Abstract
Cyclooxygenase-2 (COX-2) has been implicated in the development of gastrointestinal malignancies. The aim of the present study was to determine COX-2 expression/activity throughout stages of experimental and human pancreatic neoplasia. COX-2 immunohistochemistry was performed in pancreata of hamsters subjected to the carcinogen N-nitrosobis-(2-oxopropyl)amine (BOP) and in human pancreatic tumors. COX-2 activity was determined by prostaglandin E2 assay in tumor versus matched normal pancreatic tissues. The activity of the COX inhibitor sulindac was tested in the PC-1 hamster pancreatic cancer model. COX-2 expression was elevated in all pancreatic intraepithelial neoplasias (PanINs) and adenocarcinomas. In BOP-treated hamsters, there were significant progressive elevations in COX-2 expression throughout pancreatic tumorigenesis. In human samples, peak COX-2 expression occurred in PanIN2 lesions and remained moderately elevated in PanIN3 and adenocarcinoma tissues. COX-2 activity was significantly elevated in hamster and human pancreatic cancers compared to pair-matched normal pancreas. Furthermore, hamster pancreatic tumor engraftment/formation in the PC-1 hamster pancreatic cancer model was reduced 4.9-fold by oral administration of sulindac. Increased COX-2 expression is an early event in pancreatic carcinogeneses. The BOP-induced hamster carcinogenesis model is a representative model used to study the role of COX-2 in well-differentiated pancreatic tumorigenesis. COX inhibitors may have a role in preventing tumor engraftment/formation.
Keywords: Pancreatic neoplasms, PanIN, COX-2, chemical carcinogenesis, sulindac
Introduction
Pancreatic cancer is the fourth most common malignancy in the United States. The annual incidence of pancreatic cancer is approximately 33,730 cases [1]. Treatment options for pancreatic cancer are very limited [2]. A minority of patients are surgical candidates, and even those patients who undergo surgical treatment have, at most, a 5-year survival rate of 15%. All other treatments for pancreatic cancer are, for the most part, palliative and provide little hope of cure.
A better understanding of the molecular changes that occur in the process of pancreatic carcinogenesis may lead to better treatment and/or chemopreventive strategies. Cyclooxygenase-2 (COX-2) has been implicated in the development of colon and other human epithelial malignancies [3–6]. COX-2 catalyzes the conversion of arachidonic acid to prostaglandin E2 (PGE2) and a variety of other prostanoids, some of which appear to stimulate cancer cell proliferation, inhibit apoptosis, and/or induce angiogenesis [5,7–13]. COX-2 inhibitors have been demonstrated to cause the regression of adenomatous polyps—the precursor lesions of colon cancer [4,6].
The role of COX-2 in pancreatic carcinogenesis is less clear. Several investigators have demonstrated elevated COX-2 protein or mRNA in pancreatic adenocarcinomas compared to pair-matched normal tissues [10,14–25]. The principal aim of our study was to further characterize the role of COX-2 in pancreatic carcinogenesis by measuring COX-2 protein expression in each of the successive stages of both experimental and human pancreatic carcinogeneses. These stages are characterized by pancreatic intraepithelial neoplasia (PanIN) [26] (i.e., PanIN1, PanIN2, and PanIN3) and, ultimately, invasive adenocarcinoma. For these experiments, we used a N-nitrosobis-(2-oxopropyl)amine (BOP) chemically induced hamster pancreatic cancer model system because it is widely used in pancreatic cancer studies, and we compared the results to those obtained from studies on the human pancreas. Secondly, we tested the hypothesis that COX-2 enzymatic activity is elevated in pancreatic cancer by assaying PGE2 levels in hamster and human tumors versus pair-matched normal tissues. Finally, we tested the antitumor activity of the nonselective COX inhibitor sulindac in the PC-1 hamster pancreatic cancer model. Our findings lend further support for the hamster BOP model system as a relevant model for human pancreatic neoplasia and suggest that COX-2 may be relevant both early in neoplasia and later in tumor formation and engraftment.
Materials and Methods
Assurances
These studies have been conducted in strict compliance with the Indiana University School of Medicine Institutional Review Board and with the Indiana University-Purdue University Indianapolis Science Institutional Animal Care and Use Committee.
Animals
Experimental pancreatic carcinogenesis was initiated by exposing male Syrian golden hamsters to the carcinogen BOP. Beginning at time 0, 30 hamsters were given three weekly (20 mg/kg) intraperitoneal injections of BOP. On week 42, the animals were euthanized, and the pancreas was preserved in 10% neutral-buffered formalin. The tissues were processed and embedded in paraffin, 5-µm sections from four different areas of each pancreas were stained with hematoxylin and eosin (H&E), and neoplastic lesions were scored by a pathologist, as described previously [27]. An adjacent section from each sample was processed for COX-2 immunohistochemistry (see below). A related model (PC-1 hamster pancreatic cancer model) was employed to measure in vivo PGE2 levels and to assess the effects of the nonselective COX inhibitor sulindac. Male Syrian golden hamsters weighing 90 to 100 g were injected subcutaneously with the PC-1 cell line (1 x 106 cells) [28], which was originally established from a BOP-induced Syrian golden hamster pancreatic ductal adenocarcinoma in the model described above. Tumor-bearing animals were euthanized, and PGE2 levels were measured in PC-1-derived tumors and in normal pancreas from the same animal. In the sulindac study, hamsters were given a purified, nutritionally complete Teklad hamster diet 96224 (Harlan-Teklad, Indianapolis, IN) [27] or sulindac (Sigma, St. Louis, MO) as 0.01 % (wt/wt) of the same diet for 5 days before the flank injection of PC-1 tumor cells. Animals continued on their respective diets throughout the experiment. The presence or absence of a tumor > 2 mm in diameter was determined twice weekly for 4 weeks, and tumor incidence percentage was calculated as: (number of tumor-bearing animals/total number of animals) x 100.
Immunohistochemistry
Immunohistochemistry was performed after deparaffinizing the slides in xylene followed by three ethanol and one phosphate-buffered saline (PBS) washes. Slides were then microwave-heated in 5 mM sodium citrate (Fisher, Pittsburgh, PA) buffer, allowed to cool, and washed with PBS, 3% hydrogen peroxide (Sigma), and PBS again. Slides were then blocked for 15 minutes with nonfat dry milk (0.3 g/10 ml water), incubated with the primary COX-2 antibody (Cayman Chemical, Ann Arbor, MI) for 90 minutes at 37°C, and then washed in PBS. Slides were then exposed to secondary antirabbit antibody (Biogenex, San Ramon, CA) for 30 minutes at room temperature and then washed with PBS. This was followed by exposure to streptavidin peroxidase (Biogenex) for 30 minutes at room temperature, followed by washes in PBS and water. α-Ethyl carbazol (Zymed, South San Francisco, CA) and diaminobenzidine tetrahydrochloride (Sigma) were used as chromagens for the hamster and human slides, respectively. Hematoxylin (Fisher) was used as counterstain. COX-2 expression was determined in human and hamster pancreas independently by board-certified pathologists. Hamster lesions were graded according to the most advanced lesion present in the pancreata on a point intensity scale from 0 to 2 (0 = absent/weak; 1 = intermediate; 2 = intense staining). A separate pathologist independently graded the human lesions by intensity and frequency of staining. The intensity of staining was scored on a scale of 0 to 3 (0 = absent; 1 = faint; 2 = intermediate; 3 = strong), and the percentage of cells stained was scored on a scale of on a point scale from 0 to 4 (0 = 0%; 1 = 1–5%; 2 = 6–25%; 3 = 26–50%; 4 = greater than 50%). Differences in COX-2 expression were determined in normal ductules, normal ducts, PanIN1-PanIN3 lesions, and invasive cancers. A normal ductule was defined as a duct surrounded by normal-appearing acinar architecture that had no more than one-epithelial-cell-layer-thick connective tissue stroma. A normal duct in our study was defined as a duct with greater than one-cell-thick stroma surrounding it, which is not necessarily surrounded by normal acinar architecture. COX-2 expression was also assessed in cancers according to differentiation status. Contiguous lesions or lesions that appeared architecturally (on serial slices) to connect to other lesions within the ductal system were not double-counted and were considered part of the same lesion. In invasive cancers and occasionally in PanIN lesions, there was heterogeneity of COX-2 staining. In such cases, the tumor was divided randomly into x20 field regions analyzed. The number of x20 fields depended on the size of the tumor represented on the slide. Within each x20 field, the intensity and frequency of COX-2 staining were determined. The average of the x20 fields served as the COX-2 score for each lesion that had significant heterogeneity.
Statistical analysis in human specimens was performed by generating a COX-2 expression score for each ductule, duct, PanIN1-PanIN3, and invasive cancer, which was the multiplicative factor of the intensity times the frequency of cells stained. All of the human data were then analyzed according to neoplastic stage with an overall “average score,” where equal weighting was given for each identified lesion of the same stage within the same specimen. For example, if a single human specimen had three separate PanIN2 lesions identified, each PanIN2 lesion would count in the average of all PanIN2 lesions across all of the specimens. Because of the possibility of bias with this method, all of the human data were also analyzed according to neoplastic stage with a “composite score,” where each specimen could only count once toward any particular stage of neoplasia. For example, if a single human specimen had three separate PanIN2 lesions identified, their scores would be averaged to generate only one “composite” score in the average of all PanIN2 lesions across all of the specimens.
PGE2 Assay
Cryopreserved tumor specimens and pair-matched normal tissues were homogenized in ice-cold 50 mM Tris-HCl, pH 7.4 (5 ml/g tissue), containing 10 µg/ml of the COX inhibitor indomethacin and pelleted by centrifugation (150g x 10 minutes). The resultant pellet was discarded, and homogenates were adjusted to a final protein concentration of approximately 6 mg/ml. PGE2 was extracted from 0.5 ml of the resulting homogenate by adding 0.5 ml of a water/ethanol (1:4) solution and 10 µl of glacial acetic acid, mixing gently, and incubating at room temperature for 5 minutes. After spinning, supernatants were loaded onto a primed Amprep C18 minicolumn (Amersham, Piscataway, NJ). The column was washed with distilled water followed by hexane. PGE2 was eluted with ethyl acetate, and resulting fractions were evaporated to dryness under nitrogen. Samples were reconstituted in 200 µl of assay buffer (supplied in PGE2 kit), and 10 µl was assayed for PGE2. Each sample was assayed in duplicate following the recommended protocol. The assay is based on the competition between unlabeled PGE2 and a fixed quantity of peroxidase-labeled PGE2 for binding to a PGE2-specific antibody bound to a plate coated with goat antimouse immunoglobulin. The amount of bound PGE2 peroxidase can be measured by the addition of the substrate. After the addition of sulfuric acid to stop the reaction, the plate is read at 450 nm. PGE2 levels were compared with COX-2 expression in the same samples to determine the functional activity correlated with COX-2 protein expression.
Western Blot Analysis
Cryopreserved tumor specimens and pair-matched normal tissues were homogenized in ice-cold 50 mM Tris-HCl (pH 7.4) containing 1 mM EDTA, 1 mM sodium vanadate, and 1 tablet of Complete Protease Inhibitor (contains leupeptin, aprotinin, and phenylmethylsulfonyl fluoride [PMSF]) per 50 ml. Tissues were then homogenized and pelleted by centrifugation (150g x 10 minutes). The resultant pellet was discarded, and the supernatant was reconstituted in homogenization buffer. Tissue homogenates (10 µg of total protein) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% or on 4% to 20% gradient gels (Invitrogen, San Diego, CA) and transferred to Immobilon P membranes. The blots were probed with primary antibodies specific for human COX-2 (polyclonal; Cayman Chemical) and actin (I-19; Santa Cruz Biotechnology, Santa Cruz, CA), according to the manufacturer's protocol, followed by secondary antibody (Amersham) and ECL detection (Amersham).
Results
COX-2 Expression in Pancreatic Carcinogenesis
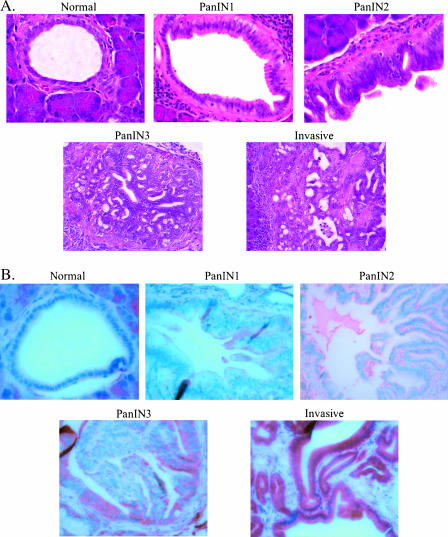
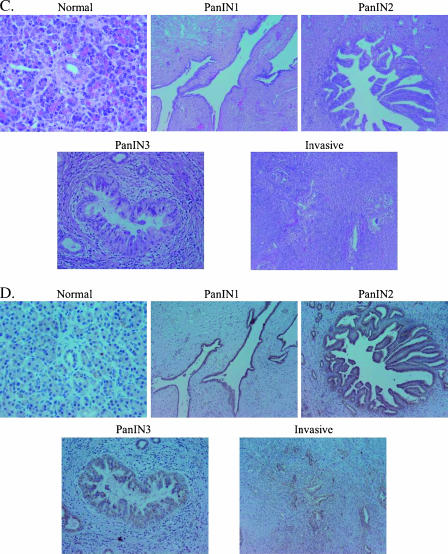
A number of published studies have reported elevated COX-2 expression in pancreatic adenocarcinomas or collectively in PanINs versus normal pancreatic ducts. However, any specific changes in COX-2 expression at each distinct pathological stage in pancreatic neoplasia have not been described. We first employed a BOP-induced model of hamster pancreatic carcinogenesis for the measurement of COX-2 expression at every stage of experimental pancreatic neoplasia. The highest-grade lesion in each pancreas was identified in an H&E-stained section, and an adjacent section was used to detect COX-2 protein expression by immunohistochemistry. Thirty invasive ductal adenocarcinomas, 47 PanIN lesions (23 PanIN1, 7 PanIN2, and 17 PanIN3), and 39 normal ducts were identified for analysis. As depicted in Figure 1A, the normal ducts, PanINs, and invasive adenocarcinomas of hamsters are strikingly similar to those of the human pancreas (Figure 1C).
Figure 1.
(A) H&E-stained normal hamster pancreas, hamster pancreatic intraepithelial neoplasms (PanIN1, PanIN2, and PanIN3), and invasive pancreatic adenocarcinoma. (B) COX-2 immunohistochemistry in normal hamster pancreas, hamster pancreatic intraepithelial neoplasms (PanIN1, PanIN2, and PanIN3), and invasive pancreatic adenocarcinoma. (C) H&E-stained normal human pancreas, human pancreatic intraepithelial neoplasms (PanIN 1, PanIN2, and PanIN3), and invasive pancreatic adenocarcinoma. (D) COX-2 immunohistochemistry in normal human pancreas, human pancreatic intraepithelial neoplasms (PanIN1, PanIN2, and PanIN3), and invasive pancreatic adenocarcinoma.
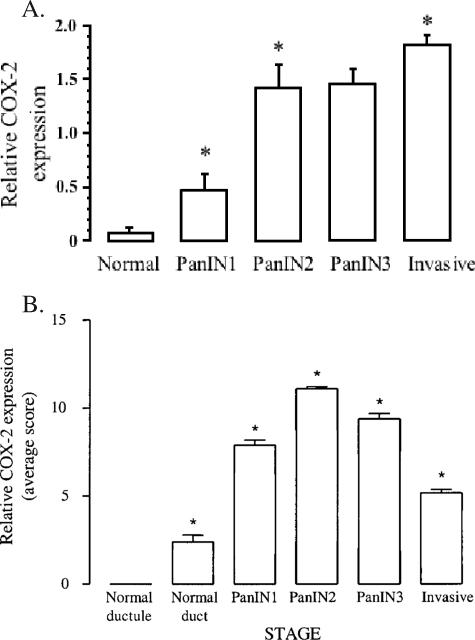
In Figure 1B, representative examples of COX-2 expression in normal hamster pancreatic ducts, PanIN1-PanIN3 lesions, and an invasive ductal adenocarcinoma are shown, and quantitative COX-2 expression data are presented in Figure 2A and Table 1. Little to no COX-2 staining was observed in normal ductal cells. There was a marked stepwise increase in staining as the PanIN lesions progressed toward malignancy, with the most intense staining in invasive lesions. Average COX-2 expression increased significantly from normal to PanIN1 lesions, and then again from PanIN1 to PanIN2 [Figure 2A; P < .001 by analysis of variance (ANOVA)]. There was little change in COX-2 expression between PanIN2 and PanIN3 lesions, and a slight but statistically significant further increase in invasive lesions (P < .001, ANOVA). As shown in Table 1, the majority of normal ducts and most PanIN1 lesions showed little or no COX-2 staining, PanIN2 and PanIN3 lesions had intermediate or intense staining in approximately equal proportions, and nearly all invasive adenocarcinomas exhibited intense COX-2 staining.
Figure 2.
(A) COX-2 expression (average score) in hamster pancreatic neoplasms. Hamster pancreatic cancer was initiated at time 0 with the chemical carcinogen BOP. On week 42, all hamsters were euthanized, and lesions were scored by neoplastic stage. COX-2 expression was measured by immunohistochemistry in the most advanced lesion in each pancreas, and each lesion was assigned a value of 0 (absent/weak staining), 1 (intermediate staining), or 2 (intense staining). The data represent the mean ± SEM. ANOVA indicated that COX-2 expression significantly (P < .001) increased with each stage of neoplastic progression, except PanIN2 to PanIN3. (B) COX-2 expression in human pancreatic neoplasms. COX-2 expression was measured by immunohistochemistry. The COX-2 average expression score for each ductule, duct, PanIN1-3, and invasive cancer was the multiplicative factor of the intensity (0–3) times the frequency (0–4) of cells stained for COX-2. The data represent mean ± SEM. Analysis of variance indicated that COX-2 expression was significantly different in all stages of neoplasia compared to normal ductules and normal ducts (P < .001) and increased with each stage of neoplastic progression up to the PanIN2 stage (P < .05).
Table 1.
COX-2 Expression in Hamster Pancreatic Carcinogenesis.
| Tissue | Relative COX-2 Expression [n (%)] | ||
| Absent/Weak | Intermediate | Intense | |
| Normal duct | 36/39 (92.3) | 3/39 (7.7) | 0/39 (0) |
| PanIN1 | 14/23 (60.9) | 7/23 (30.4) | 2/23 (8.7) |
| PanIN2 | 0/7 (0) | 4/7 (57.1) | 3/7 (42.9) |
| PanIN3 | 0/17 (0) | 9/17 (52.9) | 8.17 (47.1) |
| Invasive ductal adenocarcinoma | 1/30 (3.3) | 3/30 (10) | 26/30 (86.7) |
Hamster pancreatic cancer was initiated at time 0 with the chemical carcinogen BOP. On week 42, all hamsters were euthanized and scored according to the state of neoplastic progression. COX-2 expression was measured by immunohistochemistry in the most advanced lesion in each pancreas, and each lesion was assigned a value of 0 (absent/weak staining), 1 (intermediate staining), or 2 (intense staining). The data represent the number of animals with tumors staining at the indicated level/total number of animals and the percentage of animals with tumors staining at the indicated level.
COX-2 expression was then detected by immunohistochemistry in 30 human pancreatic adenocarcinomas. Figure 1C demonstrates H&E sections of human tumors and demonstrates a representative example of a normal ductule, PanIN1-PanIN3, and an invasive lesion. Correspondingly, Figure 1D shows COX-2 staining in a normal ductule, PanIN1-PanIN3, and invasive human pancreas lesions.
An analysis was undertaken to identify normal ductules, normal ducts, PanIN lesions, and invasive lesions within 30 human pancreas specimens from patients with pancreatic adenocarcinoma. Normal ductules and ducts were also identified in five intraductal papillary mucinous neoplasms (IPMNs), one ampullary cell cancer specimen, and one acinar cell cancer specimen. Together, the analysis of these specimens identified 55 normal ductules, 42 normal ducts, 22 PanIN1 lesions, 18 PanIN2 lesions, 13 PanIN3 lesions, and 30 invasive adenocarcinomas. PanINs were identified in 25 of 30 patients with pancreatic adenocarcinoma, and many of these had more than one PanIN. Figure 2B depicts the average COX-2 expression score of all normal ductules, ducts, and lesions identified in a particular stage of pancreatic carcinogenesis. COX-2 expression was significantly higher in all stages of human pancreatic neoplasia than in normal ductules (P < .001), and it increased in a stepwise manner with each stage of neoplastic progression up to the PanIN2 stage (P < .05, ANOVA). PanIN3 lesions also had high COX-2 expression (higher than PanIN1 but less than PanIN2), whereas average COX-2 expression was relatively lower in invasive cancers. Interestingly, among invasive lesions, well-differentiated lesions had the highest COX-2 expression, with an average score of 6.1 followed by moderately differentiated lesions at 5.5 and poorly differentiated lesions at 3.6. A tumor involving lymph nodes represented on the slides had an average COX-2 expression score of 8, similar to that of PanIN3. Alternatively, we also measured the composite score, whereby each of the 30 human cancer specimens examined received one score for each normal ductule, duct, or lesion stage (e.g., PanIN1) represented within that specimen. This method revealed very similar results (data not shown). Importantly, the overall pattern of COX-2 expression is consistent with the pattern observed within individual specimens (i.e., within each individual specimen, there is a general trend of increasing COX-2 expression through the different stages of pancreatic carcinogenesis that were represented).
Our pathologist also examined the topography and character of COX-2 staining as a function of the lesion stage of pancreatic tumorigenesis. Through successive PanIN stages, there is a higher degree of perinuclear stippling-type staining. This stippling-type staining is typically very strong in intensity. In invasive lesions, particularly those with poor differentiation, there is loss of perinuclear stippling, which is largely replaced by diffuse cytoplasmic staining. This diffuse-type staining is typically very faint in intensity.
Finally, in a separate analysis of five I PMNs (precancerous lesions similar to PanINs in that they can progress to invasive pancreatic cancer), we observed a similar trend with increasing neoplastic grade. Average scores were as follows: ductules, 2; “normal ducts,” 10.7; adenoma, 12; low-grade dysplasia, 8.6; high-grade dysplasia, 8.5; overall invasive lesions, 4.25; well-differentiated lesions, 7.5; moderately differentiated lesions, 4. The trend in the topography and character of COX-2 staining is mirrored in IPMNs. As a negative control, we examined acinar cell carcinoma of the pancreas and ampullary carcinoma, neither of which demonstrated COX-2 staining of ductal cells.
PGE2 Level (COX-2 Activity) in Pancreatic Cancer
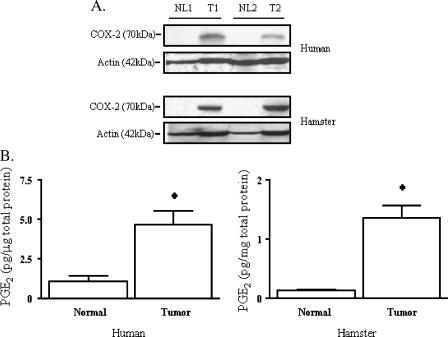
Having established that COX-2 is overexpressed throughout hamster and human pancreatic neoplasia, we next sought to determine if this overexpression corresponded to an increased enzymatic activity of COX-2 in vivo. Two pair-matched samples of human invasive pancreatic cancer versus normal tissue were cryopreserved and then analyzed for COX-2 protein expression by Western blot analysis (Figure 3A). As expected, in both hamster and human specimens, COX-2 expression in tumor tissue was greater than in pair-matched normal tissues. Actin is shown, but in inflammatory-based cancers, such as pancreatic (and hepatocellular) cancer, higher amounts of actin protein are typically expressed in tumors than in normal tissues. Coomassie blue staining of the same samples (data not shown) demonstrates nearly equivalent protein loading.
Figure 3.
(A) COX-2 expression in human and hamster pancreatic neoplasms. Pair-matched samples of human or hamster invasive pancreatic cancer versus normal tissue were cryopreserved and then analyzed for COX-2 expression by Western blot analysis. A representative Western blot is shown of both human and hamster pancreatic neoplasms (T, tumor) compared to pair-matched normal control tissues from the same patient or hamster (NL, normal). Actin, which is known to be elevated in tumor samples, is also shown. Gels stained with Coomassie blue confirmed an equivalent overall protein loading of the samples (data not shown). (B) COX-2 activity in human and hamster pancreatic neoplasms. Pair-matched samples of human or hamster invasive pancreatic cancer versus normal tissue were cryopreserved and then analyzed for COX-2 activity by PGE2 assay. The assay is based on competition between unlabeled PGE2 and a fixed quantity of peroxidase-labeled PGE2 for binding to a PGE2-specific antibody bound to a plate coated with goat antimouse immunoglobulin. The human tumor PGE2 level [4.7 ± 0.8pg/µg total protein (mean ± SEM)] was significantly higher than that of normal tissue [1.1 ± 0.3pg/µg total protein (mean ± SEM), n = 3, t-test, P < .05]. Similarly, the hamster tumor PGE2 level [1.4 ±0.2 pg/µg total protein (mean ± SEM)] was significantly higher than that in normal tissue [0.1 ±0.0 pg/µg total protein (mean ± SEM), n = 7, t-test, P < .05].
The same pair-matched samples were then subjected to PGE2 assay to determine PGE2 tissue concentrations. Figure 3B demonstrates a significantly greater average of 4.7 ± 0.8 pg/µg total protein for PGE2 in human pancreatic tumor versus an average level of 1.1 ± 0.3 pg/µg total protein of PGE2 in pair-matched normal tissue (P < .05, Student's t test). COX-2 activity was then measured by PGE2 production in the hamster PC-1 pancreatic ductal adenocarcinoma cell line-derived tumor and then compared with a normal pancreas isolated from the same animal. The level of PGE2 in hamster pancreas tumors (1.4 ± 0.2 pg/µg total protein) was significantly higher than that in normal pancreas (0.1 ± 0.0 pg/µg total protein; P < .05, Student's t test; Figure 3B).
Anticancer Activity of the Nonselective COX Inhibitor Sulindac
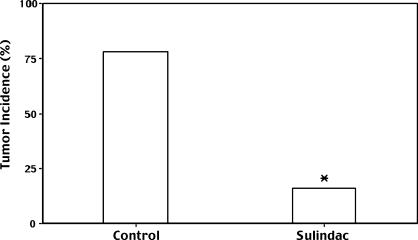
Based on these results, we hypothesized that sulindac, a COX inhibitor, would have anticancer activity in a hamster pancreatic cancer model. Hamsters were treated with either control or a 0.01 % (wt/wt) sulindac diet starting 5 days before subcutaneous flank injection of a suspension of PC-1 hamster pancreatic ductal adenocarcinoma cells (derived from a BOP-induced pancreatic adenocarcinoma). After 4 weeks, tumor incidence in control animals was 78% (18/23) vs 16% (2/12) in the sulindac group (P < .0005, chi-square analysis; Figure 4). Sulindac had little effect on hamster tumor growth when administered orally to animals bearing already established PC-1 tumors (D. A. Hertzler and P. L. Crowell, unpublished observations), leading us to conclude that sulindac may have the greatest effect on tumor engraftment/formation.
Figure 4.
Inhibition of PC-1 hamster pancreatic cancer engraftment/formation with the nonselective COX inhibitor sulindac. Male Syrian golden hamsters were fed either control or 0.01% (wt/wt) sulindac diet starting 5 days before the injection of hamster PC-1 pancreatic adenocarcinoma cells. The data represent the percentage of 23 control and 12 sulindac animals with tumor 4 weeks after PC-1 cell injection (*P <. 0005 vs control by chi-square analysis).
Discussion
This study was undertaken to investigate the degree of COX-2 expression during the multistep process of pancreatic neoplasia both in experimental and in human pancreatic cancer. We have found, as other investigators have previously identified, that COX-2 expression is elevated in invasive adenocarcinomas compared to normal ductal tissues [10,14–25]. Unlike previous studies, however, we have determined that normal ductules do not express COX-2. Furthermore, we report the novel findings of COX-2 expression in each type of PanIN lesion, the correlation of COX-2 enzymatic activity (PGE2 level) with COX-2 expression, and the direct comparison of COX-2 expression and activity in the BOP-induced hamster pancreatic carcinogenesis model versus human pancreatic tissues.
COX-2 expression varies markedly throughout the pathological process of pancreatic neoplasia. In PanIN lesions, we determined that there is a highly significant stepwise increase in the expression of COX-2 from normal ducts to PanIN1 and from PanIN1 to PanIN2 in both human and experimental pancreatic cancers. The difference between PanIN2 and PanIN3 lesions is less marked, and, in human tissues, may be somewhat decreased. COX-2 expression in hamster invasive adenocarcinomas was higher than in PanIN2 or PanIN3 lesions, whereas the average level of COX-2 expression in human invasive adenocarcinomas was lower than in any PanIN lesions, albeit higher than in normal ducts. The hamster invasive tumors were typically well differentiated, whereas the human invasive tumors were a mixture of well differentiated to poorly differentiated states. In human tissues, therefore, we evaluated COX-2 expression in invasive lesions as a function of differentiation status. Well-differentiated lesions expressed COX-2 to the highest degree, and there was less expression of COX-2 in moderately and poorly differentiated lesions. Thus, the different trends for COX-2 expression in hamster versus human invasive cancers may be attributable to the different states of differentiation in the tumors.
We found that COX-2 activity (PGE2 level) correlated well with COX-2 expression. COX-2 activity was significantly elevated in pair-matched human tumors versus normal specimens. In addition, in specimens that did not have a pair match, aggregate data suggest that the tumor has an activity greater than the average activity expressed by normal nonpair-matched samples. COX-2 activity was also examined in the PC-1 hamster pancreatic cancer model. Mirroring human data, COX-2 activity was significantly elevated in the tumor versus the normal pancreas, although the fold difference of PGE2 detected in hamster tumors versus normal tissues (∼ 14-fold) was higher than in humans (∼ 4-fold), possibly due to the very low levels of PGE2 detected in normal hamster control pancreas. Correspondingly, the higher basal level of PGE2 in “normal” human pancreas may be secondary to this tissue being adjacent to cancer (unlike the normal control pancreas in the hamster). Human pancreatic cancer often results in fibrosis and an intense stromal reaction in the rest of the pancreas that may be responsible for the elevation of PGE2. Interestingly, COX-2 expression even in “normal” ducts (pre-PanIN) had a stepwise increase as the degree of stromal reaction surrounding the duct increased. Thus, the effect of the microenvironment on these tumors in relation to COX-2 may be quite significant.
From these data, it appears that COX-2 may be very important in, and perhaps even a contributor to, pancreatic neoplasia. Based on the pattern of expression of COX-2 observed, we hypothesize that it may be very important in the early stages of human pancreatic carcinogenesis; namely, the PanIN1 and PanIN2 lesions. The BOP-induced hamster pancreatic carcinogenesis model appears to be quite suitable for studying the role of COX-2 in pancreatic carcinogenesis, particularly in the early PanIN1 and PanIN2 stages.
COX-2 has been shown in other cancers to play a significant role in carcinogenesis. In colorectal cancer, it has been shown to have a chemotherapeutic and chemopreventive role in treating the disease [4]. Some demographic data suggest that individuals who take aspirin have a lower risk of developing pancreatic cancer [29], although other studies have reported no effect or an increased risk [30–33]. We report that oral administration of sulindac reduced the incidence of pancreatic tumor formation in the PC-1 hamster model, suggesting that sulindac or other nonsteroidal anti-inflammatory drugs (NSAIDs) may also have chemotherapeutic potential in pancreatic cancer cells. By Western blot analysis, PC-1 cells express COX-2 protein but no detectable levels of COX-1. This suggests that, in the PC-1 hamster pancreatic cancer model, sulindac is targeting COX-2, other COX-independent pathways, or both. COX-2 inhibitors, such as nimesulide and celecoxib (Celebrex; Pfizer Inc., Ann Arbor, MI), have been previously shown to inhibit pancreatic tumorigenesis in the BOP-induced hamster model [34,37,38]. Even if COX-1 does not effect tumorigenesis, the absence of COX-1 inhibition, as is the case with COX-2-selective inhibitors, may have untoward effects on an organism's normal cells. COX-2-selective inhibitors have recently come under scrutiny by the Food and Drug Administration due to apparent cardiovascular side effects with long-term use; thus, a nonselective COX inhibitor, such as sulindac, employed in these studies may be more suitable for treatment.
Although NSAIDs are known to target COX, antitumor effects may be mediated by targeting COX-independent pathways as well, such as NF-κB and cGMP-dependent phosphodiesterase [39]. Future studies should further explore the role of COX-2 in the chemoprevention of experimental and human pancreatic cancers, with emphasis on intervention in the early stages of carcinogenesis. The hamster pancreatic carcinogenesis models are reasonable models to use in undertaking these studies [34–38].
Abbreviations
- COX
cyclooxygenase
- PGE2
prostaglandin E2
- PanIN
pancreatic intraepithelial neoplasia
- PBS
phosphate-buffered saline
Footnotes
This work was supported by a Central Surgical Association Enrichment Award 2002 (to C.M.S.) and an American Institute for Cancer Research grant (99B047 to P.L.C.).
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;2:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.McKenna S, Eatock M. The medical management of pancreatic cancer: a review. Oncologist. 2003;8:149–160. doi: 10.1634/theoncologist.8-2-149. [DOI] [PubMed] [Google Scholar]
- 3.Ding XZ, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001;1:291–299. doi: 10.1159/000055827. [DOI] [PubMed] [Google Scholar]
- 4.Ricchi P, Zarrilli R, Di Palma A, Acquaviva AM. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br J Cancer. 2003;88:803–807. doi: 10.1038/sj.bjc.6600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Straus WL, Sandler RS. Chemoprevention of gastrointestinal malignancies with nonsteroidal antiinflammatory drugs. Cancer. 2002;94:950–963. [PubMed] [Google Scholar]
- 7.Chu J, Lloyd FL, Trifan OC, Knapp B, Rizzo MT. Potential involvement of the cyclooxygenase-2 pathway in the regulation of tumor-associated angiogenesis and growth in pancreatic cancer. Mol Cancer Ther. 2003;2:1–7. [PubMed] [Google Scholar]
- 8.Ding XZ, Tong WG, Adrian TE. Blockade of cyclooxygenase-2 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Anticancer Res. 2000;20:2625–2631. [PubMed] [Google Scholar]
- 9.Eibl G, Reber HA, Wente MN, Hines OJ. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas. 2003;26:33–41. doi: 10.1097/00006676-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 11.Perugini RA, McDade TP, Vittimberga FJ, Jr, Duffy AJ, Callery MP. Sodium salicylate inhibits proliferation and induces G1 cell cycle arrest in human pancreatic cancer cell lines. J Gastrointest Surg. 2000;4:24–32. doi: 10.1016/s1091-255x(00)80029-3. (discussion, 32–23) [DOI] [PubMed] [Google Scholar]
- 12.Tseng WW, Deganutti A, Chen MN, Saxton RE, Liu CD. Selective cyclooxygenase-2 inhibitor rofecoxib (Vioxx) induces expression of cell cycle arrest genes and slows tumor growth in human pancreatic cancer. J Gastrointest Surg. 2002;6:838–843. doi: 10.1016/s1091-255x(02)00061-6. (discussion, 844) [DOI] [PubMed] [Google Scholar]
- 13.Yip-Schneider MT, Sweeney CJ, Jung SH, Crowell PL, Marshall MS. Cell cycle effects of nonsteroidal anti-inflammatory drugs and enhanced growth inhibition in combination with gemcitabine in pancreatic carcinoma cells. J Pharmacol Exp Ther. 2001;298:976–985. [PubMed] [Google Scholar]
- 14.Agoff SN, Crispin DA, Bronner MP, Dail DH, Hawes SE, Haggitt RC. Neoplasms of the ampulla of Vater with concurrent pancreatic intraductal neoplasia: a histological and molecular study. Mod Pathol. 2001;14:139–146. doi: 10.1038/modpathol.3880270. [DOI] [PubMed] [Google Scholar]
- 15.Aoki T, Nagakawa Y, Tsuchida A, Kasuya K, Kitamura K, Inoue K, Ozawa T, Koyanagi Y, Itoi T. Expression of cyclooxygenase-2 and vascular endothelial growth factor in pancreatic tumors. Oncol Rep. 2002;9:761–765. [PubMed] [Google Scholar]
- 16.Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333–338. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Kong G, Kim EK, Kim WS, Lee KT, Lee YW, Lee JK, Paik SW, Rhee JC. Role of cyclooxygenase-2 and inducible nitric oxide synthase in pancreatic cancer. J Gastroenterol Hepatol. 2002;17:914–921. doi: 10.1046/j.1440-1746.2002.02829.x. [DOI] [PubMed] [Google Scholar]
- 18.Koshiba T, Hosotani R, Miyamoto Y, Wada M, Lee JU, Fujimoto K, Tsuji S, Nakajima S, Doi R, Imamura M. Immunohistochemical analysis of cyclooxygenase-2 expression in pancreatic tumors. Int J Pancreatol. 1999;26:69–76. doi: 10.1007/BF02781733. [DOI] [PubMed] [Google Scholar]
- 19.Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Wilentz RE. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- 20.Merati K, said Siadaty M, Andea A, Sarkar F, Ben-Josef E, Mohammad R, Philip P, Shields AF, Vaitkevicius V, Grignon DJ, et al. Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol. 2001;24:447–452. doi: 10.1097/00000421-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Niijima M, Yamaguchi T, Ishihara T, Hara T, Kato K, Kondo F, Saisho H. Immunohistochemical analysis and in situ hybridization of cyclooxygenase-2 expression in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2002;94:1565–1573. doi: 10.1002/cncr.10358. [DOI] [PubMed] [Google Scholar]
- 22.Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, et al. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018–2024. [PubMed] [Google Scholar]
- 23.Schlosser W, Schlosser S, Ramadani M, Gansauge F, Gansauge S, Beger HG. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26–30. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 25.Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 26.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Burke YD, Ayoubi AS, Werner SR, McFarland BC, Heilman DK, Ruggeri BA, Crowell PL. Effects of the isoprenoids perillyl alcohol and farnesol on apoptosis biomarkers in pancreatic cancer chemoprevention. Anticancer Res. 2002;22:3127–3134. [PubMed] [Google Scholar]
- 28.Egami H, Takiyama Y, Cano M, Houser WH, Pour PM. Establishment of hamster pancreatic ductal carcinoma cell line (PC-1) producing blood group-related antigens. Carcinogenesis. 1989;10:861–869. doi: 10.1093/carcin/10.5.861. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KE, Johnson TW, Lazovich D, Folsom AR. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst. 2002;94:1168–1171. doi: 10.1093/jnci/94.15.1168. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs EJ, Connell CJ, Rodriguez C, Patel AV, Calle EE, Thun MJ. Aspirin use and pancreatic cancer mortality in a large United States cohort. J Natl Cancer Inst. 2004;96:524–528. doi: 10.1093/jnci/djh084. [DOI] [PubMed] [Google Scholar]
- 31.Schernhammer ES, Kang JH, Chan AT, Michaud DS, Skinner HG, Giovannucci E, Colditz GA, Fuchs CS. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst. 2004;96:22–28. doi: 10.1093/jnci/djh001. [DOI] [PubMed] [Google Scholar]
- 32.Menezes RJ, Huber KR, Mahoney MC, Moysich KB. Regular use of aspirin and pancreatic cancer risk. BMC Public Health. 2002;2:18. doi: 10.1186/1471-2458-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomark Prev. 2000;9:119–123. [PubMed] [Google Scholar]
- 34.Furukawa F, Nishikawa A, Lee IS, Kanki K, Umemura T, Okazaki K, Kawamori T, Wakabayashi K, Hirose M. A cyclooxygenase-2 inhibitor, nimesulide, inhibits postinitiation phase of N-nitrosobis(2-oxopropyl)amine-induced pancreatic carcinogenesis in hamsters. Int J Cancer. 2003;104:269–273. doi: 10.1002/ijc.10965. [DOI] [PubMed] [Google Scholar]
- 35.Schuller HM, Zhang L, Weddle DL, Castonguay A, Walker K, Miller MS. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525–532. doi: 10.1007/s00432-002-0365-y. [DOI] [PubMed] [Google Scholar]
- 36.Standop J, Schneider MB, Ulrich A, Pour PM. Experimental animal models in pancreatic carcinogenesis: lessons for human pancreatic cancer. Dig Dis. 2001;19:24–31. doi: 10.1159/000050650. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Furukawa F, Toyoda K, Sato H, Hasegawa R, Imaida K, Hayashi Y. Effects of various prostaglandin synthesis inhibitors on pancreatic carcinogenesis in hamsters after initiation with N-nitrosobis(2-oxopropyl)amine. Carcinogenesis. 1990;11:393–395. doi: 10.1093/carcin/11.3.393. [DOI] [PubMed] [Google Scholar]
- 38.Wenger FA, Kilian M, Achucarro P, Heinicken D, Schimke I, Guski H, Jacobi CA, Muller JM. Effects of Celebrex and Zyfloon BOP-induced pancreatic cancer in Syrian hamsters. Pancreatology. 2002;2:54–60. doi: 10.1159/000049449. [DOI] [PubMed] [Google Scholar]
- 39.Hwang DH, Fung V, Dannenberg AJ. National Cancer Institute workshop on chemopreventive properties of nonsteroidal anti-inflammatory drugs: role of COX-dependent and -independent mechanisms. Neoplasia. 2002;4(2):91–97. doi: 10.1038/sj.neo.7900226. [DOI] [PMC free article] [PubMed] [Google Scholar]