 44.Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 45.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 46.Zhu J, Leng X, Dong N, Liu Y, Li G, Du R. Expression of mitogen-activated protein kinase and its upstream regulated signal in human hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 2002;40:1–16. [PubMed] [Google Scholar]
- 47.Sebolt-Leopold JS. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene. 2000;19:6594–6599. doi: 10.1038/sj.onc.1204083. [DOI] [PubMed] [Google Scholar]
- 48.Nottage M, Siu LL. Rationale for Ras and raf-kinase as a target for cancer therapeutics. Curr Pharm Des. 2002;8:2231–2242. doi: 10.2174/1381612023393107. [DOI] [PubMed] [Google Scholar]
- 49.Marshall SJ, Senis YA, Auger JM, Feil R, Hofmann F, Salmon G, Peterson JT, Burslem F, Watson SP. GPIb-dependent platelet activation is dependent on Src kinases but not MAP kinase or cGMP-dependent kinase. Blood. 2004;103(7):2601–2609. doi: 10.1182/blood-2003-09-3319. [DOI] [PubMed] [Google Scholar]
- 50.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, et al. Multi-center phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 52.Lorusso P, Krishnamurthi S, Rinehart JR, Nabell L, Croghan B, Varterasian M, Sadis SS, Menon SS, Leopold J, Meyer MB. A Phase 1–2 Clinical Study of a Second Generation Oral MEK Inhibitor, PD 0325901 in Patients with Advanced Cancer. Orlando, FL: American Society of Clinical Oncology; 2005. [Google Scholar]
- 53.Mitsui H, Takuwa N, Maruyama T, Maekawa H, Hirayama M, Sawatari T, Hashimoto N, Takuwa Y, Kimura S. The MEK1-ERK map kinase pathway and the PI 3-kinase-Akt pathway independently mediate anti-apoptotic signals in HepG2 liver cancer cells. Int J Cancer. 2001;92:55–62. [PubMed] [Google Scholar]
- 54.Duesbery NS, Resau J, Webb CP, Koochekpour S, Koo HM, Leppla SH, Vande Woude GF. Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple MEK pathways. Proc Natl Acad Sci USA. 2001;98:4089–4094. doi: 10.1073/pnas.061031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 56.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 57.Collisson EA, De A, Suzuki H, Gambhir SS, Kolodney MS. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res. 2003;63:5669–5673. [PubMed] [Google Scholar]
- 58.Kramer BW, Gotz R, Rapp UR. Use of mitogenic cascade blockers for treatment of C-Raf induced lung adenoma in vivo: CI-1040 strongly reduces growth and improves lung structure. BMC Cancer. 2004;4:24. doi: 10.1186/1471-2407-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]