Abstract
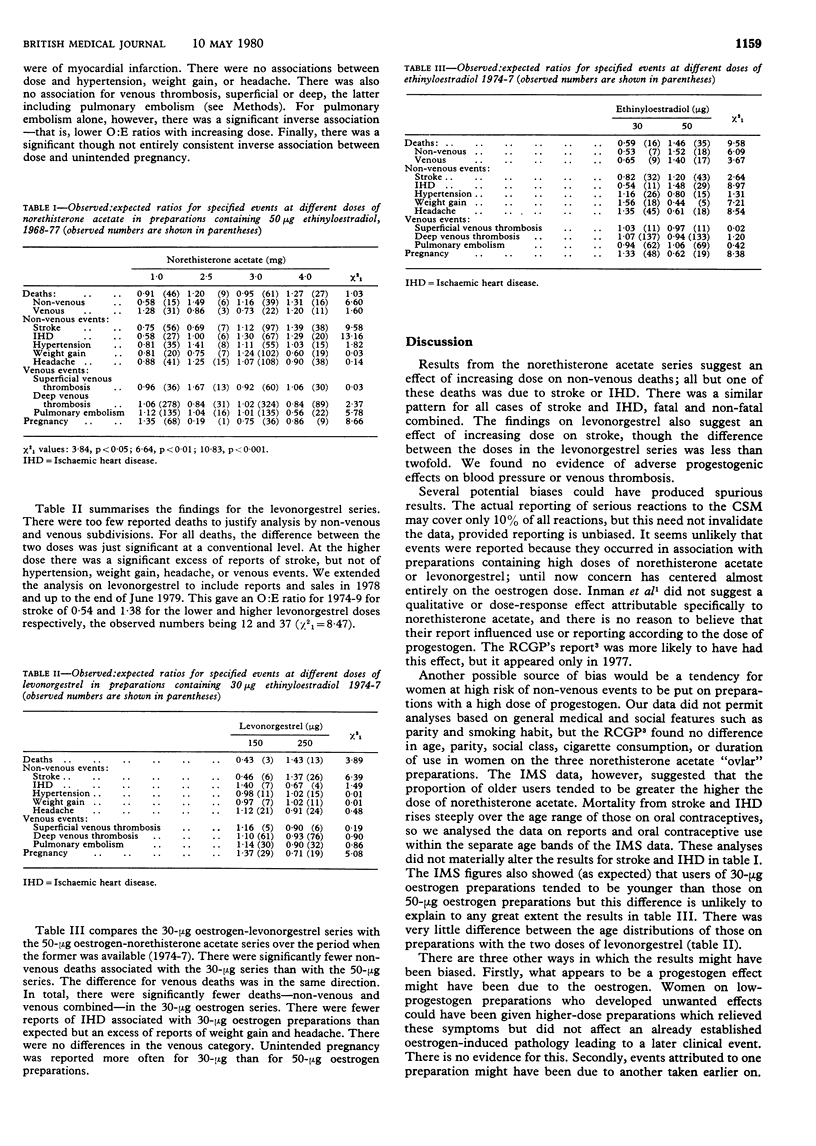
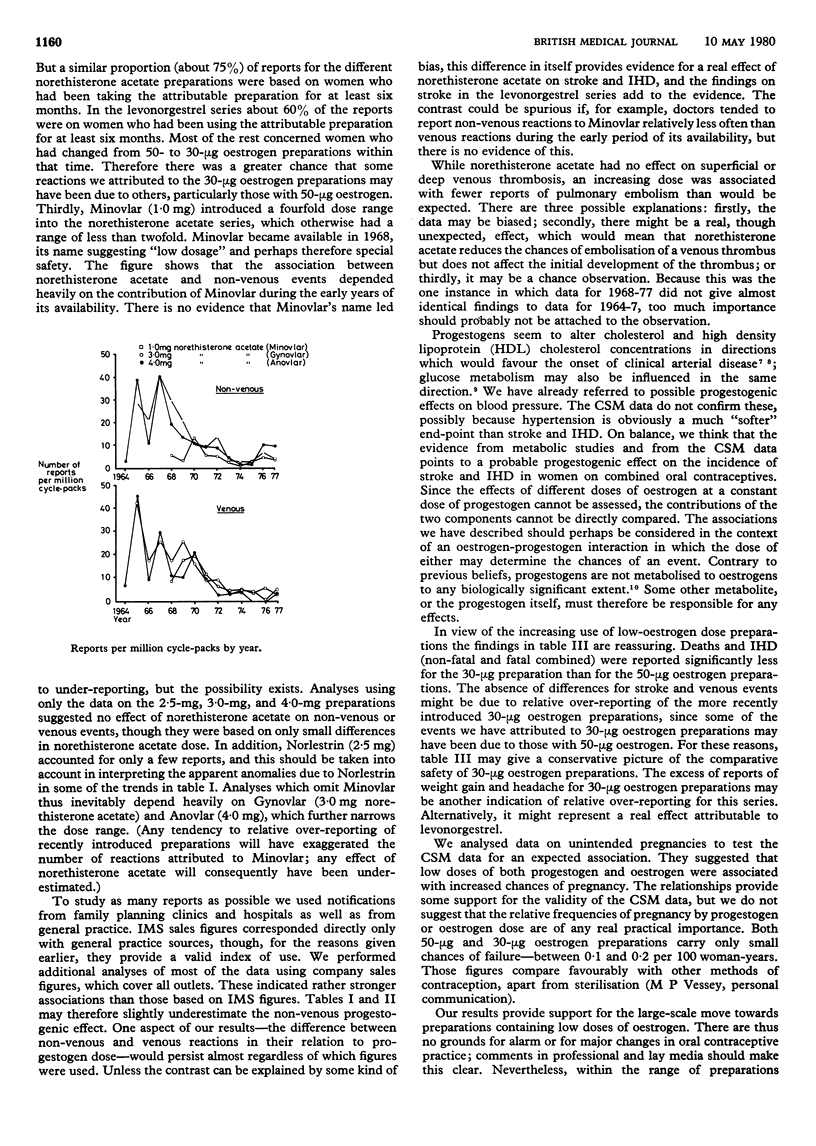
Progestogens probably have metabolic effects that may contribute to the increased risk of cardiovascular reactions associated with combined oestrogen-progestogen oral contraceptives. This possibility was investigated by a study of nearly 2000 reports to the Committee on Safety of Medicines from 1964 to 1977. The reports concerned preparations in which norethisterone acetate in doses of 1.0, 2.5, 3.0, or 4.0 mg was combined with 50 microgram of ethinyloestradiol and those in which levonorgestrel in doses of 150 or 250 microgram was combined with 30 microgram of ethinyloestradiol. Observed and expected numbers of reports were compared, using retail pharmacy purchase figures as a measure of the use of different preparations. There was a significant positive association between the dose of norethisterone acetate and deaths from stroke and ischaemic heart disease (IHD); this association was also found for all cases of these two conditions, fatal plus non-fatal. There were no associations of dose of norethisterone acetate with hypertension or venous thrombosis. The higher dose of levonorgestrel was associated with a possible excess of deaths, non-venous plus venous, and an excess of strokes. There was no association between dose of levonorgestrel and hypertension or venous thrombosis. The reports were also used to assess the relative safety of 30-microgram and 50-microgram oestrogen preparations. Those with 30 microgram of oestrogen were associated with significantly fewer reports of death and IHD (both fatal, and fatal plus non-fatal) than those with 50 microgram of oestrogen. In view of the large-scale move towards preparations with progressively lower oestrogen doses, there are no grounds for major changes in oral contraceptive practice. Within the range of preparations currently in use, however, there is a case for minimising the dose of progestogen to reduce the chances of thromboembolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. D., Wingerd J., Petitti D. B., Krauss R. M., Ramcharan S. Serum high-density-lipoprotein cholesterol in women using oral contraceptives, estrogens and progestins. N Engl J Med. 1978 Jul 6;299(1):17–20. doi: 10.1056/NEJM197807062990104. [DOI] [PubMed] [Google Scholar]
- Inman W. H., Vessey M. P., Westerholm B., Engelund A. Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. Br Med J. 1970 Apr 25;2(5703):203–209. doi: 10.1136/bmj.2.5703.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade T. W., Haines A. P., North W. R., Chakrabarti R., Howarth D. J., Stirling Y. Haemostatic, lipid, and blood-pressure profiles of women on oral contraceptives containing 50 microgram or 30 microgram oestrogen. Lancet. 1977 Nov 5;2(8045):948–951. doi: 10.1016/s0140-6736(77)90888-1. [DOI] [PubMed] [Google Scholar]
- Townsley J. D., Brodie H. J. Low conversion of 19-nortestosterone to urinary oestrogens. Lancet. 1970 Nov 14;2(7681):1039–1039. doi: 10.1016/s0140-6736(70)92860-6. [DOI] [PubMed] [Google Scholar]
- Wynn V., Adams P. W., Godsland I., Melrose J., Niththyananthan R., Oakley N. W., Seed M. Comparison of effects of different combined oral-contraceptive formulations on carbohydrate and lipid metabolism. Lancet. 1979 May 19;1(8125):1045–1049. doi: 10.1016/s0140-6736(79)92949-0. [DOI] [PubMed] [Google Scholar]


