Abstract
Evidence from epidemiological studies suggests that plant-based diets can reduce the risk of prostate cancer. However, very little information is available concerning the use of botanicals in preventing prostate cancer. As a first step toward developing botanicals as prostate cancer preventives, we examined the effect of Nexrutine on human prostate cancer cells. Nexrutine is a herbal extract developed from Phellodendron amurense. Phellodendron extracts have been used traditionally in Chinese medicine for hundreds of years as an anti-diarrheal, astringent, and anti-inflammatory agent. The present study investigated its potential antitumor effect on human prostate cancer cells. Our results suggest that it inhibits tumor cell proliferation through apoptosis induction and inhibition of cell survival signaling. The results of the present study indicate that Nexrutine treatment 1) inhibits the proliferation of both androgen-responsive and androgen-independent human prostate cancer cells through induction of apoptosis; 2) reduces levels of pAkt, phosphorylated cAMP response-binding protein (pCREB), and CREB DNA-binding activity; and 3) induces apoptosis in prostate cancer cells stably overexpressing Bcl-2. Further Akt kinase activity was reduced in cells treated with Nexrutine, and ectopic expression of myristoylated Akt protected from Nexrutine induced inhibition of proliferation, implicating a role for Akt signaling.
Keywords: Akt/CREB signaling, Bcl-2-independent apoptosis, Herbal extracts, Nexrutine, CREB DNA binding
Introduction
Prostate cancer (PCA) is the most common malignancy in men especially in the Western world. Approximately 230,000 men are diagnosed with PCA every year in the United States alone and 30,000 of them die [1]. Although genetic and epigenetic factors play a major role in the development and progression of PCA, both experimental and epidemiological studies show that several agents found in the human diet may prevent the effects of carcinogenic agents or suppress the promotional activity in already initiated neoplastic prostatic cells. Even though PCA is the second leading cause of cancer-related deaths in men, it has a long latency period that provides opportunities for intervention with strategies including the use of dietary phytochemicals. Such strategies may delay disease initiation or progression or will prevent the development of clinically significant disease. It is interesting that incidence of PCA is low in the population consuming phytochemicals, which are chemical compounds derived from plants such as fruits and vegetables. For example, genistein, the most abundant phytochemical component of soy; resveratrol, found in grapes and peanuts; curcumin, a dietary spice; and green tea polyphenols have been implicated in the prevention of PCA [2–7]. Therefore, the use of dietary supplements including nutritional and natural products is gaining ground in modulating the risk of development and/or for treating PCA [7,8]. Estimates also show approximately 40% of Americans use alternative remedies including herbal medicine for disease prevention and therapy [7–9]. Such herbal-based dietary supplements contain a large array of phytochemicals with polyphenolic constituents (flavonoids) that may mediate physiological functions related to cancer suppression. An extract of eight Chinese herbs called PC-SPES has been shown to inhibit the growth of PCA cells through various mechanisms involving modulation of apoptosis, androgen receptor expression, and microtubule synthesis. Evaluation of PC-SPES also showed a significant response in patients with PCA with acceptable toxicity profile. However, it has been recalled by the US Food and Drug administration because of contamination with the synthetic estrogen DES, anticoagulant warfarin, and the anti-inflammatory indomethacin (reviewed in Refs. 7,8; 10,11).
Consumption of tomato powder rather than lycopene (an active tomato ingredient) inhibits prostate carcinogenesis, suggesting that compounds present in the tomato powder in addition to lycopene modify prostate carcinogenesis [12]. These data suggest that botanicals and whole foods may work better than the single biologically active component. Nexrutine is a fractionated extract of the traditional Chinese medicine Phellodendron amurense. Phellodendron extracts have been used traditionally in Chinese medicine for hundreds of years as an antidiarrheal, astringent, and anti-inflammatory agent [13,14]. However, it is not known whether Nexrutine could be developed as a dietary supplement for prevention or treatment of PCA. In this study we examined the effect of Nexrutine on PCA cells and showed that it has a potential to be developed as an agent for PCA therapy.
Materials and Methods
Preparation of Nexrutine
Nexrutine was provided by Next Pharmaceuticals (Irvine, CA). Stock solution of Nexrutine was prepared by dissolving 10 mg of Nexrutine in 10 ml DMSO to obtain a concentration of 1 mg/ml. This was diluted in growth medium to obtain concentrations of 1 to 10 µg/ml.
Prostate Cancer Cell Lines
Human PCA cell lines, androgen-responsive LNCaP, and androgen-independent PC-3 cells were grown and maintained as described earlier [15–17]. PC-3 Neo and PC-3 Bcl-2 cells were grown in the presence of 400 µg/ml G 418 [18].
Cell Proliferation Assays
Cancer cells were plated in 96-well plates at a density of 4000 cells per well in triplicate. After attachment (after 24 hours), cells were treated with different concentrations of Nexrutine (0.5, 1, 2.5, 5, and 10 µg/ml). Control cells received only the solvent (DMSO). Cell proliferation was detected after 72 hours of incubation with Nexrutine by using CellTiter 96 Aqueous One (Promega Corporation, Madison, WI) solution assay as described by the manufacturer [15,16]. Cell cycle analysis was performed as described in Kumar et al. [15,16].
Colony Formation Assay
Logarithmically growing LNCaP or PC-3 cells were plated at a density of 13,000 cells/ml in 0.5% agarose plates in triplicate without and with increasing concentrations of Nexrutine (2.5, 5, 7.5, and 10 µg/ml). Plates were prepared fresh by adding 0.5 g agar (FMC 50102) to 100 ml of complete growth medium. After incubation at 44°C, 2 ml of this agarose was evenly layered in six-well plates and allowed to solidify for 30 minutes. Then 2 ml complete medium containing 0.5% agar was added to 40,000 cells. After mixing, 1 ml medium containing cells was poured on top of the 0.5% medium in the six-well plate. A plate containing no cells was used as a negative control. After 14 days, cells were stained with 0.02% p-iodonitrotetrazolium. After 5 hours, the colonies that stained dark pink were counted in 10 different fields from each well as described earlier [19].
Detection of Apoptosis
LNCaP, PC-3, PC-3 Neo, or PC-3 Bcl-2 cells were plated at a density of 1 x 105 in 60-mm dishes. At 70% to 80% confluency, cells were treated with different concentrations of Nexrutine. After incubation, both adherent and floating cells were collected by trypsinization for detection of apoptosis by morphologic analysis; TUNEL assay and 4′-6-diamidino-2-phenylindole (DAPI) staining. DAPI staining can be used to distinguish live from apoptotic cells based on the morphology of their nuclei. DAPI forms fluorescent complexes with double-stranded DNA, and DAPI-stained nuclei show a bright blue fluorescence when observed under a fluorescent microscope with DAPI filter.
Preparation of Cell Extracts and Western Blotting
Cells were treated with Nexrutine (5 µg/ml for 24 hours or at different concentrations and time points as indicated in the figures) and subsequently lysed in a buffer containing [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% NP40, 50 mM NaF, 1 mM NaVO4, 1 mM phenylmethylsufonyl fluoride, 25 µg/ml leupeptin, 25 µg/ml aprotinin, 25 µg/ml pepstatin, and 1 mM DTT]. After the lysate was passed through a 25G needle, cell debris was removed by centrifugation at 12,000 rpm for 30 minutes. Nuclear extracts were prepared according to the method of Dignam, and protein content of the extracts was determined by the method of Bradford as described earlier [15,16].
Equal amounts of extracts were fractionated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel. After electrophoresis, proteins were electrophoretically transferred to a nitrocellulose membrane. The blotted membrane was blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (blocking solution), and incubated with indicated polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; Cell Signaling Technology, Inc., Beverly, MA; and Upstate Cell Signaling Solutions, Lake Placid, NY) followed by incubation with HRP-conjugated anti-rabbit IgG antibody (Sigma, St. Louis, MO) in blocking solution. Bound antibody was detected by enhanced chemiluminescence using Supersignal West Pico Chemiluminescent Substrate, following the manufacturer's directions (Pierce, Rockford, IL). All the blots were stripped and reprobed with β-actin to ensure equal loading of protein. Each experiment was repeated thrice using different sets of extracts.
Preparation of RNA
LNCaP cells at 70% to 80% confluency were treated with 5 µg/ml of Nexrutine for 24 hours. Total RNA was isolated with the TRIZOL reagent (Ambion, Austin, TX) according to the manufacturer's recommendations as described earlier [20].
Fast Activated Cell-Based ELISA for Akt
We measured the levels and activity of Akt by multiple approaches including Western blotting, Fast Activated Cell-Based ELISA (FACE), and immunocytochemistry. We monitored the phosphorylation of Akt using a more sensitive kit that specifically measures activated Akt and/or total Akt quantitatively (Active Motif, Carlsbad, CA). Briefly, cells in 96-well plates were treated with different concentrations of Nexrutine for 6 hours. After treatment, cells were rapidly fixed and incubated with a primary antibody that recognizes either phosphorylated or total Akt. This was followed by incubation with secondary HRP-conjugated antibody followed by colorimetric development. The relative number of cells in each well was determined using crystal violet reagent for normalization.
Transient Expression Assays
Transient transfections were performed in LNCaP cells by using a lipofectin reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Cells were transfected with myristoylated Akt or vector alone (pcDNA3) plasmid (1 µg per well) and incubated for 48 hours. Forty-eight hours after transfection, cells were treated with Nexrutine for 24 hours. After treatment, cells were trypsinized, and trypan exclusion method was used to determine cell viability as described earlier [16].
cAMP Response Element Binding Protein DNA-Binding Activity
cAMP response element binding protein (CREB) DNA-binding activity was measured in LNCaP and PC-3 nuclear extracts using TransAM CREB (Active Motif). The sequence of the wild-type CREB was 5′-AGAGATTGCCTGACGTCAAGAGAGCTAG-3′ (mutated nucleotides are shown in bold). Nuclear extracts were prepared from cells treated with Nexrutine (5 µg/ml) for different periods (0,3,6,12, and 24 hours). Briefly, the control and Nexrutine-treated nuclear extracts were incubated with CREB consensus oligonucleotide that was immobilized in a 96-well plate. A primary antibody specific for an epitope on the bound and active form of the CREB was added followed by subsequent incubation with secondary antibody and developing solution. After this incubation, CREB activity was measured colorimetrically at 450 nm. Nuclear extracts prepared from human fibroblast WI-38 cells stimulated with forskolin (CREB activator) was used as positive control. For competition experiments, the wells containing immobilized oligo were preincubated with 100-fold molar excess of wild-type and mutant oligonucleotide for 30 min before addition of the nuclear extract.
Immunocytochemistry
Slides with fixed cells (as described earlier) treated or untreated with Nexrutine were used. The slides were washed with TBS and 0.2% Triton X-100, followed by incubation with a blocking buffer containing 5.5% horse serum in 1 x phosphate-buffered saline (PBS) for 1 hour at room temperature. After another wash with TBS, the slides were incubated with the primary antibody (pAkt), diluted in blocking buffer (based on the manufacture's recommendations) overnight at 4°C with gentle rocking. After three washes with TBS/Triton, the slides were incubated with a Universal Secondary (Invitrogen Corporation, Carlsbad, CA) antibody for 1 hour at room temperature. After washes with TBS and TBS/Triton, the slides were incubated with 0.6% hydrogen peroxide for 30 minutes. The slides were washed once more with TBS and TBS/Triton and stable DAB was added to each spot on the slide for 15 to 20 minutes. Slides were then rinsed with water and mounted with a coverslip. Pictures of cells were taken at 20x and 40x magnification.
Statistical Analysis
Data were presented as average ± S.D. and significance was determined by using Student's t test. Differences between the experimental groups were considered significant at P < .05.
Results
We examined the effect of Nexrutine on proliferation of androgen-responsive LNCaP and androgen-independent PC-3 human PCA cells using the CellTiter 96 Aqueous One solution assay as described earlier [15,16]. Exponentially growing cells were treated with 0, 1, 2.5, 5, 10, and 25 µg/ml Nexrutine and cell proliferation was measured after 72 hours as described in Materials and Methods. As shown in Figure 1A, 5 µg/ml of Nexrutine was necessary to inhibit the growth of LNCaP cells by about 50%; more than 10 µg/ml was necessary for a similar effect in PC-3 cells. These results were also confirmed by measuring cell viability using the trypan blue exclusion method (data not shown). Because a characteristic feature of transformed cells is their ability to grow in an anchorage-independent fashion [21], we tested the effect of increasing concentrations of Nexrutine on growth of colonies in soft agar. We found that the number of colonies formed with Nexrutine treatment decreased with the concentration of Nexrutine (Figure 1B), which was consistent with the above cell-proliferation data. These data collectively indicate that Nexrutine inhibits the anchorage-dependent and-independent growth of PCA cells.
Figure 1.
Nexrutine inhibits anchorage-dependent (A) and anchorage-independent (B) growth of human PCA cells. Androgen-responsive (LNCaP) and androgenindependent (PC-3) cells were plated in 96-well plates as described in Materials and Methods and treated with indicated concentrations of either Nexrutine (in micrograms per milliliter) or solvent control. Cell proliferation was determined using Cell Titer96 Aqueous One solution assay at 72 hours. Absorbance at 570 nm determined by using a SpectraMaxPlus plate reader (Molecular Devices, Sunnyvale, CA) is shown. The data shown are averages ± S.D. of three replicate wells and is representative of four independent experiments. (B) Anchorage-independent growth cells were plated in triplicate in 35-mm dishes on 0.5% agarose containing medium as described in Materials and Methods. After 14 days' incubation, cells were stained with 0.5 ml of 0.02% p-iodonitrotetrazolium and colonies were counted in 10 different fields from each plate. The results are expressed as means ± S.D. and are representative of two independent experiments.
We examined if exposure to Nexrutine may activate apoptotic pathway in these cells. Cells treated with Nexrutine were examined for apoptotic features by using a combination of methods including 1) light microscopy to detect morphologic changes associated with the induction of apoptosis and 2) TUNEL assay. Exponentially growing LNCaP cells at 70% to 80% confluence were treated with either solvent control or different concentrations of Nexrutine (0, 1, 2.5, and 5 µg/ml for 24 hours). The cells were observed microscopically for treatment-associated morphologic changes. Significant changes in the morphology of the cells indicative of apoptosis including rounding of cells with granular appearance were evident after treatment with Nexrutine at a concentration of as low as 1 µg/ml. This resulted in detachment of cells from culture dishes and a significant proportion of the cells started to float by 24 hours (Figure 2A). Under identical conditions, the vehicle did not induce any features of apoptosis. The TUNEL assay used here was a colorimetric assay that involves incorporation of a biotinylated deoxyuridine 5-triphosphate (dUTP) into 3′-hydroxyl groups that were generated due to cleavage of apoptotic DNA. Incorporation of dUTP is detected using streptavidin-HRP conjugate and the chromogen (diaminobenzidine) is detected as a dark brown precipitate. As shown in Figure 2B, the nuclei of Nexrutine-treated cells showed characteristic brown staining indicative of DNA fragmentation. These data demonstrate that Nexrutine induces apoptosis of both LNCaP and PC-3 cells. We also examined the effect of Nexrutine on cell cycle distribution by measuring cellular DNA content in propidium iodide-stained cells by flow cytometry. As shown in Figure 2C, incubation of LNCaP or PC-3 cells with 5 µg/ml of Nexrutine for 24 hours showed a moderate G1-checkpoint block. Time course studies are being conducted to determine whether the observed G1-checkpoint block is responsible for apoptosis induction.
Figure 2.
Nexrutine treatment-induced apoptosis. (A) Morphological alterations; (B) apoptosis determined by TUNEL staining. LNCaP and PC-3 cells were treated with either DMSO or Nexrutine (0, 1,2.5, and 5 µg/ml) for 24 hours. (A) Photomicrographs of cells by phase-contrast microscopy using a Nikon Microscope with a digital camera system Coolpix 995 (Nikon Corporation, Tokyo, Japan). Original magnification x 20. (B) TUNEL staining of LNCaP and PC-3 cells treated with 5 µg/ml Nexrutine for 24 hours. Brown-stained nuclei are indicative of apoptosis. (C) Effect of Nexrutine for 24 hours on cell cycle distribution. The data shown are averages ± S.D. of two independent experiments.
Apoptosis is a complex program involving participation of several different genes as well as deregulation of cell survival signaling pathways [22,23]. The Bcl-2 family containing both proapoptotic and antiapoptotic members has been shown to play a critical role in the induction of apoptosis in various systems including prostate [24,25]. To investigate the mechanistic basis of Nexrutine-induced apoptosis in LNCaP cells, we examined the levels of Bcl-2 and Bax by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for RT-PCR and β-actin for Western blot analysis. Results presented in Figure 3A show the amplification products of the Bcl-2 and Bax along with GAPDH (bottom band). As shown here, there was no significant difference in the total levels of Bcl-2 after normalization to GAPDH. However, RNA from Nexrutine-treated samples showed increased levels of Bax (after normalization to GAPDH) in three different experiments. As shown in Figure 3B, the ratio of Bcl-2/Bax decreases in a time-dependent fashion in response to Nexrutine. Apoptosis is regulated by relative ratio of anti-apoptotic (Bcl-2) and proapoptotic (Bax) family members. Increased ratio of Bcl-2/Bax favors the formation of anti-apoptotic heterodimerization of Bcl-2/Bax and homodimerization between Bcl-2/Bcl-2 leading to inhibition of apoptosis. However, decrease in the ratio of Bcl-2/Bax favors the formation of Bax/Bax homodimerization leading to induction of apoptosis. It is possible that the observed decrease in the ratio of Bcl-2/Bax in response to Nexrutine may favor the formation of Bax/Bax homodimers with consequent induction of apoptosis. Similar results were also observed in the protein level as determined by Western blot analysis (Figure 3C). These data indicate that Bcl-2 may not participate directly in Nexrutine-induced apoptosis in these cells. This also suggests a possibility that Nexrutine may be useful in inhibiting the growth of tumor cells overexpressing Bcl-2.
Figure 3.
Bcl-2 may not be a direct target of Nexrutine-induced biologic effects. (A). Expression of Bcl-2 and Bax was examined using quantitative RT-PCR and Western blotting. Total RNA and whole-cell protein extracts were prepared from LNCaP cells treated with Nexrutine (5 µg/ml for different time intervals). RNA was used in quantitative RT-PCR with Bcl-2/GAPDH or Bax/GAPDH primers. A representative gel is shown. The bottom band in each lane is GAPDH. (B). Levels of Bcl-2, Bax, and GAPDH were quantified with KODAK MI software using KODAK Image Station 4000R digital imaging system (Eastman Kodak Company, Kodak Imaging Systems, New Haven, CT). The ratio of Bcl-2/Bax expression was determined after normalization with GAPDH internal control and shown as a graph. (C) Equal amounts of the extracts were fractionated on a 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. The blotted membrane was blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (blocking solution), and incubated with indicated antibodies followed by incubation with HRP-conjugated anti-rabbit IgG antibody (Sigma) in blocking solution. Bound antibody was detected by enhanced chemiluminescence using Supersignal West Pico Chemiluminescent Substrate following the manufacturer's directions (Pierce, Rockford, IL). The blot shown is a representative blot of three different experiments. All the blots were stripped and reprobed with β-actin to ensure equal protein loading. (D) PC3-Neo (Neo) and PC-3 Bcl-2 (Bcl-2) cells were used in proliferation assay. Cells were plated in 96-well plates as described in Figure 1A and treated with indicated concentrations of either Nexrutine or solvent control. Cell proliferation was measured by Cell Titer96 Aqueous One solution assay at 72-hour time point by determining the absorbance at 570 nm using SpectraMaxPlus plate reader (Molecular Devices). Statistical significance of the data was determined by Student's t test. (E) PC-3 Neo and PC-3 Bcl-2 cells treated with Nexrutine (5 µg/ml for 24 hours) were trypsinized and used in DAPI staining as described in Materials and Methods. Bright fluorescence indicates viable cells.
To study the role of Bcl-2 in Nexrutine-induced apoptosis we used PC-3 cell line that is engineered to overexpress Bcl-2 protein (PC3Bcl-2) and a control cell line that expresses Bcl-2 at low levels (PC-2Neo [18]). Such high levels of Bcl-2 expression may be similar to those in human tumors. After examining the levels of Bcl-2 in these cell lines, we evaluated the functionality of the Bcl-2 transgene by testing the sensitivity of these clones to etoposide treatment. Consistent with the published data, etoposide inhibited the growth of PC-3Neo cells, but high levels of Bcl-2 in PC-3 Bcl-2 clones provided partial protection from etoposide-induced growth inhibition (data not shown). Then we tested the effect of Nexrutine on the proliferation and apoptosis of PC-3 Bcl-2 and PC-3 Neo cells. As shown in Figure 3D, Nexrutine inhibited the proliferation of PC-3-Bcl-2 cell lines significantly (P < .034 and < .001 with 5 and 10 µg/ml Nexrutine, respectively, between control versus treated). We used DAPI staining to determine its effect on apoptosis in these cells. As shown in Figure 3E, untreated PC-3Neo or PC-3 Bcl-2 cells showed bright homogeneous fluorescence staining indicating viable cells. However, PC-3 Neo cells treated with Nexrutine showed morphologic features indicative of apoptosis. PC-3 Bcl-2 cells underwent apoptosis to a lesser extent in response to Nexrutine treatment. When PC-3 Neo and PC-3 Bcl-2 cells treated with etoposide (positive control) were used in DAPI staining, we found apoptotic features only in PC-3 Neo cells and bright homogenous fluorescence in PC-3 Bcl-2 cells, indicating that overexpression of Bcl-2 was protecting from etoposide-induced apoptosis (data not shown). These data indicate Nexrutine-induced apoptosis is not protected by overexpression of Bcl-2, suggesting it may not be a direct target.
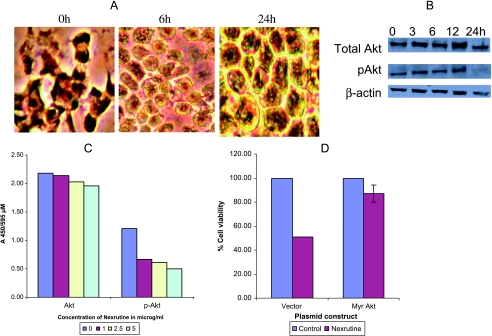
Because Nexrutine induced apoptosis in the presence of Bcl-2 we hypothesized that the mechanistic basis of the observed phenomenon could be through modulation of cell survival signaling pathways. Akt is an antiapoptotic serine-threonine kinase that regulates a number of critical cellular pathways including those leading to cellular proliferation and inhibition of apoptosis [26–30]. Akt is activated in response to diverse stimuli by recruitment to the cell membrane through the actions of phosphotidylinositol 3 kinase (PI3K), which in turn is regulated by the phosphatase and tensin homologue deleted from chromosome ten (PTEN). Mutations of PTEN are most commonly encountered in human cancers including prostate cancer [31]. After activation by PI3K, Akt translocates to the nucleus where it is thought to regulate various biologic processes including cell survival through downstream targets such as GSK, mTOR, caspase 9, and Bad and transcription factors such as forkhead transcription factor (FKHR), nuclear factor κB (NFκB), and CREB; [32,33]). To investigate if Nexrutine acts through such a mechanism, we measured the expression of Akt and pAkt immunocytochemically. To validate staining, we used paraffin-embedded LNCaP cell pellets that are either untreated or treated with LY294002, a PI3K inhibitor. Control LNCaP cells showed positive staining for pAkt that was reduced after treatment with LY294002 (data not shown) and in response to Nexrutine treatment (Figure 4A). We also performed Western blot analysis with extracts prepared from PC-3 cells treated with Nexrutine (5 µg/ml) for different periods. As shown in Figure 4B, there was no significant change in the levels of total Akt. However, levels of pAkt were undetectable from 24-hour treated extract. Similar results were obtained in LNCaP cells (data not shown). We also monitored the phosphorylation of Akt using FACE kit that specifically measures activated Akt and/or total Akt quantitatively (Active Motif). As shown in Figure 4C, the levels of phosphorylated Akt decreased, whereas the levels of total Akt did not change significantly, which is consistent with the Western blot data presented above. To show the direct involvement of Akt inactivation in Nexrutine-induced growth inhibition and apoptosis, we measured cell viability and apoptosis in LNCaP cells ectopically expressing constitutively active myristoylated Akt (transfected either with pCMVMyrAkt, which bypasses the requirement for pleckstrin homology domain-mediated membrane recruitment, or with control vector, pCMV) as described earlier [16]. As shown in Figure 4D, Nexrutine treatment inhibited the growth of LNCaP cells transfected with control vector and is consistent with the above cell proliferation data. In contrast, overexpression of constitutively active Akt protected LNCaP cells from growth-inhibitory activity of Nexrutine. Microscopic observations of vector-transfected cells show characteristic features of apoptosis unlike MyrAkt-transfected cells after treatment with Nexrutine (data not shown). These results demonstrate that inhibition of Akt activation is an important signaling event induced by Nexrutine in these cells. However, it is not known whether Akt inactivation is the primary cause or the observed effects are a consequence of Akt inactivation.
Figure 4.
Nexrutine treatment reduces the expression of pAkt. (A) Cells treated with Nexrutine (5 µg/ml for 24 hours) were fixed on slides using paraformaldehyde. Fixed slides were blocked in horse serum as described in Materials and Methods and incubated with the selected primary antibody (pAkt), diluted in blocking buffer (based on the manufacturer's recommendations) overnight at 4°C with gentle rocking. After three washes with TBS/Triton, the slides were incubated with a Universal Secondary antibody for 1 hour at room temperature. After washes with TBS and TBS/Triton, the slides were incubated with 0.6% hydrogen peroxide for 30 minutes. The slides were washed once more with TBS and TBS/Triton and stable DAB was added to each spot on the slide for 15 to 20 minutes. Slides were then rinsed with water and mounted with a coverslip. (B) Logarithmically growing PC-3 cells were treated with 5 µg/ml Nexrutine or with solvent control for different periods (0, 3, 6, 12, and 24 hours) and whole-cell extracts were prepared. Western blotting was performed as described above for Figure 3C. The blot shown is a representative blot that was repeated three different times. (C) To measure levels of total andphosphorylated Akt using FACE-Akt kit, exponentially growing cells in 96-well plates were treated with different concentrations of Nexrutine. Cells were rapidly fixed and incubated with a primary antibody that recognizes either phosphorylated or total Akt. This was followed by incubation with secondary HRP-conjugated antibody followed by colorimetric development. Relative number of cells in each well was determined using crystal violet reagent for normalizing the data. The data shown here is a representative of three experiments and the values represent averages obtained from triplicate wells. (D) Subconfluent LNCaP cells were transfected with either pCMVMyrAkt (an activated form of Akt with the Src myristoylation signal fused in-frame to the c-Akt coding sequence) or control vector (pCMV) using Lipofectamin (Invitrogen) in triplicate dishes. Forty-eight hours after transfection, cells were treated with either 5 µg/ml Nexrutine or solvent control. Both floating and adherent cells were collected after 24 hours of treatment and assessed for cell viability by trypan blue exclusion assay. Data are averages ± S.D. of three independent experiments.
Our results are consistent with published data showing induction of apoptosis in PCA cells by blocking Akt activation independent of Bcl-2 by celecoxib, a Cox-2 inhibitor [34]. Because one of the downstream targets of Akt is CREB, we determined the levels of total CREB and phosphorylated CREB (pCREB) in nuclear extracts from LNCaP cells after treatment with Nexrutine (0, 1, 2.5 and 5 µg/ml) for 24 hours using Western blot analysis. As shown in Figure 5A, after treatment with Nexrutine the level of pCREB was reduced with no significant changes in the levels of total CREB. Nuclear extracts were prepared from PC-3 cells treated with 5 µg/ml of Nexrutine for 0, 3,6,12, and 24 hours. As shown in Figure 5B, CREB antibody detected a doublet band representing phosphorylated (indicated by an arrow) and unphosphorylated forms. However, the intensity of phosphorylated form was reduced in extracts prepared from cells treated with Nexrutine. We confirmed these observations using phosphospecific CREB antibody (Figure 5B). The identity of the bands in Figure 5B are pCREB (46 kDa); phosphorylated CREM (30 kDa), and ATF-1 (38 kDa). Forskolin-treated WI-38 cell extract was used as positive control. These data suggest that Nexrutine treatment reduces the levels of the phosphorylated form of CREB in LNCaP and PC-3 cells. Because CREB has been shown to regulate the expression of genes involved in various cellular processes including Bcl-2, we measured CREB DNA-binding activity in nuclear extracts prepared from LNCaP and PC-3 cells. As shown in Figure 5C, nuclear extracts prepared from untreated LNCaP cells showed CREB DNA-binding activity that was reduced with Nexrutine treatment. Use of 100-fold molar excess of cold competitor (wildtype CREB consensus oligo sequence) inhibited the CREB DNA binding but not the mutant CREB consensus oligo sequence, indicating that the observed DNA binding is sequence specific. Experiments using nuclear extracts prepared from PC-3 cells treated with Nexrutine for different time points are shown in Figure 5D. CREB DNA-binding activity showed a statistically significant reduction in a time-dependent manner (P < .01 at 12-hour time point). We also investigated if Nexrutine-mediated biologic effects involve modulation of Akt kinase activity. As shown in Figure 5E, extracts prepared from untreated PC-3 cells showed Akt kinase activity. However, treatment with Nexrutine (5 µg/ml) led to undetectable levels of Akt kinase activity within 3 hours. These data indicate constitutive levels of Akt kinase activity protect PC-3 cells from undergoing apoptosis and that treatment with Nexrutine induces apoptosis possibly by inhibiting Akt kinase activity. It is also noteworthy to mention that CREB has been shown to be a substrate for various kinases including Akt [33]. Our findings showing reduction in the levels of pCREB and reduced CREB DNA-binding activity in Nexrutine-treated extracts implicate a potential role for Akt/CREB signaling in mediating Nexrutine-induced biologic response.
Figure 5.
Nexrutine treatment reduces the levels of pCREB and CREB DNA-binding activity in LNCaP and PC-3 cells. Logarithmically growing LNCaP cells were treated with Nexrutine (0, 1, 2.5, and 5 µg/ml for 24 hours) or with solvent control and nuclear extracts were prepared. In the case of PC-3 cells, cells were treated with 5 µg/ml of Nexrutine for different periods (0, 3, 6, 12, and 24 hours). Western blotting was performed as described for Figure 4B. (A) Western blotting of LNCaP extracts. (B) Western blotting of extracts prepared from PC-3 cells. Nuclear extracts prepared from human fibroblast WI-38 cells stimulated with forskolin (CREB activator) was used as positive control (Std). (C) CREB DNA-binding activity was measured in LNCaP nuclear extracts by using TransAM CREB (Active Motif). Briefly, the control and Nexrutine-treated nuclear extracts were incubated with CREB consensus oligonucleotide that was immobilized in 96-well plates. A primary antibody specific for an epitope on the bound and active form of the CREB was then added followed by subsequent incubation with secondary antibody and developing solution. After this incubation with the developing solution, CREB activity was measured colorimetrically at 450 nm. Nuclear extracts prepared from human fibroblast WI-38 cells stimulated with forskolin (CREB activator) were used as positive control. For competition experiments, the wells containing immobilized oligo were preincubated with 100-fold molar excess of wild-type and mutant oligonucleotide for 30 min before addition of the nuclear extract. (D) CREB DNA-binding activity in PC-3 cells using TransAM CREB (Active Motif) as described above for (C). (E) Akt kinase activity in extracts prepared from PC-3 cells treated with Nexrutine (5 µg/ml) for different time intervals. Endogenous Akt was immunoprecipitated with Akt antibody. Kinase reaction was performed in the presence of cold ATP and GSK-3 substrate as per manufacturer's recommendations (Cell Signaling Technology). Phosphorylation of GSK-3 was measured by Western blotting using an anti-phospho GSK-3 antibody. The blot shown is a representative blot of two independent experiments.
Discussion
Although evidence from epidemiological and animal studies suggests that increased consumption of plant-based diet can reduce the risk of PCA, presently, little information is available concerning the use of botanicals in preventing PCA. As a first step toward developing botanicals as PCA preventives, we examined the effect of Nexrutine on human PCA cell lines. Overall, our results suggest that Nexrutine inhibits tumor cell proliferation by a combination of apoptosis induction and inhibition of cell survival signaling. The results of the present study demonstrate that Nexrutine treatment 1) inhibits the proliferation of human PCA cells through induction of apoptosis independent of Bcl-2; 2) results in reduction in the levels of pAkt, and overexpression of Akt protects cells from Nexrutine-induced inhibition of cell proliferation implicating a role for Akt signaling; and 3) reduces the levels of pCREB and CREB DNA-binding activity. Because overexpression of Bcl-2 is associated with advance hormone-refractory prostate tumors, the observed induction of apoptosis in response to Nexrutine in the presence of Bcl-2 is novel. This also indicates a potential for this agent not only in the treatment of metastatic PCA, but also in the therapy of neoplasms other than prostate that overexpress Bcl-2.
The significant outcome of this study is the finding that enforced activation of Akt largely blocked Nexrutine-induced inhibition of cell proliferation and the induction of apoptosis. These implicate a direct role for Akt signaling in PCA growth inhibition in response to Nexrutine. This observation also raises the possibility that this agent might prove useful in neoplasms characterized by PTEN mutations in which inappropriate Akt activation plays a role in enhanced survival [29]. We also observed a correlation between Nexrutine-induced apoptosis and levels of pCREB and CREB DNA-binding activity. Because Akt has been shown to phosphorylate CREB, it is possible that Akt-mediated activation of CREB plays an important role in prostate carcinogenesis [33].
The transcription factor CREB regulates genes involved in various cellular processes by binding to cAMP response element (CRE) sequences present in their promoter regions [35,36]. A number of growth factors, hormones, and a variety of protein kinases including protein kinase A (PKA), mitogen-activated PKs (MAPKs), Ca2+/calmodulin-dependent PKs (CaMKs), and Akt have been shown to activate CREB [35,36]. Transgenic mice overexpressing dominant-negative CREB induces apoptosis in Tcells after growth factor stimulation, implicating CREB as an important factor for cell survival [37]. More importantly, it has been shown that interactions between CREB and androgen receptor (AR) signaling pathways may be one of the contributing factors involved in the development of androgen-independent PCA [38]. The widely used serum marker for PCA, namely, prostate-specific antigen gene, contains a putative binding site for CREB located at -3196/-3189 [38]. Recently, it has been demonstrated that interactions between AR and CREB through coactivator proteins including CBP/p300 regulate prostate-specific antigen gene expression. Thus, it is reasonable to speculate that Nexrutine may exert its growth inhibitory effects by disruption of AR/CREB interactions directly or through reduction in the levels of coactivator protein CBP/p300 or other unidentified proteins. Nonetheless, disruption of Akt signaling by Nexrutine may also reduce the levels of pCREB because Akt has been shown to phosphorylate CREB. However, it is not known if Nexrutine acts upstream of Akt at the level of PI3K signaling, and such studies are currently in progress. The role of the other Akt downstream signaling pathways including NFκB, FKHR, etc. is not ruled out.
The results of this study could have implications for incorporation of herbal extracts such as Nexrutine into the prevention/therapeutic strategies against PCA and other cancers. An added advantage of Nexrutine is that it is safe to consume and biologically active in human test subjects. A contract research company (Dennis and Company Research, Stanford, CT) conducted an open-label, homeuse trial on Nexrutine. Fifty-three dietary supplement users who suffered from joint or muscle pain took one or two Nexrutine capsules once or twice daily for 2 weeks. The study included 29 men and 24 women (La Valle, 2006, personal communication). The average age of the subjects was 49 years. Seven of 10 subjects rated the product as effective, reporting that their muscles were less sore after physical activities. Nine of 10 felt Nexrutine was gentle on the stomach and reported minimal side effects. Our data show that Nexrutine induces apoptosis involving multiple mechanisms in part by blocking the activation of an anti-apoptotic kinase Akt and transcription factor CREB in a Bcl-2-independent fashion. More importantly, inhibition of these multiple signaling pathways with Nexrutine resulted in reduction in the colony-forming efficiency in a soft agar assay indicating that inhibition of this pathway plays an important role in oncogenecity in these cells. Based on our results, we speculated that Akt-mediated activation of CREB may play an important role in the PCA progression and that Nexrutine blocks this pathway. We cannot rule out the possibility that MAPKs such as ERK and p38 may also act as upstream regulators in the activation of CREB. Nevertheless, our findings indicate that this pathway plays a role in the progression of PCA and that control of this pathway may have a potential in the prevention of PCA. Furthermore, the nature of the biologically active component in this extract is not known. Additional studies, including preclinical studies, to identify the biologically active principle and the precise molecular pathways regulating apoptotic response to this agent are currently in progress in the laboratory.
Acknowledgements
Part of this work was conducted in the Center for Cancer Causation and Prevention at AMC Cancer Research Center, Denver, CO. We acknowledge receiving Nexrutine from Next Pharmaceuticals (Irvine, CA) and Akt expression plasmids from Arthur Weiss (Howard Hughes Medical Institute, University of California, San Francisco, CA). We greatly appreciate receiving the paraffin-embedded LNCaP cell pellets treated with LY294002 (PI3K inhibitor) to validate the immunohistochemical staining of pAkt from Cell Signaling Technology. We thank Bob Garrison (Next Pharmaceuticals) and Rita Ghosh and Ian Thompson (both from University of Texas Health Science Center, San Antonio, TX) for reviewing the manuscript.
Footnotes
This study was supported in part by grants R21 CA 98744 and NCI CA 46934-15S1 (A.P.K.).
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 3.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:2030S–2031S. doi: 10.1093/jn/135.8.2030S. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JH, Kristal AR, Stanford JL. Fruits and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Kolonel LN, Hankin JH, Whittemor AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 7.Nelson PS, Montgomery B. Unconventional therapy for prostate cancer: good, bad or questionable? Nat Rev Cancer. 2003;3:845–858. doi: 10.1038/nrc1210. [DOI] [PubMed] [Google Scholar]
- 8.Ho S-M. Estrogens and antiestrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Von Rompay M, Kessler RC. Trends in alternative medicine use in the United states, 1990–1997. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 10.Oh WK, Kantoff PW, Weinberg V, Jones G, Rini BI, Derynck MK, Bok R, Smith MR, Bubley GJ, Rosen RT, et al. Prospective multicenter randomized phase II trial of the herbal supplement PC-SPES and diethylstilbestrol in patients with androgen independent prostate cancer. J Clin Oncol. 2004;22:3705–3712. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 11.White J. PC-SPES—a lesson for future dietary supplement research. J Natl Cancer Inst. 2002;94:1261–1263. doi: 10.1093/jnci/94.17.1261. [DOI] [PubMed] [Google Scholar]
- 12.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU) testosterone-treated rats fed tomato powder, lycopene, or energy restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 13.Mori H, Fuchigami M, Inoue N, Nagai H, Koda A, Nishioka I, Meguro K. Principle of the bark Phellodendron amurense to suppress the cellular immune response: effect of phellodendrine on cellular and humoral immune response. Planta Med. 1995;61:45–49. doi: 10.1055/s-2006-957997. [DOI] [PubMed] [Google Scholar]
- 14.Cuellar MJ, Giner RM, Recio MC, Manez S, Rios JL. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72:221–229. doi: 10.1016/s0367-326x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 15.Kumar AP, Garcia GE, Slaga TJ. 2-Methoxyestradiol blocks cell-cycle progression at G(2)/M phase and inhibits growth of human prostate cancer cells. Mol Carcinog. 2001;31:111–124. doi: 10.1002/mc.1046. [DOI] [PubMed] [Google Scholar]
- 16.Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003;5:255–266. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia GE, Wisniewski H-G, Scott Lucia M, Arevalo N, Slaga TJ, Kraft SL, Strange R, Kumar AP. 2-Methoxyestradiol (2-ME) inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate (TRAMP): role of TNFα-stimulated gene 6 (TSG-6) Clin Cancer Res. 2006;12:980–988. doi: 10.1158/1078-0432.CCR-05-2068. [DOI] [PubMed] [Google Scholar]
- 18.Kyprianou N, King ED, Bradbury D, Rhee JG. Bcl-2 overexpression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. Int J Cancer. 1997;70:341–348. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Kumar AP, Garcia GE, Orsborn J, Levin VA, Slaga TJ. 2-Methoxyestradiol interferes with NFκB transcriptional activity in primitive neuroectodermal brain tumors: Implications for management. Carcinogenesis. 2003;24:209–216. doi: 10.1093/carcin/24.2.209. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh R, Nadiminty N, Fitzpatrick J, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280:5812–5819. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- 21.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 22.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 23.Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol Cell Biochem. 2004;256:141–155. doi: 10.1023/b:mcbi.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- 24.Zeng L, Kyprianou N. Apoptotic regulators in prostatic intra-epithelial neoplasia (PIN); value in prostate cancer detection and prevention. Prostate cancer prostatic diseases. 2005;8:7–13. doi: 10.1038/sj.pcan.4500757. [DOI] [PubMed] [Google Scholar]
- 25.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML. Expression of the protooncogene Bcl-2 in the prostate and its association with emergence of androgen independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 26.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 27.Vivanco I, Sawyers CL. The phosphotidylinositol-3-kinase Akt pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto H, Altuwaijri S, Cai Y, Messing EM, Chang C. Inhibition of the Akt, cyclooxygenease-2 and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: potential therapeutic approaches for prostate cancer. Mol Carcinog. 2005;44:1–10. doi: 10.1002/mc.20121. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Ittmann MM, Ayala G, Tsai M-J, Amato RJ, Wheeler TM, Miles BJ, Kadmon D, Thompson TC. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate cancer andprostatic diseases. 2005;8:108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Hung M-C. Signaling intricacies take center stage in cancer cells. Cancer Res. 2005;65:2511–2515. doi: 10.1158/0008-5472.CAN-05-0189. [DOI] [PubMed] [Google Scholar]
- 31.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 32.Nakamura N, Rmaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du K, Montiminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 34.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 35.Mayr B, Montiminy M. Transcriptional regulation by the phosphorylation dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 36.Montimony M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 37.Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Jia L, Stallcup MR, Coetzee GA. The role of protein kinase A pathway and cAMP responsive element binding protein in androgen receptor-mediated transcription at the prostate-specific antigen locus. J Mol Endocrinol. 2005;34:107–118. doi: 10.1677/jme.1.01701. [DOI] [PubMed] [Google Scholar]