Abstract
This work analyzes the hypothesis that human CD38 may cooperate with MHC Class II by acting as coreceptor in a superantigen-induced activation. The initial evidence is that CD38 ligation by specific monoclonal antibodies inhibits superantigen-induced T lymphocyte proliferation. Inhibitory effects become apparent after engagement of CD38 expressed by monocytes, whereas ligation of CD38 expressed by T lymphocytes does not apparently affect activation. The inhibition requires a cell-to-cell interaction, followed by the relevant transmembrane signaling that is reproduced by CD38 ligation. Indeed, CD38 ligation on monocytes induces tyrosine phosphorylation of several intracellular proteins including the protooncogene product c-cbl and the fgr and hck tyrosine kinases. The receptorial nature of the CD38-mediated events is confirmed by the observation of an intracellular calcium flux in monocytes secondary to CD38 ligation. These effects are additive with the similar events elicited by MHC Class II ligation, a likely indication that CD38 and MHC Class II share a common activation pathway. This conclusion is strengthened by results of comodulation experiments, indicating that CD38 and MHC Class II display lateral associations on monocytes. These results attribute to CD38 expressed by monocytes a role in the transduction of signal(s) involved in superantigen-induced activation, operating in synergy with MHC Class II.
Human CD38 is a 45-kDa transmembrane glycoprotein with a short amino-terminal cytoplasmic domain and a long carboxyl-terminal extracellular domain (1). Its expression is correlated with differentiation or activation of human T and B lymphocytes (2). Human, mouse, and rat CD38 molecules display amino acid sequences similar to that of Aplysia ADP ribosyl cyclase, an enzyme that catalyzes the formation of cyclic ADP ribose from NAD+1. CD38 catalyzes not only the hydrolysis of NAD+ but also the formation and hydrolysis of cyclic ADP ribose (3–6). A variety of cellular functions can be elicited by ligation of CD38 with specific mAbs (7–12). Comodulation experiments demonstrated that CD38 is physically associated with the CD3/TCR, BCR/CD19/CD21 and CD16 complexes on T, B, and NK cells, respectively (13).
Superantigens (Sag) constitute a peculiar family of ligands able to transduce signal via MHC Class II molecules (14). Sag are of viral (15, 16) or bacterial origin, such as the enterotoxins of Staphylococcus aureus or the toxic shock syndrome toxin (TSST-1). They share the characteristic ability of directly binding MHC Class II molecules (17) and TCR Vβ region (18). The formation of the trimolecular complex results in activation of all T lymphocytes bearing the particular TCR Vβ family. Although T cell activation by Sag was reported as being MHC Class II molecule dependent, the expression of MHC Class II molecules on some antigen-presenting cells is not always sufficient to induce a T cell response to the Sag (19–21), leading to the conclusion that costimulatory molecules are necessary in addition to the MHC Class II molecules to obtain an efficient presentation of Sag.
The present study provides conclusive evidence that CD38 expressed by peripheral blood monocytes cooperates with MHC Class II molecules as coreceptor in Sag-induced activation of T cells.
Materials and Methods
Antibodies.
CD38 mAbs used in this study were: IB4 (IgG2a) (2), SUN 4B7 (IgG1) (22), OKT10 (IgG1) (23), T16 (IgG1) (a gift of A. Martin (Etablissement de Transfusion Sanguine, Rennes, France) (24), and BB51 (IgG1) (25). Other mAbs used in this study were the CD2 mAb, O275 (IgG1) and the CD3 mAb, UCHT1 (IgG1), provided during the Vth International Workshop (Boston) (25). A. Bernard (Institut National de la Santé et de la Recherche Médicale U343, Nice, France) kindly provided the cytotoxic CD3 mAb. 12E7, an IgG1 CD99 mAb, was kindly provided by R. Levy (Howard Hughes Medical Institute, Stanford, CA) (26). The MHC Class II mAb used was the 1.35-mAb (IgG1) (27). The antiphosphotyrosine mAb, PY20, was purchased from Transduction Laboratories (Lexington, KY). The affinity-purified rabbit polyclonal Abs anti-c-Cbl, anti-fgr, and anti-hck were purchased from Santa Cruz Biotechnology. mAbs used to purify monocytes were the CD3 mAb, CBT3 (IgG2a), the CD19 mAb, CB19 (IgG1), the CD20 mAb, CB 20 (IgG1), and the CD16 mAb, CB16 (IgG1).
Cell Preparations.
Peripheral blood mononuclear cells (PBMC) were prepared by Ficoll–Paque (Pharmacia) density centrifugation of buffy coats obtained from the local blood bank. Purified T cells were obtained by the following negative selection procedure: two cycles of plastic adherence and one cycle of complement-dependent lysis with L1.L12 mAb (a cytotoxic anti-HLA Class II mAb, locally produced) and rabbit complement (Filorga, Paris). The remaining unlysed cells contained ≤0.5% CD14+ cells.
When indicated, T cells were further purified into CD38+ and CD38− subsets: T cells were initially labeled with the BB51 mAb (5 μg/ml) and then incubated with goat anti-mouse Ig-coated magnetic beads (Miltenyi Biotec, Bergisch Gladstadt, Germany). Labeled and unlabeled cells were separated by using a MACS system (Miltenyi Biotec) by two passages through a magnetic separation column.
Monocytes were purified from PBMC preparations by negative selection. Briefly, PBMC underwent two cycles of incubation (20 min at 4°C) with a mixture of CD19, CD20, CD3, and CD16 mAbs. These mAbs were previously conjugated to immunomagnetic beads (Dynal, Oslo; 2 μg of purified mAb/mg beads) by incubation at 4°C for 12 h. Conjugated beads were used with a ratio of 10 beads/1 cell. This approach enabled us to obtain monocytes ≥98% pure, as assessed by CD14 expression. Monocytes were also fixed by treatment with 1% paraformaldehyde for 20 min at room temperature, followed by three washes with culture medium. Fixed monocytes were kept at least 1 wk at 4°C before use. When indicated, monocytes were treated before fixation with mAb by incubation for 1 h at 4°C with the diluted relevant mAb.
Proliferation Assays.
Cells were cultured in RPMI-1640 medium (Biochrom, Berlin) supplemented with 2 mM l-glutamine/10 μg/ml penicillin/10 unit/ml streptomycin (GIBCO)/10% heat-inactivated FCS (Seromed, Biochrom). PBMC or purified T cells were cultured for 4 days in 96-well round-bottom plates (Nunc) at a concentration of 4 × 104 cells/well (total volume of 0.16 ml). Some experiments included fixed or irradiated autologous monocytes (8 × 103/well) mixed with T cells. Cultures were added with Staphylococcus enterotoxin A (SEA) (recombinant SEA, kindly provided by W. Mourad, Centre Hospitalier Universitaire, Laval, Québec) or TSST-1 (Toxin Technology, Sarasota, FL) at 1 ng/ml. mAbs were used at a concentration of 5 μg/ml. Proliferation was measured at day 4 as DNA synthesis by adding 1 μCi/well of [3H]thymidine (Amersham) for the last 8 h of culture, after which plates were harvested with a TOMTEC harvester (Tomtec, Orange, CT), and the radioactivity was measured in a scintillation counter (1450 Microbeta Plus, Wallac, Turku, Finland). All cultures were performed in triplicate. In all proliferation experiments, results of one experiment representative of five are expressed either as mean cpm or as percentage inhibition of T cell proliferation because of the addition of mAbs. SDs were typically <15%.
Analysis of Intracellular Calcium Flux.
The analysis was performed as previously reported (28). Briefly, unstimulated monocytes were loaded with indo-1 by incubation with its acetomethylester (Molecular Probes). Cells were kept in the dark before analysis on a cytofluorograph (Epics Elite ESP Flow Cytometer, Coultronics, Roissy, France), the ratio of indo-1 violet to blue fluorescence was calculated for each individual cell. Cells were exposed to the indicated mAb (10 μg/ml) followed by crosslinking with F(ab′)2 IgG preparation of a rabbit anti-mouse Ig serum (Cappel).
Determination of Protein Tyrosine Phosphorylation.
Purified monocytes were incubated with the indicated mAbs (10 μg/ml) for 10 min on ice. After washing, an F(ab′)2 fraction of goat anti-mouse IgG (Cappel) was added for 10 min as a crosslinker. Cells were then stimulated for 3 min at 37°C and immediately lysed in 0.5% Nonidet P-40 lysis buffer (29). After centrifugation to remove nuclei, an aliquot of lysates was diluted in Laemmli sample buffer, boiled for 5 min, and stored at −80°C before being run on SDS/PAGE. The remainder of lysates was used for either fgr or hck immunoprecipitations: lysates were precleared with recombinant protein A-Sepharose (Repligen) and subsequently incubated with either anti-fgr or anti-hck Abs. Immune complexes were recovered with recombinant protein A-Sepharose beads and eluted by boiling in SDS nonreducing sample buffer. Preparations obtained were run on an 8% SDS/PAGE and transferred on polyvinilydene difluoride membranes. After blocking in 1% BSA in Tris-buffered saline-Tween (10 mM Tris, pH 7.4/100 mM NaCl/0.1% Tween-20), filters were probed with PY20, an antiphosphotyrosine mAb, followed by horseradish peroxidase-conjugated anti-mouse IgG (Promega) and developed with enhanced chemiluminescent reagents (Amersham). Membranes were then stripped, blocked again with 5 to 2% nonfat dry milk in TBS-T, and reprobed with anti-c-Cbl, anti-hck, or anti-fgr Abs.
Antigenic Modulation.
Purified monocytes (1 × 106 cells/ml) were incubated in complete medium for 18 h at 37°C in the presence of an excess of mAb (20 μg/ml) of the indicated specificity. Cells were then washed, labeled with phycoerythrine (PE) conjugated CD38 or MHC Class II mAbs (Immunotech, Luminy, France) or with FITC-conjugated CD99 mAb (PharMingen). Cells were then analyzed by flow cytometry by using a FACScan flow cytometer (Becton Dickinson).
Results
Involvement of CD38 in the Activation of PBMC by Sag.
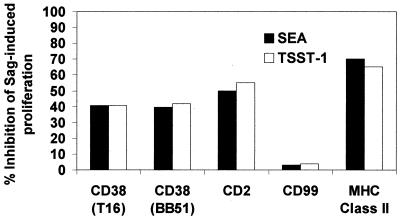
The results of the proliferative responses of PBMC to SEA and TSST-1 toxins in the presence of the selected mAbs are presented in Fig. 1. This proliferation is reported to be inhibited by mAbs directed against MHC Class II and CD2 (17, 30). The inhibitory effects observed in the present experiments were in the range of 65% for the MHC Class II and 50% for CD2 (Fig. 1). With CD38 mAbs, proliferation was reduced by ≈40%. The activation was constantly unaffected by the isotype-matched CD99 mAb.
Figure 1.
The proliferative response of PBMC to SEA and TSST-1 is inhibited by CD38 mAbs. PBMC (4 × 104 cells/well) were cultured with SEA or TSST-1 (1 ng/ml) in the presence of mAbs of the indicated specificity (5 μg/ml). [3H]thymidine incorporation measured at day 4 for PBMC in the absence of blocking mAb was 102.205 ± 11.242 cpm in SEA and 95.802 ± 8.622 cpm in TSST-1.
The inhibitory effects exerted by CD38 mAbs are specific (see above) and dose dependent (data not shown).
Evaluation of the Role of Surface T Cell CD38 in T Cell Proliferation Induced by Sag.
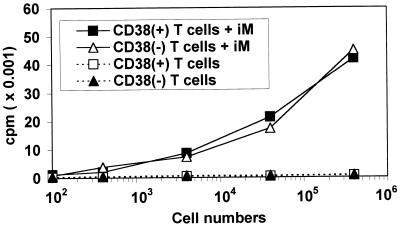
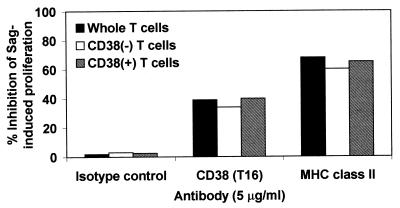
T lymphocytes were separated on the basis of CD38 expression. The CD38-enriched population (≥95% CD38+ cells) and the CD38-depleted population (≤6% CD38+ cells) was tested for their ability to respond to bacterial toxins in the presence of irradiated autologous monocytes. Both populations proliferate in response to SEA (Fig. 2). The proliferation of the CD38− T cells was quantitatively similar to that of the CD38+ T cells. Furthermore, the overlapping of the proliferation curves can be taken as an indication that CD38+ and CD38− T cells are equally responsive to the toxins. The addition of CD38 mAbs to the two populations was followed by an almost identical inhibition (≈35–40%) (Fig. 3). These results seem to exclude any detectable role played by surface CD38 expressed by T lymphocytes in the activation induced by Sag. Almost identical results were obtained when SEA was replaced by TSST-1 (data not shown).
Figure 2.
CD38− T lymphocytes proliferate in response to SEA. CD38+ or CD38− T cells were cultured by using different cell numbers, with or without irradiated autologous monocytes (8 × 103 cells/well) in the presence of SEA. Results are expressed as mean cpm measured at day 4. In all experiments, mean cpm of unstimulated CD38+ or CD38− T cells cultured in the presence of irradiated autologous monocytes was ≈0.6 × 103 cpm.
Figure 3.
Evaluation of the role of surface T cell CD38. Whole, CD38+, or CD38− purified T cells (4 × 104 cells/well) were cultured with irradiated monocytes (8 × 103 cells/well) with or without mAb of the indicated specificity (5 μg/ml) in the presence of SEA. [3H]thymidine incorporation measured at day 4 in the absence of blocking mAb was 40.710 ± 4.050 cpm for T cells, 45.100 ± 4.059 cpm for CD38− T cells, and 42.200 ± 4.642 cpm for CD38+ T cells.
Evaluation of the Role of Surface Monocyte CD38 in T Cell Proliferation Induced by Sag.
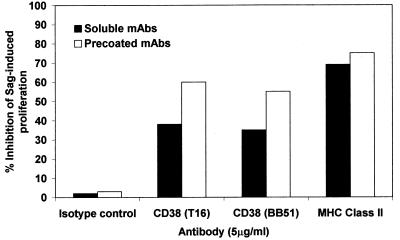
We next turned our attention to CD38 present on the surface of the majority of monocytes. Purified T cells were tested in the presence of fixed monocytes either pretreated or not with CD38 mAbs. Although purified T cells were unable to proliferate in response to the toxins, the addition of fixed monocytes allowed a partial restoration of the response (Fig. 4). The addition of CD38 mAbs in solution to purified T cells with fixed monocytes was followed by a significant inhibition of the proliferation. Similar results were obtained after using the control anti-MHC Class II mAb. The use of mAbs not in solution but prelinked to monocytes brought to a significantly higher degree of inhibition (60%). Almost identical results were obtained when SEA was replaced by TSST-1 (data not shown). The inhibition levels observed in these conditions were clearly greater than those observed by adding soluble mAb to the culture, suggesting that the direct interaction of the mAb with the CD38 molecule expressed by monocytes is responsible for the inhibition observed. The conclusion is that CD38 on the surface of monocytes is involved in the response to bacterial toxins.
Figure 4.
Evaluation of the role of surface monocyte CD38. Whole purified T cells (4 × 104 cells/well) were cultured with SEA in the presence of fixed monocytes (8 × 103 cells/well) with mAbs (soluble or precoated) of the indicated specificity. [3H]thymidine incorporation measured at day 4 for T cells in the presence of fixed monocytes was 30.547 ± 2.596 cpm in the absence of blocking mAb.
CD38 Signaling on Monocytes Induced Ca2+ Mobilization.
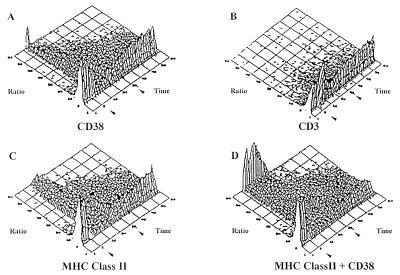
The results obtained prompted us to test the hypothesis that surface CD38 may act by delivering signals of biological relevance. Indeed, CD38 ligation by T16 mAb on CD14+ cells purified from PBMC was followed by a rapid mobilization of Ca2+ that involved ≈40% of the cells (Fig. 5A). The isotype-matched CD3 mAb induced no effect, ruling out the participation of FcR (Fig. 5B) The calcium flux was not modified by the subsequent crosslinking of the CD38 molecule by a rabbit antimurine Ig serum. Signaling through the MHC Class II molecule induced almost identical effects on Ca2+: a rapid Ca2+ flux that involved ≈50% of the CD14+ cells (Fig. 5C). Additive effects were observed when CD14+ cells were simultaneously exposed to CD38 and MHC Class II mAbs: ≈60% of the cells mobilized Ca2+ (Fig. 5D). Identical results were obtained when the T16 mAb was replaced by the BB51 mAb (data not shown).
Figure 5.
An intracellular calcium flux is mediated via CD38 on monocytes. Purified monocytes were loaded with indo-1, washed, and tested for calcium mobilization. After measurement of basal level, mAbs of the following specificity were added (first arrow): (A) CD38, (B) CD3, (C) anti-MHC Class II, (D) CD38 + anti-MHC Class II. After 5 min, these were crosslinked by addition of a rabbit anti-mouse F(ab′)2 fragment at a predetermined concentration known not to mobilize intracellular calcium (second arrow).
CD38 Signaling on Monocytes Induced Tyrosine Phosphorylation of Cytoplasmic Substrates. Involvement of the Protooncogene Product c-Cbl.
The results obtained with the analysis of Ca2+ fluxes suggested to us that we extend the observation to the phosphorylation of cytoplasmic substrates elicited on CD38 signaling.
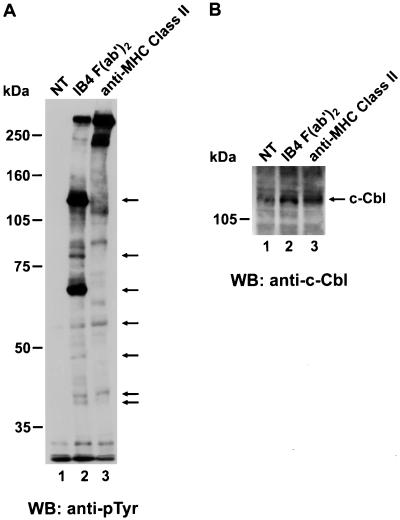
A phosphotyrosine immunoblot of lysates from purified monocytes is shown in Fig. 6. We compared the effects of CD38 and MHC Class II engagement on tyrosine phosphorylation. To exclude FcR contribution, a F(ab′)2 preparation of the IB4 mAb was used for CD38 stimulation. On CD38 ligation, several substrates became phosphorylated, as indicated by arrows. Among those substrates, the upper bands at ≈120, 84, and 70 kDa were the more prominent. Moreover, they appeared to be selectively phosphorylated after CD38, but not MHC Class II triggering. However the two pathways share the 59-, 50-, 43-, and 41-kDa substrates that appeared phosphorylated, although to a lesser extent, after either CD38 or MHC Class II engagement. Thus, CD38 delivered a tyrosine-phosphorylation signal even more potent that the one highlighted through MHC Class II stimulation. Unstimulated cells showed no significant tyrosine phosphorylation, thus confirming that no improper trigger was given during the purification procedure and adding specificity to the signals obtained with the mAbs of interest.
Figure 6.
Protein tyrosine phosphorylation is induced via CD38 on human monocytes. (A) Purified monocytes were either unstimulated (lane 1) or stimulated with the appropriate mAb. Cells were then lysed and subjected to SDS/PAGE before transferring to nitrocellulose membranes and immunoblotting with antiphosphotyrosine mAb. The same quantity of protein was loaded in each well. Molecular mass markers are indicated. (B) Filter shown in A was stripped and reprobed with an anti-Cbl Ab. The position of Cbl is indicated by an arrow.
The upper 120-kDa band showed an apparent molecular weight compatible with that of the protooncogene product c-cbl. This substrate plays a pivotal role in signal transduction via CD38 in HL60 (31) and THP-1 (32) cells. Indeed, reprobing of the upper part of the filter with a specific anti-c-cbl Ab clarified the identity of the 120-kDa band as c-cbl (Fig. 6B). Moreover, the protein was highlighted in all lanes, confirming that equal amounts of proteins were loaded in each well and that the 120-kDa band observed with the antiphosphotyrosine mAb on CD38-stimulated cells was a highly phosphorylated c-cbl species.
CD38 Ligation Induces Tyrosine Phosphorylation of the hck and fgr Tyrosine Kinases.
We next focused our phosphorylation studies on two substrates that play a key role in monocyte signaling, namely fgr (33) and hck (34). These proteins are tyrosine kinases of the src family known to be phosphorylated after MHC Class II engagement (35). Indeed, phosphorylated bands were visualized around 59–50 kDa, a molecular weight compatible with these two proteins. Moreover, a suggestive pattern of tyrosine phosphorylation was constantly observed after CD38 ligation with different CD38 mAbs (Fig. 7): OKT10, Sun 4B7, IB4 F(ab′)2 and BB51. This pattern was also visualized when monocytes were activated with the anti-MHC Class II mAb.
Figure 7.
Protein tyrosine phosphorylation of substrates around 55–59 kDa is induced on human monocytes with different CD38 mAbs. Purified monocytes were either unstimulated or stimulated with the indicated mAbs. Cells were then lysed and subjected to SDS/PAGE before transferring to nitrocellulose membranes and immunoblotting with antiphosphotyrosine mAb. The same quantity of protein was loaded in each well. Molecular mass markers are indicated.
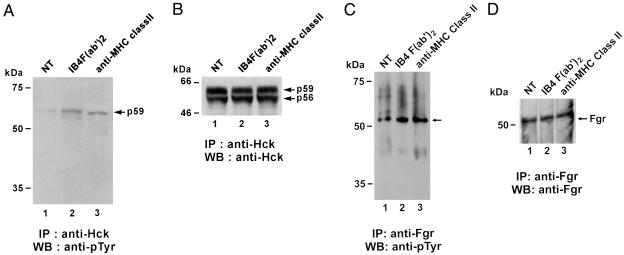
To investigate the role of hck, a lysate of monocytes (activated with F(ab′)2 IB4 or MHC Class II mAbs or untreated) was immunoprecipitated with a specific anti-hck Ab, followed by Western blot analysis with an anti-pTyr mAb. The p59 isoform was tyrosine phosphorylated after CD38 or MHC class II engagements (Fig. 8A). Both p56 and p59 isoforms of hck are equally immunoprecipitated from lysates of unstimulated cells, CD38-activated cells, or MHC Class II-activated cells (Fig. 8B).
Figure 8.
CD38 ligation on human monocytes induces tyrosine phosphorylation of the fgr and hck tyrosine kinases. After incubation with either no mAb, CD38 mAb, F(ab′)2 IB4, or anti-MHC Class II mAb, purified monocytes were lysed and immunoprecipitates prepared by using either an anti-hck Ab (A and B) or an anti-fgr Ab (C and D). After blotting, filters were probed with an antiphosphotyrosine mAb (A and C). The filter shown in A was stripped and reprobed with an anti-hck Ab revealing identical amounts of hck in all samples (B). The filter shown in C was stripped and reprobed with an anti-fgr Ab revealing identical amounts of fgr in all samples (D).
The same analysis was performed on fgr. The addition of the IB4 F(ab′)2 fraction or the MHC Class II mAbs to monocytes was followed by a significant tyrosine phosphorylation of fgr (Fig. 8C). Subsequent reprobing with anti-fgr Ab (Fig. 8D) confirmed the identity of the highlighted bands and the equal loading in each lane.
CD38 Comodulates with MHC Class II in Monocytes.
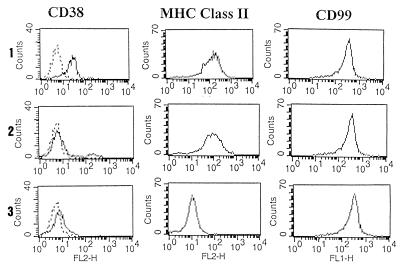
The synergistic effects on cytoplasmic Ca2+ fluxes after CD38 and MHC Class II signalings and the phosphorylation of similar cytosolic substrates prompted us to study the existence on monocytes of a physical association between the two molecules. The expression of CD38 was significantly reduced after surface modulation of MHC Class II molecules (Fig. 9). In these conditions, the expression of the CD99 molecule remained identical to that of untreated cells. However, surface modulation of the CD38 molecule did not modify the expression of the MHC Class II molecule on monocytes.
Figure 9.
The CD38 expression is reduced by surface modulation of MHC Class II molecules. The expression of CD38 and MHC Class II molecules was analyzed on purified monocytes incubated for 18 h in medium alone (1), with an excess of BB51 mAb (2) or with an excess of D1.12 mAb (3). The fluorescence was analyzed on a FACScan flow cytometer with logarithmic amplification. x-axis, fluorescence intensity/cells; y-axis, number of cells/channel. Number of cells analyzed, 10.000.
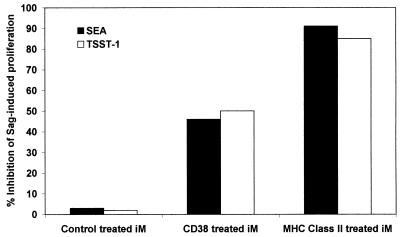
After modulating the surface expression of CD38 and MHC Class II, monocytes were tested for their ability to support Sag-induced T cell activation. As already shown in Fig. 3, purified T cells were able to proliferate in response to the toxins when untreated irradiated monocytes were added. In contrast, the MHC Class II-treated monocytes (which are CD38− MHC Class II−, as shown in Fig. 9) were unable to restore the T cell response to the Sag (Fig. 10). The response was ≈90% reduced as compared with the levels observed in presence of untreated monocytes. The addition of CD38-treated monocytes (which are CD38− MHC Class II+, as shown in Fig. 9) was followed by only a partial restoration of the proliferative response, in line with the inhibition results observed after exposure to a CD38 mAb.
Figure 10.
Effect of CD38 and MHC class II modulation on the Sag-induced T cell proliferation. Whole purified T cells (4 × 104 cells/well) were cultured with SEA (1 ng/ml) in the presence of irradiated monocytes (8 × 103 cells/well) pretreated or not by CD38 or MHC class II mAbs as described in Fig. 9. [3H]thymidine incorporation measured at day 4 for T cells in the presence of untreated irradiated monocytes was 54.410 ± 5.275 cpm.
Discussion
The starting point of this work was the search of the coaccessory molecule necessary to implement a valid signal during T cell activation by Sag. The candidate coreceptor we took into consideration was CD38, a surface molecule with pleiotropic functions (12, 31, 32, 36–41).
The first results obtained indicated that CD38 ligation on PBMC was followed by a slight but constant and significant inhibition of the proliferative response to SEA and TSST-1. This initial finding appears contradictory to previous results demonstrating costimulatory effects exerted by CD38 mAbs on T cell proliferation induced via CD3 (7). Indeed, the inhibitory effects observed in this model after CD38 ligation are restricted to the response to Sag, because no such inhibitory effect was detected on the PHA or CD3-induced proliferation (data not shown).
Moreover, the present results clearly demonstrate that the inhibition is mediated via the CD38 molecule on the surface of monocytes, as demonstrated by the absence of T cell activation in the presence of fixed autologous monocytes pretreated with a CD38 mAb and by the equivalent response of the CD38+ and CD38− T cell populations to the toxins. It thus appears that the ligation of the CD38 molecule expressed by monocytes is responsible for the inhibitory effects observed initially.
The effects are likely to be mediated by the signals implemented by the engagement of CD38, as shown by the increased intracellular Ca2+ fluxes and by the tyrosine phosphorylation observed when the molecule expressed by monocytes is ligated by specific mAbs.
Our results clearly indicate that several intracellular substrates became phosphorylated on CD38 ligation, one of the more prominent being the protooncogene product, c-Cbl. This result is in agreement with independent studies demonstrating that c-Cbl is tyrosine phosphorylated after CD38 engagement in T cells (29), B cells (40), and myeloid cells (31, 32). Among the many others proteins that display increased tyrosine phosphorylation in activated monocytes, we next focused our attention on the substrates activated via either CD38 or MHC Class II. Monocytes express several src-type phosphotyrosine kinases, some of which are relatively restricted to myelomonocytic cells, e.g., hck (33) and fgr (34). Besides highlighting the phosphorylation cascade elicited by CD38 in freshly purified monocytes, the present study offers evidence that these two pivotal kinases, hck and fgr, are involved in the CD38-mediated signaling in the myeloid lineage. Indeed, the analysis of the signals mediated via CD38 in myeloid cell lines (31) has shown the tyrosine phosphorylation of three major phosphorylated proteins of 120, 87, and 77 kDa. However, these observations were made by using HL60 cells, where CD38 expression was induced after treatment with retinoic acid. Consequently, the fgr- and hck-phosphorylated proteins may be distinctive markers of normal and uncultured monocytes exposed to CD38 signaling. Involvement of fgr and hck in MHC Class II-mediated signals has been reported after stimulation of human peripheral blood monocytes with a Sag (35). In agreement with these results, we have observed that the p59 isoform of hck is the predominantly phosphorylated form.
The results obtained indicate that CD38 plays a role as a coreceptor in the activation of T lymphocytes induced by Sag and is endowed with the ability to transduce signals efficiently across the membrane in fresh monocytes. The first conclusion confirms previous reports that hypothesized the presence of unknown surface molecules cooperating with MHC Class II molecules (19–21). In this context, the CD38 monocyte molecule would play the role of an accessory molecule providing the second signal in monocytes necessary for a full Sag-induced T cell activation. However, we cannot actually exclude the hypothesis of another, yet to be defined, essential monocyte costimulatory molecule that would be down-regulated after CD38 signaling. The second conclusion on the receptorial nature of CD38 leads us to depict a likely scenario of cooperative interactions occurring on the surface of monocytes. Indeed, the cytoplasmic portion of the molecule is inept by itself in transducing signals: the only possibility so far devised is the lateral association with a structure specialized in signaling. One of the unique features of the CD38 molecule is that such associations vary according to the lineage (13). Inoue et al. hypothesized that a candidate molecule on monocytes could be FcRII (42). However, the data obtained in the present work seem to support the view that in the Sag model the role of FcR is negligible or secondary. The molecule that CD38 “parasites” for its signaling is MHC Class II, as confirmed by the phosphorylation of similar cytosolic substrates and by Ca2+ mobilization with identical timing and distribution. Furthermore, we observed marked additive effects between the pathways driven by MHC Class II and CD38, suggesting the use of common substrates. The observation that CD38 molecules may comodulate with MHC Class II molecules favors the hypothesis that these molecules may be physically and functionally associated on monocytes.
Another partner that is expected to contribute to the network is CD38-ligand, characterized as CD31 (43). It is reported that CD38/CD31 interaction reproduces the biological effects attributed to CD38 ligation with agonistic CD38 mAbs, including Ca2+ release and cytokine mRNA synthesis (37).
The plot drawn by these results will be resolved when the contribution of all these receptors and ligands will be singularly investigated and defined by means of soluble CD38 and CD31 and by transfectants expressing the human molecules.
Acknowledgments
This work was supported by Association pour la Recherche sur le Cancer (Villefuif, France), Institut National de la Santé et de la Recherche Médicale (Paris), Assistance Publique-Hôpitaux de Paris (Paris), Associazione Italiana per la Ricerca sul Cancro (Milan), and Telethon (Rome). S.G. was supported by a fellowship from the M. Merieux Fondation (Lyon, France). S.D. is a student of the Postgraduate School of Oncology, University of Torino Medical School, Torino, Italy.
Abbreviations
- Sag
Superantigen, SEA, Staphylococcus enterotoxin A
- TSST-1
toxic shock syndrome toxin 1
- PBMC
peripheral blood mononuclear cells
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050583197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050583197
References
- 1.Jackson D G, Bell J I. J Immunol. 1990;144:2811–2817. [PubMed] [Google Scholar]
- 2.Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 3.Howard M, Grimaldi J C, Bazan J F, Lund F E, Santos A L, Parkhouse R M, Walseth T F, Lee H C. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 4.Takasawa S, Tohgo A, Noguchi N, Komuga T, Nata K, Sugimoto T, Yonekura H, Okamoto H. J Biol Chem. 1993;268:26052–26054. [PubMed] [Google Scholar]
- 5.Deterre P, Gelman P, Gary-Gouy H, Arrieumerlou C, Berthelier V, Tixier J-M, Ktorza S, Goding J, Schmitt C, Bismuth G. J Immunol. 1996;157:1381–1388. [PubMed] [Google Scholar]
- 6.Hoshino S, Kukimoto I, Kontani K, Inoue S, Kanda Y, Malavasi F, Katada T. J Immunol. 1997;158:741–747. [PubMed] [Google Scholar]
- 7.Funaro A, Spagnoli G C, Ausiello C M, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F. J Immunol. 1990;145:2390–2396. [PubMed] [Google Scholar]
- 8.Santos A L, Teixera C, Preece G, Kirkham P A, Parkhouse R M. J Immunol. 1993;151:3119–3130. [PubMed] [Google Scholar]
- 9.Dianzani U, Funaro A, DiFranco D, Garbarino G, Bragardo M, Redoglia V, Buonfiglio D, De M L, Pileri A, Malavasi F. J Immunol. 1994;153:952–959. [PubMed] [Google Scholar]
- 10.Zupo S, Rugari E, Dono M, Taborelli G, Malavasi F, Ferrarini M. Eur J Immunol. 1994;24:1218–1222. doi: 10.1002/eji.1830240532. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai M, Coustan S E, Murray D J, Silvennoinen O, Murti K G, Evans W E, Malavasi F, Campana D. J Exp Med. 1995;181:1101–1110. doi: 10.1084/jem.181.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ausiello C M, Urbani F, La Sala A, Funaro A, Malavasi F. Eur J Immunol. 1995;25:1477–1480. doi: 10.1002/eji.1830250554. [DOI] [PubMed] [Google Scholar]
- 13.Funaro A L, De Monte L B, Dianzani U, Forni M, Malavasi F. Eur J Immunol. 1993;23:2407–2411. doi: 10.1002/eji.1830231005. [DOI] [PubMed] [Google Scholar]
- 14.Marrack P, Kappler J. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 15.Lafon M, Lafage M, Martinez-Arends A, Ramirez R, Vuillier F, Charron D, Lotteau V, Scott-Algara D. Nature (London) 1992;358:507–510. doi: 10.1038/358507a0. [DOI] [PubMed] [Google Scholar]
- 16.Acha-Orbea H, Held W, Waanders G A, Shakov A N, Scarpellino L, Lees R K, McDonald H R. Immunol Rev. 1993;131:5–25. doi: 10.1111/j.1600-065x.1993.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher H, Dohlsten M, Lindvall H, Sjögren H, Carlsson R. J Immunol. 1989;142:3151–3157. [PubMed] [Google Scholar]
- 18.Seth A, Stern L J, Ottenhoff T H, Engel I, Owen M J, Lamb J R, Klausner R D, Wiley D C. Nature (London) 1994;369:324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- 19.Rott O, Tontsh U, Fleischer B. J Immunol. 1993;150:87–95. [PubMed] [Google Scholar]
- 20.Nickoloff B J, Mitra R S, Green J, Zheng X G, Shimizu Y, Thompson C, Turka L A. J Immunol. 1993;150:2148–2159. [PubMed] [Google Scholar]
- 21.Yagi J, Uchiyama U, Janeway C A. J Immunol. 1994;152:1154–1162. [PubMed] [Google Scholar]
- 22.Deaglio S, Dianzani U, Horestein A L, Fernandez J E, Van Kooten C, Bragardo M, Garbarino G, Funaro A, DiVirgilio, Banchereau J, Malavasi F. J Immunol. 1996;156:727–734. [PubMed] [Google Scholar]
- 23.Reinherz E L, Kung P C, Goldstein G, Levey R H, Schlossman S F. Proc Natl Acad Sci USA. 1980;77:1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knapp W, Dörken B, Gilks W R, Rieber E P, Schmidt R E, Stein H, vom dem Borne A E. Leucocyte Typing IV. Oxford: Oxford Univ. Press; 1989. p. 1182. [Google Scholar]
- 25.Schlossman S F, Boumsell L, Gilks W, Harlan J M, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, et al. Leucocyte Typing V. Oxford: Oxford Univ. Press; 1995. p. 2023. [Google Scholar]
- 26.Levy R, Dalley J, Fox R I, Warnke R. Proc Natl Acad Sci USA. 1979;76:6552–6556. doi: 10.1073/pnas.76.12.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charron D J, Mc Dewitt H O. J Exp Med. 1980;152:18–36. [Google Scholar]
- 28.Grynkiewicz G, Poenie M, Tsein T. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 29.Zubiaur M, Izquierdo M, Terhorst C, Malavasi F, Sancho J. J Immunol. 1997;159:193–205. [PubMed] [Google Scholar]
- 30.Mittrücker H W, Fleisher B. Cell Immunol. 1992;139:108–117. doi: 10.1016/0008-8749(92)90104-w. [DOI] [PubMed] [Google Scholar]
- 31.Kontani K, Kukimoto I, Nishina H, Hoshino S, Hazeki O, Kanaho Y, Katada T. J Biol Chem. 1996;271:1534–1537. doi: 10.1074/jbc.271.3.1534. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo T, Hazeki K, Tsujimoto N, Inoue S, Kurosu H, Kontani K, Hazeki O, Ui M, Katada T. FEBS Lett. 1996;397:113–116. doi: 10.1016/s0014-5793(96)01151-9. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler S F, Marth J C, Lewis D B, Perlmutter R M. Mol Cell Biol. 1987;7:2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ley T J, Connolly N L, Katamine S, Cheah M S C, Senior M R M, Robbins K C. Mol Cell Biol. 1989;9:92–99. doi: 10.1128/mcb.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morio T, Geha R S, Chatila T. Eur J Immunol. 1994;24:651–658. doi: 10.1002/eji.1830240325. [DOI] [PubMed] [Google Scholar]
- 36.Cesano A, Visonneau S, Deaglio S, Malavasi F, Santoli D. J Immunol. 1998;160:1106–1115. [PubMed] [Google Scholar]
- 37.Deaglio S, Morra M, Mallone R, Ausiello C M, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- 38.Morra M, Zubiaur M, Terhorst C, Sancho J, Malavasi F. FASEB J. 1998;12:581–592. doi: 10.1096/fasebj.12.7.581. [DOI] [PubMed] [Google Scholar]
- 39.Kitanaka A, Ito C, Nishigaki H, Campana D. Blood. 1996;88:590–598. [PubMed] [Google Scholar]
- 40.Silvennoinen O, Nishigali H, Kitanaka A, Kumagai M, Ito C, Malavasi F, Lin Q, Conley M E, Campana D. J Immunol. 1996;156:100–107. [PubMed] [Google Scholar]
- 41.Kitanaka A, Ito C, Coustan-Smith E, Campana D. J Immunol. 1997;159:184–192. [PubMed] [Google Scholar]
- 42.Inoue S, Kontani K, Tsujimoto N, Kanda Y, Hosoda N, Hoshino S, Hazeki O, Katada T. J Immunol. 1997;159:5226–5232. [PubMed] [Google Scholar]
- 43.Stockinger H, Gadd S J, Eher R, Madjic O, Schreiber W, Kazinrerk W, Straa B, Schnabl E, Knapp W. J Immunol. 1990;145:3889–3897. [PubMed] [Google Scholar]