Abstract
Carcinoma in situ (CIS) represents the preinvasive stage of human germ cell tumors, but the mechanism leading to pubertal proliferation and invasive malignancy remains unknown. Among testicular gap junctional proteins, connexin 43 (Cx43) represents the predominant Cx, and, previously, an inverse correlation between synthesis of Cx43 protein and progression of tumor development was detected. In the present study, using cDNA microarray analysis, in situ hybridization, semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) from tissue homogenates, RT-PCR from microdissected tubules with normal spermatogenesis and CIS, and seminoma cells from invasive seminoma, we asked whether reduction of Cx43 protein is accompanied by a change of Cx43 transcripts. We detected a significant downregulation of Cx43 at mRNA level in Sertoli and germ cells starting in seminiferous tubules infiltrated with CIS and resulting in a complete loss in seminoma cells. It was demonstrated that downregulation of Cx43 expression in neoplastic human testis takes place at the transcriptional level and starts in CIS. This reduction of Cx43 expression further suggests that early intratubular derangement in Cx43 gene expression and disruption of intercellular communication between Sertoli cells and/or Sertoli and preinvasive tumor cells may play a role in the progression phase of human seminoma development.
Keywords: Carcinoma in situ, seminoma, downregulation, connexin 43, gene expression
Introduction
Testicular germ cell tumor is the most common cancer among men aged 17 to 45 years [1]. It comprises a variety of histologic features with two major subtypes, seminoma and nonseminoma [2]. It is known that there are two periods of major changes in gene expression: first at the transition from primordial germ cells to pre-spermatogonia, and later during the pubertal switch from mitotic to meiotic cell division [3]. The transformation of the common precursor, the carcinoma in situ (CIS) cell, to atypical germ cells has been suggested to take place in gonocytes at 7 to 10 weeks of gestation [4–6]. It has further been suggested that testicular cancer progression may be correlated with an impaired status of Sertoli cell differentiation [7–11]. In recent years, several genes and chromosomal aberrations have been identified that are considered to be involved in the pathogenesis of testicular germ cell tumorigenesis [12–14], but the molecular mechanism leading to an increased proliferation of CIS cells at puberty followed by progression to invasive malignancy after long latency remains still unknown.
Gap junctions contain channels that connect neighboring cells and constitute the basis for direct intercellular communication [15]. These channels are composed of connexin (Cx) protein subunits. Different subtypes of Cx are expressed in an overlapping spatiotemporal and cell-type-specific pattern [16]. Thus, Cx can be used as specific cellular differentiation markers [17]. In addition, gap junctions play an important role in the maintenance of normal cell growth so that genes for the Cx proteins form a family of tumor suppressor genes [18]. The occurrence of gap junctions in the seminiferous epithelium of different species has been firmly established [19]. Among testicular Cx, Cx43 is the predominant Cx and can be expressed by Sertoli cells, spermatogonia, spermatocytes, peritubular cells, and Leydig cells [19–22]. Testicular gap junctions containing Cx43 seem to be necessary for the regulation of differentiation of Sertoli cells, the controlled interaction and communication of Sertoli and germ cells, and the coordinated start of differentiation and proliferation of normal germ cells at puberty [19–22]. Cx43 is also present in the endothelial cells of large- and small-caliber blood vessels, where the protein coexists with Cx40 and Cx37 [23,24]. Cx37 is known to be expressed in the vascular endothelium including testicular blood vessels and seems to play a role in vascular development [16,25,26].
Cx43 as a phosphoprotein demonstrates multiple electrophoretic isoforms when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), including a faster migrating form that includes nonphosphorylated (P0 or NP) Cx43 and at least two slower migrating forms, commonly termed P1 and P2. Both P1 and P2 comigrate with P0 following alkaline phosphatase treatment, suggesting that phosphorylation is the primary covalent modification detected in SDS-PAGE analysis [27,28]. This phosphorylation of Cx43 in its life cycle has been implicated in the regulation of a broad variety of Cx processes, such as the trafficking, assembly/disassembly, degradation, as well as the gating of gap junction channels [29,30]. In addition, Cx43 phosphorylation is known to play a role in gap junctional plaque formation and activity [27].
So far, some studies have been published investigating the presence of gap junctions and Cx43 in seminiferous tubules and pathologic human testes. Seminiferous tubules showing spermatogenic arrest at the level of spermatogonia, those with Sertoli-cell-only (SCO) syndrome and infiltrated with CIS cells display an intratubular reduction or loss of Cx43 protein [10,21,22,31]. In a recent study, an aberrant cytoplasmic localization of Cx43 protein was detected in tumor cells of four patients with seminoma and in neoplastic cells originating from the JKT-1 seminoma cell line [32]. Thus, alterations of Cx43 protein synthesis can be correlated to various testicular disorders and male infertility including cancer [10,21,31–35].
To date, there are no reports associating abnormalities in intratubular Cx43 gene expression with spermatogenic impairment in the context of the development of testicular human seminoma from CIS. The present work at mRNA level extends an earlier study on the expression of the tumor suppressor Cx43 in CIS of adult patients [10] that revealed an inverse correlation of Cx43 protein synthesis between Sertoli cells and abnormal preinvasive germ cells. As alterations of Cx protein synthesis in neoplasms are not always paralleled by changes in mRNA expression and because some tumors can express steady-state transcripts without observable immunoreactivity, indicating that they are able to downregulate Cx immunoreactivity independently of mRNA abundance [36,37], we aimed to elucidate whether a reduction of Cx43 protein is accompanied by a downregulation of Cx43 transcripts using cDNA microarray analysis, in situ hybridization (ISH), semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) from tissue homogenates, and RT-PCR from microdissected tubules with normal spermatogenesis and CIS, and from seminoma cells from invasive seminoma.
Material and Methods
Testicular Tissue
After obtaining written informed consent, investigations were performed on biopsies from 10 patients (age 28–52 years; mean 32.4 years) with obstructive azoospermia and histologic normal spermatogenesis, 4 patients (age 58–67; mean 62 years) orchiectomized for prostate carcinoma revealing histologic normal spermatogenesis, 20 patients (age 20–45 years; mean 33.8 years) with CIS of the testis and impaired spermatogenesis, and 40 patients (age 25–57 years; mean 39 years) with different stages of seminoma (22 x pT1, 14 x pT2 and 4 x pT3). Patients were not treated with any drugs before surgery.
Fixation, Embedding, and Histologic Evaluation
Testicular biopsy specimens were cut into two equal pieces. One part was fixed by immersion in Bouin's fixative and embedded in paraffin wax using standard techniques. Sections (5µm) were stained with hematoxylin and eosin and scored, according to Bergmann and Kliesch [38]. The other part was snap-frozen in liquid nitrogen. Diagnosis of CIS and/or seminoma was established by histologic and morphologic examination of the biopsy specimen and by placental alkaline phosphatase (PIAP) immunostaining.
cDNA Microarray Analyses
We compared the gene expression profiles of cells of the 40 seminoma from different patients described above in different stages (pT1, pT2, pT3) with three normal testes using tissue homogenates and cDNA microarrays focusing on the predominant testicular Cx, Cx43 (probe set ID 32531 _at, accession no. NM_000165; corresponding Cx primer sequences can be viewed using probe set IDs under http://www.affymetrix.com/analysis/netaffx/). Additionally, the expression pattern of other Cx was investigated: Cx31 (probe set ID 41076_at, accession no. AF099730), Cx31.1 (38903_at, accession no. AF099731), Cx32 (39598_at, accession no. X04325), Cx37 (40687_at, accession no. M96789), Cx47 (39504_at, accession no. AF014643, and 39505_at, accession no. AW007036), and Cx50 (31778_at, accession no. U34802). Testicular tissues used for microarray analyses were lysed in TRIzol (Life Technologies, Karlsruhe, Germany) and total cellular RNA was prepared using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. RNA quality was controlled spectrophotometrically and by gel electrophoresis. Microarray analyses were carried out applying HG-U95Av2 microarrays (Affymetrix, Santa Clara, CA) from 5 µg of total RNA. Fragmentation of cRNA, hybridization, washing, staining, and scanning was performed following the manufacturer's protocols. Expression values were obtained using Affymetrix Microarray Suite Version 5.0. This includes the background correction of the average of the lowest two percentiles of intensities on a 4 x 4 grid on the chip, the introduction of an “ideal mismatch” forced to be lower than the corresponding perfect match, and the use of Tukey's biweight to elicit an expression value out of single probe intensity pairs. Annotation of the probe sets was taken from the annotation files provided on the Affymetrix home page (version updated in October 2003). Exploratory data analysis, Wilcoxon paired nonparametric tests for difference, and the Mann-Whitney test for the nonparametric independent two-group comparisons were performed with the program SPSS 10 for Windows (SPSS Inc, Chicago, IL) and Microsoft Excel 2000 (Microsoft Corp, Redmond, WA). Differences with P < .05 were regarded as statistically significant.
To confirm the Cx43 (accession no. NM_000165) expression data, we performed RT-PCR with tissue homogenate from 20 patients (5 normal testes and 15 seminoma tissues) using the following primer pairs: 5′-AGG AGT TCA ATC ACT TGG CGT GAC-3′ as forward primer and 5′-GGC TCA GCG CAC CAC TGG TCG CA-3′ as reverse primer. Because most of the frequently used housekeeping genes were differentially expressed in seminoma [39,40], the ubiquitin B (UBB) housekeeping gene, which was found to be constantly expressed in seminoma, was chosen for this approach and coamplified as internal standard control. For semiquantitative examination, UBB primer pairs (5′-GGC TTT GTT GGG TGA GCT TG-3′ as forward primer and 5′-CTG GGC TCC ACC TCC AGA GT-3′ as reverse primer) were added in the same tube to the Cx43 primers. PCR conditions were as follows: 1 x 94°C for 2 minutes, 30 x (94°C for 1 minute, 63°C for 1 minute, 72°C for 1 minute), 72°C for 4 minutes. The generated PCR products were size-fractionated on a 2% agarose gel and detected by ethidium bromide staining. The degree of Cx43 expression was evaluated densitometrically applying the Scion Image software (Scion Corporation, Frederick, MD) and correlated to the expression of UBB. Experiments were repeated at least three times.
UV Laser-Assisted Microdissection, Laser Pressure Catapulting, RNA Extraction, and RT-PCR
Microdissection followed by RT-PCR was performed on biopsies from four patients with histologically normal spermatogenesis, on four patients showing CIS, and on three biopsies from patients with seminoma (1 x pT1, 1 x pT2, 1 x pT3) using the PALM MicroBeam system (PALM, Bernried, Germany). The system consisted of a nitrogen laser of high beam precision (wavelength 337 nm), which was coupled to an inverted microscope (Axiovert 135; Zeiss, Jena, Germany) using the epifluorescence illumination path. The microscope stage was digitally controlled and moved by a computer mouse. The PALM Membrane Slides were irradiated with an ultraviolet lamp for 30 minutes to achieve better adhesion of the sections. For histologic evaluation, the sections were stained with hematoxylin and dehydrated. Tubules of interest were identified by light microscopy and PIAP immunostaining (CIS tubules) and separated from the adjacent interstitial tissue by laser microdissection. With a laser shoot the chosen seminiferous tubules or cells were catapulted into the cap of a test tube (laser pressure catapulting) filled with mineral oil. The same number of seminiferous tubules (n = 20) or the same number of seminoma cell clusters of approximately the same size but from different locations (n = 20) were collected per tube. Isolated cell profiles were then dissolved in 350 µl lysis buffer containing β-mercaptoethanol and 5 µl carrier RNA (RNeasy Micro Kit, Qiagen). RNA from microdissected human testicular tissue was extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer's protocol including the use of carrier RNA and DNase-I digestion. Equal amounts of RNA were used for first-strand cDNA synthesis using Sensiscript II reverse transcriptase, as recommended by the manufacturer (Qiagen). PCR analysis from microdissected seminiferous tubules or seminoma cells was carried out with the following primer pairs (accession no, NM_000165): 5′-CCA TCT CTA ACT CCC ATG CAC AGC-3′ as forward primer and 5′-TGG CAC GAC TGC TGG CTC TGC TT-3′ as reverse primer, resulting in a 137-bp amplicon. PCR conditions were as follows: 1 x 95°C for 3 minutes, 15 x (95°C for 1 minute, 66°C for 1 minute, 72°C for 2 minutes), 45 x (95°C for 1 minute, 62°C for 1 minute, 72°C for 2 minutes), 72°C for 10 minutes. Ten microliters of cDNA was added to 5 ′l 10x PCR buffer (including 15 mM MgCl2; Qiagen), 10 µl5x Q solution, 1 µl dNTPs (Promega, Mannheim, Germany), 0.5 µl Taq polymerase (5 U/µl; Qiagen), 1 µl of each primer (10 µM), and DEPC-H2O to a final volume of 50 µl. For internal (methodological) control due to low amounts of mRNA and for evaluation of RNA quality, we performed RT-PCR for the housekeeping gene β-actin (ACTB) from the same RNA samples that were used for Cx43 amplification. The primer sequences for ACTB (accession no. BC013835) were 5′-TTC CTT CCT GGG CAT GGA GT-3′ (forward) and 5′-TAC AGG TCT TTG CGG ATG TC-3′ (reverse) resulting in a 90-bp amplicon. PCR conditions were 1 x 95°C for 2 minutes, 50 x (94°C for 1 minute, 55°C for 1 minute, 72°C for 1 minute), and 1 x 72°C for 10 minutes. PCR products were separated on a 2% agarose gel, visualized by SYBR Green (Sigma-Aldrich, Munich, Germany), and sequenced by Qiagen. Microdissection using serial sections followed by RT-PCR was repeated at least three times per patient.
Production of Digoxigenin-Labeled cRNA Probes for ISH
Digoxigenin (DIG)-labeled cRNA probes were generated as described previously [41]. Briefly, the 137-bp PCR product of the human Cx43 gene was subcloned in pGEM-T (Promega). Plasmids were transformed in the XL1-Blue E. coli strain (Stratagene, Heidelberg, Germany) and extracted by column purification according to the manufacturers instruction (Qiagen). After sequencing, vectors containing the Cx43 insert were digested with NcoI and NotI (New England Biolabs, Frankfurt, Germany) for the production of sense cRNA (NcoI) and antisense cRNA (NotI), respectively. Subsequently, in vitro transcription was performed using 10x RNA-DIG Labeling Mix (Boehringer Mannheim, Mannheim, Germany) and RNA polymerases T7 and SP6 (Promega).
In Situ Hybridization
In situ hybridization was performed on biopsies of 10 patients with normal spermatogenesis, on biopsies of 10 patients with CIS, and on 20 biopsies of patients with seminoma (10 x pT1, 6 x pT2, 4 x pT3) with minor changes as described previously [41,42]. Briefly, deparaffinized tissue sections were incubated in active DEPC water for 2 x 12 minutes at 40°C, postfixed in 4% paraformaldehyde for 10 minutes, exposed to 20% acetic acid, and prehybridized in 20% glycerol for 30 minutes. Sections were then incubated with the DIG-labeled sense or antisense cRNA probes. Both cRNAs were used at a dilution of 1:25 in hybridization buffer containing 50% deionized formamide, 10% dextran sulfate, 2x standard saline citrate (SSC), 1x Denhardt's solution, 10 µg/ml salmon sperm DNA (Sigma, Deisenhofen, Germany), and 10 µg/ml yeast t-RNA (Sigma). Hybridization was performed overnight at 40°C in a humidified chamber containing 50% formamide in 2x SSC after posthybridization washes. Subsequently, sections were incubated with the anti-DIG Fab antibody conjugated to alkaline phosphatase (Boehringer) overnight at 4°C. Staining was visualized by developing sections with nitroblue-tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (KPL, Gaithersburg, MD) in a humidified chamber protected from light. Finally, sections were rehydrated for 5 minutes in deionized water and then dehydrated through successive baths of ethanol and xylol, and mounted in Eukitt resin (Merck, Darmstadt, Germany). For each test, negative controls were performed using DIG-labeled cRNA sense probes. Mouse testes were used as positive control. ISH was repeated at least twice.
Protein Extraction and Western Blot Analysis
For Western blot analysis, testicular biopsies from eight men were snap frozen in liquid nitrogen and stored at -80°C until further processing. Histologic evaluation of cryosections of these biopsies before Western blot experiments revealed normal spermatogenesis in four cases and seminoma in four cases (2 x pT2, 2 x pT3). Protein extraction was carried out using TRIzol reagent as recommended by the manufacturer (Life Technologies). Cx43 represents a phosphoprotein and was therefore incubated (10 U/µl, 60 minutes, 37°C) with calf intestinal alkaline phosphatase (CIP, New England Biolabs) to elucidate whether variations in electrophoretic mobility may reflect different phosphorylation states of this protein. Briefly, same amounts of protein per lane were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% resolving gels under reducing conditions using the Mini-PROTEAN system (BioRad, Munich, Germany). Proteins were then blotted onto a Westran polyvinylidene fluoride transfer membrane (Schleicher & Schuell, Dassel, Germany) and blocked with 5% nonfat dry milk dissolved in 0.1 M phosphate-buffered saline (PBS; pH 7.4) containing 0.1% Tween for 1 hour under gentle agitation before treatment with the polyclonal anti-Cx43 antibody overnight (1:1000; Zytomed, Berlin, Germany). A biotinylated goat anti-rabbit antibody (1:1000; DAKO, Hamburg, Germany) was used as secondary antibody. The primary and secondary antibodies were dissolved 1:1000 in PBS buffer. Finally, the membrane was treated with Vectastain Elite ABC Kit (Vector, Burlingame, CA) and developed with True Blue Peroxidase Substrate (KPL). Control Western blots were carried out by omitting the primary antibody. Western blot experiments were repeated three times.
Immunohistochemistry (Single and Double Immunostaining)
Single immunohistochemical staining for Cx43 was performed on consecutive sections of the same patients used for ISH, as described previously [10]. Briefly, sections were microwave treated for 30 minutes at 1000 W in sodium citrate buffer (pH 6.0), blocked with 3% BSA for 30 minutes and incubated with the polyclonal anti-Cx43 primary antibody (1:500; Zytomed) overnight at 4°C. Sections were then exposed to the secondary antibody (1:50, mouse anti-rabbit IgG, DAKO) followed by the third antibody (1:50, rabbit anti-mouse IgG, DAKO) and, finally, mouse alkaline phosphatase anti-alkaline phosphatase (APAAP) antibody complex (1:100, DAKO) for 30 minutes each. The immunoreaction was visualized using HistoMark Red (KPL) resulting in a red color on positive cells.
Double immunostaining for Cx43 and the inhibin-a subunit for the simultaneous detection of Leydig cells in seminoma patients was performed as follows: Cx43 and inhibin-α subunit epitopes were exposed by microwaving three times for 5 minutes at 1000 W in sodium citrate buffer (pH 6.0) and then were allowed to stand for an additional 20 minutes. After blocking with 3% BSA for 30 minutes, rabbit polyclonal anti-Cx43 primary antibody (Zytomed) was used after diluting 1:500, and tissue sections were incubated overnight at 4°C, followed by the secondary antibody (1:50; mouse anti-rabbit IgG; DAKO), by the third antibody (1:50; rabbit anti-mouse IgG; DAKO), and, finally, by the mouse APAAP antibody complex (1:100, DAKO) for 30 minutes each. The immunoreaction was visualized using NBT/BCIP (KPL) to produce a blue/pink color on positive cells. Then, the sections were incubated with 3% H2O2, with 3% BSA for 30 minutes each, and with the monoclonal primary anti-inhibin-α antibody (1:50, Serotec, Oxford, UK) overnight at 4°C. On the next day, sections were incubated with the biotinylated secondary antibody (1:50; goat anti-mouse IgG, DAKO) and by incubation using the ABC Kit (Vector) for 30 minutes each. Immunoreactivity was visualized using diaminobenzidine (Biologo, Kronshagen, Germany) to produce a brown color on positive cells. Sections were finally slightly counterstained with instant hematoxylin (Shandon, Pittsburgh, PA). Foreach immunoreaction, control incubations were performed by substituting buffer for the primary antibody. Experiments were repeated three times.
Results
cDNA-Microarray Analyses
Microarray analysis identified several up- and downregulated genes including Cx43 in three normal testicular specimens and 40 seminoma of different stages (pT1, n = 22; pT2, n = 14; pT3, n = 4). The gene expression profiles of 4 samples of tumor stage pT1 were compared with those of 3 normal tissue samples resulting in 12 single comparisons representative for the 43 samples investigated. The expression of 1490 probe sets was determined as differentially expressed in at least 80% of the comparisons with a P value <.001 and >.999 for increased and decreased changes, respectively. Thereof, the expression of 635 probe sets was increased and of 855 decreased. For Cx43, two probe sets were present on the gene array: 2018_at and 32531 _at. The first showed very weak signal intensities and was given as absent, whereas the probes of 32531 _at were positive in 84% of the samples. All 3 normal testicular specimen expressed Cx43 and 32 of 40 seminoma were positive for this gene. The expression of Cx43 was significantly 9.1-fold downregulated in seminoma tissues (Figure 1A). Cx31 and Cx47 were absent in all samples investigated. Cx32 was expressed only in samples with unimpaired spermatogenesis and Cx50 was unregulated in 81% of 43 samples. Cx31.1 was expressed in 87% of 43 samples and 2.1-fold significantly increased in seminoma compared with normal testicular specimen. Cx37 was expressed in 27 of 40 seminoma tissues with a significant 1.9-fold increase compared with normal testicular tissues. The down- and upregulated Cx genes in seminoma versus normal testicular tissue obtained by microarray analysis are summarized in Table 1.
Figure 1.
(A) cDNA microarray analysis revealed a 9.1-fold downregulation of Cx43-expression in seminoma (tumor stages pT1–pT3). (B) Representative semiquantitative RT-PCR with UBB as housekeeping gene revealed a strong band for Cx43 at 827 bp in a man with normal spermatogenesis (lane 2) and a 2.8-fold decrease of Cx43 expression in patients with seminoma stages pT1 (lanes 3 and 4), pT2 (lanes 5 and 6), and pT3 (lanes 7 and 8). Lane 1: 100-bpDNA ladder. (C) Relative Cx43 mRNA expression compared with UBB and related to tumor stages pT1 to pT3. Here, the representative results from three independent PCR reactions of 5 normal testis and 15 seminoma specimens (pT1: n = 8, pT2: n = 4, pT3: n = 3) were analyzed. (D) Relative Cx43 mRNA expression in normal testis and seminoma compared with UBB. NT, normal testis; pT1-3: tumor stages 1 to 3. *P = .016.
Table 1.
Expression Data (mRNA) for Cx-Encoding Genes Present on the Microarray in 43 Tissue Samples regarding Different Tumor Stages.
| Gene | Probe Set ID | NCBI ID | P-call (%) | NT | pT1 | pT2 | pT3 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Downregulated connexins | ||||||||||||
| GJA1 | Cx43 | 32531_at | X52947 | 81 | 3804 | 1028 | 476 | 357 | 405 | 394 | 224 | 210 |
| GJA8 | Cx50 | 31778_at | U34802 | 5 | 591 | 200 | 87 | 70 | 101 | 92 | 95 | 39 |
| GJB1 | Cx32 | 39598_at | X04325 | 7 | 678 | 95 | 338 | 110 | 272 | 122 | 414 | 244 |
| GJA7 | Cx45 | 33041_at | U03493 | 65 | 429 | 67 | 406 | 262 | 352 | 96 | 547 | 478 |
| Upregulated connexins | ||||||||||||
| GJB5 | Cx31.1 | 38903_at | AF099731 | 91 | 157 | 37 | 301 | 116 | 335 | 96 | 379 | 160 |
| GJA4 | Cx37 | 40687_at | M96789 | 63 | 189 | 49 | 354 | 102 | 377 | 90 | 305 | 52 |
To confirm the reduced expression of Cx43, semiquantitative RT-PCR using UBB as housekeeping gene were carried out by investigating 5 samples from normal testicular specimen and 15 seminoma (Figure 1, B and C). The 2.8-fold downregulation of Cx43 expression in seminoma was significant (Figure 1D). However, the upregulation of Cx31.1 could not be confirmed by RT-PCR (data not shown).
RT-PCR following Laser Microdissection of Isolated Seminiferous Tubules and Seminoma Cells
To evaluate Cx43 mRNA expression, microdissection of whole seminiferous tubules and seminoma cells was performed. Same numbers of seminiferous tubules with normal spermatogenesis, with CIS cells and residual spermatogenesis, and tubules showing only CIS and Sertoli cells (CIS-only tubules), and seminoma cells from different stages were excised from testicular tissue. Subsequent RT-PCR analysis of these tubules and/or cell profiles revealed transcripts of Cx43 in tubules showing normal spermatogenesis and in tubules containing both CIS cells and residual spermatogenesis. Tubules resembling only CIS and Sertoli cells were found either positive (in very few samples) or mostly negative. Seminoma cells isolated from various sections exhibiting different seminomatous stages (pT1–pT3) showed no signal for Cx43 mRNA at all (Figure 2).
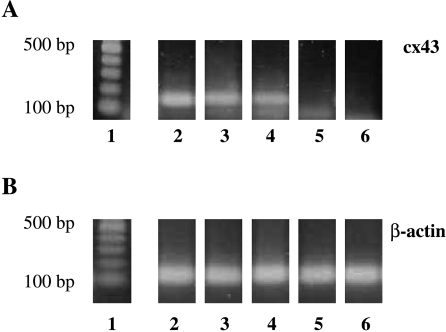
Figure 2.
Representative RT-PCR from microdissected tubules and seminoma cells using primer pairs for Cx43 (A) and β-actin (B). Seminiferous tubules with normal spermatogenesis (lane 2) and seminiferous tubules with both residual spermatogenesis and CIS (lane 3) revealed a 137-bp PCR product for Cx43. Tubules resembling only CIS and Sertoli cells (CIS-only tubules) were found positive or in most cases negative (lanes 4 and 5). Seminoma cells isolated from different seminomatous stages (pT1–pT3) showed no signal for Cx43 mRNA (lane 6). Lane 1: 100-bp DNA ladder.
In Situ Hybridization
In seminiferous tubules showing normal spermatogenesis, Cx43 mRNA was detected around the nuclei of Sertoli cells (transparent arrows), spermatogonia (transparent arrowhead) and spermatocytes (black arrow, Figure 3A). No signal was detectable in the cytoplasm of round spermatids (Figure 3A, white arrow). Seminiferous tubules from mouse testis with normal spermatogenesis used as positive control revealed similar intratubular location for Cx43 mRNA (Figure 3A, inset).
Figure 3.
(A) In normal spermatogenesis, ISH revealed a strong signal for Cx43 mRNA around the nuclei of Sertoli cells (transparent arrows), spermatogonia (transparent arrowhead), and primary spermatocytes (black arrow). Note an immunonegative spermatogonia (black arrowhead) and immunonegative round spermatids (white arrow). Seminiferous tubules from mouse testis with normal spermatogenesis used as positive control showed an identical expression pattern (inset). CIS-only tubules displayed a heterogeneous expression pattern of Cx43 mRNA. In very few tubules, a hybridization signal was mainly present in the cytoplasm of Sertoli cells (CIS cells are marked with a uniform line; Sertoli cell nuclei: transparent arrows, B). However, in most tubules the signal in Sertoli cells was either markedly reduced or completely absent (C and D). A strong signal was detectable in the cytoplasm of peritubular cells (white arrowheads, C) and in endothelial cells of small blood vessels (curved black arrow, D). In seminoma, only single cell clusters (E), single cells (curved transparent arrows, F), and/or endothelial cells (F, inset b) were found to express Cx43 mRNA, whereas seminoma cells showed no hybridization signal for Cx43. Control incubations with Cx43 cRNA sense probes were in all cases completely negative (B, inset, and F, inset a). Scale bars = 50 µm.
The expression pattern of Cx43 mRNA in CIS-only tubules (containing both Sertoli cells and CIS cells) was heterogeneous. In very few tubules, a hybridization signal was mainly present in the cytoplasm of Sertoli cells (Sertoli cell nuclei: transparent arrows; CIS cells are marked with a uniform line; Figure 3B), whereas in most CIS-only tubules the signal in Sertoli cells or tumor cells was either markedly reduced (Figure 3C) or absent (Figure 3D). Note a strong signal in the cytoplasm of peritubular cells (Figure 3C, white arrowheads) and endothelial cells of small blood vessels (Figure 3D, curved black arrow).
In patients revealing pure seminoma, only small cell clusters (Figure 3E), single cells (Figure 3F, curved transparent arrows), and/or endothelial cells (Figure 3F, inset b) were found to express Cx43 mRNA, whereas seminoma cells showed no hybridization signal for Cx43. Control incubations with Cx43 cRNA sense probes were in all cases negative (Figure 3B, inset, and F, inset a; scale bars = 50 µm).
Immunohistochemistry
In seminiferous tubules showing normal spermatogenesis, a steady Cx43 staining was detected at the level of the blood-testis barrier (BTB) between Sertoli cells, between Sertoli cells and spermatogonia, and between Sertoli cells and primary spermatocytes (Figure 4A). In addition, a strong signal was visible between interstitial Leydig cells (Figure 4A, black arrow).
Figure 4.
In seminiferous tubules showing normal spermatogenesis, a strong immunoreactivity at the level of the blood-testis barrier, apical to spermatogonia, basal to primary spermatocytes, along the Sertoli cell junctional complexes and between interstitial Leydig cells (black arrow, A) was observed. The expression pattern of Cx43 protein in CIS-only tubules was found to be heterogeneous. In very few tubules, a disorganized immunoreactive signal was present at the level of the blood-testis barrier (CIS cells: transparent arrows, B). Most of these tubules were found completely immunonegative for Cx43 protein (C). Leydig cells displayed a strong and persistent Cx43 immunoreactivity (black arrows, B and C). In seminoma, only between cells of single cell clusters within connective tissue (black arrow, D) and between endothelial cells of small blood vessels (arrowheads, E) an immunostaining for Cx43 could be detected independent of staging. Double immunostaining for Cx43 and inhibin-α clearly colocalized both proteins to the same cell type and identified these cells as Leydig cells (arrow, D). Cx43 protein (blue/pink) was imunolocalized to the plasma membranes of Leydig cells, whereas inhibin-α immunostaining (brown) was detectable in the cytoplasm of the same cell(s) (D). The tumor cells showed no immunoreaction for Cx43 at all (D and E). Scale bars = 50 µm. Representative Western blot analysis (F) from normal testicular tissue revealed three immunoreactive bands between 41 and 45 kDa (lane 2). Incubation with CIP changed the electrophoretic profiles of Cx43. Observed bands at 45 (Cx43-P2) and 43 kDa (Cx43-P1) were lost after CIP treatment, resulting in one strong band at 41 kDa (Cx43-P0, lane 3) indicating that these two former bands represent double- and single phosphorylated Cx43 isoforms. In seminoma, only a weak immunoreactive band at 45 kDa (Cx43-P2) could be demonstrated (lane 4). In addition, this band was lost after CIP treatment, resulting in the Cx43-P0 isoform (lane 5). In negative controls, no immunoreactive bands have been observed (lane 6). Lane 1: protein marker.
The expression pattern of Cx43 protein in CIS-only tubules was found to be heterogeneous, corresponding to the location of Cx43 mRNA. In very few tubules, a disorganized immunoreactive signal was present at the level of the BTB (CIS cells: tranparent arrows, Figure 4B). However, in the majority of these tubules no immunostaining for Cx43 was detectable (Figure 4C), whereas immunoreactive signals between adjacent interstitial Leydig cells persisted (Figure 4, B and C, black arrows).
In seminomatous tissue, only between cells belonging to single cell clusters within connective tissue (Figure 4D, black arrow), and between endothelial cells of small blood vessels (Figure 4E, arrowheads) immunostaining for Cx43 could be detected independent of staging. Double immunostaining for Cx43 (blue/pink) and inhibin-α (brown) clearly colocalized both proteins in the same cell type and identified these cells as Leydig cells (Figure 4D). Seminoma cells showed no immunoreaction for inhibin-α (Figure 4D) or for Cx43 (Figure 4, D and E; scale bars = 50 µm).
Western Blot Analysis
Normal testicular tissue revealed three immunoreactive bands between 41 and 45 kDa. Incubation with CIP changed the electrophoretic profiles of Cx43. Observed bands at 45 kDa (Cx43-P2) and 43 kDa (Cx43-P1) were lost after CIP treatment resulting in one strong band at 41 kDa (Cx43-P0). These results indicate that these two bands represent double- and single-phosphorylated Cx43 isoforms.
In seminoma, only a weak immunoreactive band at 45 kDa (Cx43-P2) could be demonstrated. In addition, this weak band was lost after CIP treatment resulting in the Cx43-P0 isoform (Figure 4F, arrow).
Discussion
During normal spermatogenesis, there is a close correlation between Cx43 protein synthesis and Cx43 mRNA expression, as regulation of Cx43 gene expression is tightly controlled at the transcriptional level as can be seen by its stage-dependent expression pattern [43]. Cx43 mRNA was detectable mainly in the cytoplasm of Sertoli cells, in primary spermatocytes and spermatogonia. This corresponds to the location of Cx43 protein between Sertoli cells and between Sertoli cells and germ cells indicating that these normal germ cell populations may be capable of synthesizing Cx43 protein and assembling gap junctions with Sertoli cells. These data are in accordance with previous studies showing a similar Cx43 mRNA expression pattern in mouse and human testis [31,43] and were confirmed by RT-PCR and RT-PCR from microdissected seminiferous tubules applied in this study.
For the first time, the Cx43 transcript expression pattern was demonstrated at different stages of human testicular germ cell neoplasia. The expression of Cx43 mRNA in CIS-only tubules containing both CIS and Sertoli cells was found to be variable. The hybridization signal was, in most tubules, either markedly reduced or totally absent. In very few tubules, however, a signal was mainly present in the cytoplasm of Sertoli cells corresponding with our present and previous immunohistochemical data [10]. This discrepancy of signal intensity in Sertoli cells between CIS-only tubules may be an indication of 1) the known existing partial phenotypic difference of CIS [3], 2) Sertoli cells exhibiting an altered state of maturation compared to Sertoli cells in other CIS-only tubules, or 3) a more progressive stage of tumor development toward seminoma. Specific bands for Cx43 using RT-PCR with testis homogenates from patients showing CIS or CIS-only (data not shown) and seminoma revealed that Cx43 mRNA was still expressed in neoplastic tissue, due to permanent expression of Cx43 in Leydig cells, endothelial cells and/or peritubular cells. In patients showing seminoma, endothelial cells of small blood vessels and isolated clusters of Leydig cells were found to be positive for both Cx43 mRNA and protein. Cx43 and inhibin-α double immunostaining clearly identified these cell clusters as residual Leydig cell clusters, in addition to endothelial cells, as the main source of nonneoplastic cells in seminoma expressing Cx43. Inhibin-α was chosen as this subunit is known to be expressed in human Leydig cells [44]. Furthermore, immunohistochemical stainings revealed no immunoreactive signals for Cx43 between seminoma cells, suggesting that these cells are unable to synthesize Cx43 protein and unable to communicate through Cx43 gap junction channels, and Western blot analyses performed with seminomatous tissue showed only a weak immunoreactive band for Cx43 at 45 kDa. This weak band was identified by CIP treatment as the Cx43-P2 isoform. Phosphorylation of Cx43 is known to play a role in its life cycle, as observations showed that this Cx becomes phosphorylated before its arrival at the plasma membrane and is associated with the accretion of connexons into gap junction plaques [45]. Although Cx43 in nonjunctional plasma membrane regions is not necessarily phosphorylated, Cx43 located in gap junctions has been reported to be primarily phosphorylated to the P2 form [45]. The role of Cx43-P2 in seminoma remains to be elucidated, but this Cx43 isoform probably originates from membranous Cx43 in residual Leydig cells, as can be seen in our present immunohistochemical stainings. However, the observed loss of Cx43-P0 and Cx43-P1 in Leydig cells in seminoma may indicate 1) an altered or disrupted renewal of these two Cx43 isoforms in seminoma, 2) a Leydig cell-specific property because it has been shown that different tissues (cells) exhibit their own characteristic fingerprint of Cx43 phosphorylation [46], or 3) an aberrant (compensational) hyperphosphorylated state of Cx43 in these cells, as it is known that neoplastic progression also influences the function of the remaining Leydig cells [47].
Interestingly, in CIS tubules a more disorganized staining pattern of Cx43 protein was visible that changed to a complete loss of Cx43 protein in most CIS-only tubules. The phenomenon of an aberrant localization of Cx proteins is well known [32,36,48,49] and may in these few CIS tubules be due to a disturbed intracellular trafficking of Cx43 protein or to the loss of “normal” Sertoli cells as regulators for organized synthesis and assembly of Cx43 to the plasma membrane.
Microdissection of seminiferous epithelium followed by RT-PCR analysis showed, in accordance with foregoing results, that tubules resembling only CIS and Sertoli cells were found in most cases negative, and that seminoma cells isolated from various sections exhibiting different seminomatous stages (pT1–pT3) showed no signal for Cx43 mRNA, indicating that loss of Cx43 protein is, in most cases, accompanied by a loss of Cx43 mRNA. Similar results were found in seminiferous tubules with SCO syndrome; a transcriptional regulation was evident, as there was no synthesis of Cx43 protein and expression of Cx43 mRNA [31]. Data from our cDNA microarray analysis showed a 9.1-fold decrease of Cx43 mRNA expression in patients with different stages of seminoma compared with patients revealing normal spermatogenesis, as confirmed by semiquantitative RT-PCR exhibiting a 2.8-fold decrease of Cx43 mRNA. The varying Cx43 transcript reduction using the two methods is probably caused by the different methodological approaches, i.e., primers/probes and the normalization to the housekeeping gene. However, both data indicate that the tumor suppressor gene Cx43 is significantly downregulated with progression to seminoma and that this downregulation seems to represent a process starting in CIS tubules. Development of seminoma is known to go along with an increase in the number of blood vessels, microvascular density, vascular endothelial growth factor, and thymidine phosphorylase expression [50,51]. Thus, upregulation of Cx37 expression in the present study might be explained by enhanced angiogenesis and formation of new blood vessels associated with tumor progression.
The etiological role of disorders in gap junctional intercellular communication (GJIC) in carcinogenesis was already formulated by Loewenstein and Kanno [52], indicating that tumor cells usually have a low capacity to communicate with each other. The lack of GJIC in cancer cells was extended to cancer cell-normal cell communication, and it has been suggested that this selective communicational isolation of tumor cells from surrounding normal cells helps to escape from signals keeping proliferation in normal tissues under negative control [53].
Consequently, one possible cause for the still unknown invasive potential of CIS cells could be their growing independence of adjacent Sertoli cells based on their loss of GJIC through gap junctions that contain Cx43. This is in line with studies in other organs showing that impaired expression of Cx43 has been implicated to correlate with neoplastic transformation and increased invasiveness [54,55]. Recently it has been shown that regulation of cell proliferation is mediated not by the channel but by an interaction of the C terminus of Cx43 with an intracellular signaling cascade [56]. Furthermore, the growth regulator protein CCN3 (NOV, nephroblastoma overexpressed) seems to mediate the growth suppression by binding to the C terminus of Cx43 in glioma as well as choriocarcinoma cell lines [57,58]. Thus, further investigation is needed to discriminate between intercellular and intracellular signaling for growth regulation properties of the Cx.
Consistent with our present and previous study [10], Wilgenbus et al. [59] failed to identify Cx43 in human testicular tumors by immunofluorescence. In contrast to our results, Roger et al. [32] found an aberrant intracytoplasmic Cx43 immunostaining in seminoma cells of four patients with seminoma and in the seminoma cell line JKT-1. These results may be due to the low number of patients investigated and by the fact that the authors did not consider the expression of Cx43 in Leydig cells or endothelial cells in seminomatous tissue.
Finally, it is known that normal germ cells exert reciprocal effects on Sertoli cells and that normal spermatogenic progression is required for maintenance of Sertoli cells in their fully differentiated state [60,61]. Interestingly, in human testicular cancer it has been proposed that cancer progression may be correlated with an impaired function and altered differentiation of somatic Sertoli cells [7–11]. Thus, it may be speculated that reduction of Cx43 in Sertoli cells leads to an altered state of Sertoli cell maturation and in turn to enhanced germ cell proliferation, as Sertoli cells are no longer able to transmit signals to adjacent CIS cells keeping their proliferation under negative control.
In conclusion, our data show that progression from CIS to seminoma is associated with an intratubular reduction or even loss of Cx43 gene expression and Cx43 protein synthesis mainly in Sertoli cells, indicating that regulation of their Cx43 expression takes place at transcriptional level. These changes may further be implicated in the proliferation of CIS cells in the progression phase of human seminoma development. Finally, loss of growth-suppressing signals from Sertoli cells through Cx43 gap junction channels to adjacent CIS cells may lead to uncontrolled growth of CIS cells and their increased invasive potential, which, in turn, results in an altered state of Sertoli cell maturation.
Acknowledgements
We are grateful to L. Hertle (Department of Urology, University of Münster), W. Weidner (Department of Urology and Pediatric Urology, University of Giessen), F. Franke (Department of Pathology, University of Giessen), R. Grobholz (Department of Pathology, Ruprecht-Karls-University, Heidelberg), and C. von Ostau (Department of Urology, University of Duisburg-Essen) for providing the biopsy specimens. Furthermore, we thank L. Klein-Hitpass for his excellent performance of the microarrays. The skillful technical assistance of G. Erhardt, A. Hax, A. Hild, M. Fink, and R. Weigel is gratefully acknowledged.
Abbreviations
- CIS
carcinoma in situ
- Cx
connexin
- GJIC
gap junctional intercellular communication
- SCO
Sertoli cell only
Footnotes
This work was supported by the German Research Foundation (DFG), Research Training Group 533 “Cell-Cell Interaction in Reproduction,” and the German National Genome Research Cancer Network (NGFN).
References
- 1.Adami H, Bergström R, Möhner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Ziegler H, Rahu M. Testicular cancer in nine northern European countries. Int J Cancer. 1994;59:33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- 2.Rorth M, Rajpert-De Meyts E, Andersson L, Dieckmann KP, Fossa SD, Grigor KM, Hendry WF, Herr HW, Looijenga LH, Oosterhuis JW, et al. Carcinoma in situ in thetestis. Scand J Urol Nephrol Suppl. 2000;205:166–186. doi: 10.1080/00365590050509896. [DOI] [PubMed] [Google Scholar]
- 3.Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebaek NE. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003;111:267–279. doi: 10.1034/j.1600-0463.2003.11101301.x. [DOI] [PubMed] [Google Scholar]
- 4.Skakkebaek NE. Possible carcinoma in situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 5.Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma in situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumors except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen N, Rajpert-De Meyts E, Graem N, Muller J, Giwercman A, Skakkebaek NE. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest. 1995;72:223–231. [PubMed] [Google Scholar]
- 7.Rajpert-De Meyts E, Skakkebaek NE. The possible role of sex hormones in the development of testicular cancer. Eur Urol. 1993;23:54–59. doi: 10.1159/000474570. [DOI] [PubMed] [Google Scholar]
- 8.Kliesch S, Behre HM, Hertle L, Bergmann M. Alteration of Sertoli cell differentiation in the presence of carcinoma in situ in human testis. J Urol. 1998;160:1894–1898. [PubMed] [Google Scholar]
- 9.Skakkebaek NE, Rajpert-De Meyts E, Jorgensen N, Carlsen E, Petersen PM, Giwercman A, Andersen AG, Jensen TK, Anderson AM, Müller J. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS. 1998;106:3–12. doi: 10.1111/j.1699-0463.1998.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 10.Brehm R, Marks A, Rey R, Kliesch S, Bergmann M, Steger K. Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma in situ or seminoma. J Pathol. 2002;197:647–653. doi: 10.1002/path.1140. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 12.Looijenga LH, Oosterhuis JW. Pathogenesis of testicular germ cell tumours. Rev Reprod. 1999;4:90–100. doi: 10.1530/ror.0.0040090. [DOI] [PubMed] [Google Scholar]
- 13.Skotheim RI, Lothe RA. The testicular germ cell tumour genome. APMIS. 2003;111:136–151. doi: 10.1034/j.1600-0463.2003.11101181.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoei-Hansen CE, Nielsen JE, Almstrup K, Hansen MA, Skakkebaek NE, Rajpert-DeMeyts E, Leffers H. Identification of genes differentially expressed in testes containing carcinoma in situ. Mol Hum Reprod. 2004;10:423–431. doi: 10.1093/molehr/gah059. [DOI] [PubMed] [Google Scholar]
- 15.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 16.Söhl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 17.Krutovskikh V, Troyanovsky SM, Piccoli C, Tsuda H, Asamota M, Yamasaki H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumour cell growth in vivo. Oncogene. 2000;19:505–513. doi: 10.1038/sj.onc.1203340. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151–159. doi: 10.1016/s0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- 19.Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16:300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Risley MS, Tan IP, Roy C, Saez JC. Cell-, age- and stagedependent distribution of connexin43 gap junctions in testes. J Cell Sci. 1992;103:81–96. doi: 10.1242/jcs.103.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Steger K, Tetens F, Bergmann M. Expression of connexin43 in human testis. Histochem Cell Biol. 1999;112:215–220. doi: 10.1007/s004180050409. [DOI] [PubMed] [Google Scholar]
- 22.Risley MS. Connexin gene expression in seminiferous tubules of the Sprague-Dawley rat. Biol Reprod. 2000;62:748–754. doi: 10.1095/biolreprod62.3.748. [DOI] [PubMed] [Google Scholar]
- 23.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:729–739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 24.Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res. 1998;83:1248–1263. doi: 10.1161/01.res.83.12.1248. [DOI] [PubMed] [Google Scholar]
- 25.Tan IP, Roy C, Saez RJ, Saez CG, Paul DL, Risley MS. Regulated assembly of connexin 33 and connexin 43 into rat Sertoli cell gap junctions. Biol Reprod. 1996;54:1300–1310. doi: 10.1095/biolreprod54.6.1300. [DOI] [PubMed] [Google Scholar]
- 26.Simon AM, McWhorter AR. Role of connexin37 and connexin40 in vascular development. Cell Commun Adhes. 2003;10:379–385. doi: 10.1080/cac.10.4-6.379.385. [DOI] [PubMed] [Google Scholar]
- 27.Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junctional channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 30.Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Defamie N, Berthaut I, Mograbi B, Chevallier D, Dadoune JP, Fenichel P, Segretain D, Pointis G. Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli cells. Lab Invest. 2003;83:449–456. doi: 10.1097/01.lab.0000059928.82702.6d. [DOI] [PubMed] [Google Scholar]
- 32.Roger C, Mograbi B, Chevallier D, Michiels JF, Tanaka H, Segretain D, Pointis G, Fenichel P. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J Pathol. 2004;202:241–246. doi: 10.1002/path.1509. [DOI] [PubMed] [Google Scholar]
- 33.Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–1270. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- 34.Roscoe WA, Barr KJ, Mhawi AA, Pomerantz DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biol Reprod. 2001;65:829–838. doi: 10.1095/biolreprod65.3.829. [DOI] [PubMed] [Google Scholar]
- 35.Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol. 2004;18:413–421. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Neveu M, Hully JR, Babcock KL, Hertzberg EL, Nicholson BJ, Paul DL, Pitot HC. Multiple mechanisms are responsible for altered expression of gap junction genes during oncogenesis in rat liver. J Cell Sci. 1994;107:83–95. doi: 10.1242/jcs.107.1.83. [DOI] [PubMed] [Google Scholar]
- 37.Segretain D, Decrouy X, Dompierre J, Escalier D, Rahman N, Fiorini C, Mograbi B, Siffroi JP, Huhtaniemi I, Fenichel P, et al. Sequestration of connexin43 in the early endosomes: an early event in Leydig cell tumor progression. Mol Carcinog. 2003;38:179–187. doi: 10.1002/mc.10160. [DOI] [PubMed] [Google Scholar]
- 38.Bergmann M, Kliesch S. Hodenbiopsie. In: Krause W, Weidner W, editors. Andrologie: Krankheiten der männlichen Geschlechtsorgane. Stuttgart, Germany: Enke Verlag; 1998. pp. 66–71. [Google Scholar]
- 39.Gashaw I, Grümmer R, Klein-Hitpass L, Dushaj O, Bergmann M, Brehm R, Grobholz R, Kliesch S, Neuvians TP, Schmid KW, et al. Gene signatures of testicular seminoma with emphasis on expression of ets variant gene 4. Cell Mol Life Sci. 2005;62:2359–2368. doi: 10.1007/s00018-005-5250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuvians TP, Gashaw I, Sauer CG, von Ostau C, Kliesch S, Bergmann M, Hacker A, Grobholz R. Standardization strategy for quantitative PCR in human seminoma and normal testis. J Biotechnol. 2005;117:163–171. doi: 10.1016/j.jbiotec.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Steger K, Klonisch T, Gavenis K, Drabent B, Doenecke D, Bergmann M. Expression of mRNA and protein of nucleoproteins during human spermiogenesis. Mol Hum Reprod. 1998;4:939–945. doi: 10.1093/molehr/4.10.939. [DOI] [PubMed] [Google Scholar]
- 42.Braissant O, Wahli W. A simplified in situ hybridization protocol using non-radioactively labeled probes to detect abundant and rare mRNAs on tissue sections. Biochemica. 1998;1:10–16. [Google Scholar]
- 43.Batias C, Siffroi JP, Fenichel P, Pointis G, Segretain D. Connexin43 gene expression and regulation in the rodent seminiferous epithelium. J Histochem Cytochem. 2000;48:793–805. doi: 10.1177/002215540004800608. [DOI] [PubMed] [Google Scholar]
- 44.Kommoss F, Oliva E, Bittinger F, Kirkpatrick CJ, Amin MB, Bhan AK, Young RH, Scully RE. Inhibin-alpha CD99, HEA125, PLAP, and chromogranin immunoreactivity in testicular neoplasms and the androgen insensitivity syndrome. Hum Pathol. 2000;31:1055–1061. doi: 10.1053/hupa.2000.16237. [DOI] [PubMed] [Google Scholar]
- 45.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadle R, Zhang JT, Nicholson BJ. Tissue-specific distribution of differentially phosphorylated form of cx43. Mol Cell Biol. 1991;11:363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen PM, Giwercman A, Hansen SW, Berthelsen JG, Daugaard G, Rorth M, Skakkebaek NE. Impaired testicular function in patients with carcinoma-in-situ of the testis. J Clin Oncol. 1999;17:173–179. doi: 10.1200/JCO.1999.17.1.173. [DOI] [PubMed] [Google Scholar]
- 48.Krutovskikh V, Yamasaki H. The role of gap junctional intercellular communication (GJIC) disorders in experimental and human carcinogenesis. Histol Histopathol. 1997;12:761–768. [PubMed] [Google Scholar]
- 49.Krutovskikh V, Oyamada M, Yamasaki H. Sequential changes of gap junctional intercellular communication during multistage rat liver carcinogenesis: direct measurement of communication in vivo. Carcinogenesis. 1991;12:1701–1706. doi: 10.1093/carcin/12.9.1701. [DOI] [PubMed] [Google Scholar]
- 50.Fukuda S, Shirahama T, Imazono Y, Tsushima T, Ohmori H, Kayajima T, Take S, Nishiyama K, Yonezawa S, Akiba S, et al. Expression of vascular endothelial growth factor in patients with testicular germ cell tumours as an indicator of metastatic disease. Cancer. 1999;85:1323–1330. [PubMed] [Google Scholar]
- 51.Jones A, Fujiyama C, Turner K, Fuggle S, Cranston D, Turley H, Valtola R, Bicknell R, Harris AL. Angiogenesis and lymphangiogenesis in stage 1 germ cell tumours of the testis. BJU Int. 2000;86:80–86. doi: 10.1046/j.1464-410x.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- 52.Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth; lack of communication between cancer cells. Nature. 1966;209:1248–1249. doi: 10.1038/2091248a0. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990;11:1051–1058. doi: 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- 54.Soroceanu L, Manning TJ, Sontheimer H. Reduced expression of connexin43 and functional gap junction coupling in human gliomas. Glia. 2001;33:107–117. doi: 10.1002/1098-1136(200102)33:2<107::aid-glia1010>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 55.Habermann H, Ray V, Habermann W, Prins GS. Alterations in gap junction protein expression in human benign prostatic hyperplasia and prostate cancer. J Urol. 2002;167:655–660. doi: 10.1016/S0022-5347(01)69118-3. [DOI] [PubMed] [Google Scholar]
- 56.Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- 57.Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanisms of connexin-mediated growth suppression. J Biol Chem. 2004;279:36943–36950. doi: 10.1074/jbc.M403952200. [DOI] [PubMed] [Google Scholar]
- 58.Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, et al. Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem. 2004;279:36931–36942. doi: 10.1074/jbc.M404073200. [DOI] [PubMed] [Google Scholar]
- 59.Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer. 1992;51:522–529. doi: 10.1002/ijc.2910510404. [DOI] [PubMed] [Google Scholar]
- 60.Steger K, Rey R, Louis F, Kliesch S, Behre HM, Nieschlag E, Hoepffner W, Bailey D, Marks A, Bergmann M. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathologic human testis. Hum Reprod. 1999;14:136–143. doi: 10.1093/humrep/14.1.136. [DOI] [PubMed] [Google Scholar]
- 61.Griswold MD. Interactions between germ cells and Sertoli cells in the testis. Biol Reprod. 1995;52:211–216. doi: 10.1095/biolreprod52.2.211. [DOI] [PubMed] [Google Scholar]