Abstract
Contrary to the widespread belief that stress is necessarily immunosuppressive, recent studies have shown that, under certain conditions, stress can induce a significant enhancement of a skin cell-mediated immune response [delayed-type hypersensitivity (DTH) or contact hypersensitivity]. Adrenal stress hormones and a stress-induced trafficking of leukocytes from the blood to the skin have been identified as systemic mediators of this immunoenhancement. Because γ interferon (IFNγ) is an important cytokine mediator of DTH, the studies described here were designed to examine its role as a local mediator of the stress-induced enhancement of skin DTH. The effect of acute stress on skin DTH was examined in wild-type and IFNγ receptor-deficient (IFNγR−/−) mice that had previously been sensitized with 2,4-dinitro-1-fluorobenzene. Acutely stressed wild-type mice showed a significantly larger DTH response than nonstressed mice. In contrast, IFNγR−/− mice failed to show a stress-induced enhancement of skin DTH. Immunoneutralization of IFNγ in wild-type mice significantly reduced the stress-induced enhancement of skin DTH. In addition, an inflammatory response induced by direct IFNγ administration to the skin was significantly enhanced by acute stress. Our results suggest that IFNγ is an important local mediator of a stress-induced enhancement of skin DTH. These studies are clinically relevant because, depending on the nature of the antigen, DTH reactions mediate numerous protective (e.g., resistance to viral, bacterial, parasitic, and fungal infections) or pathological (e.g., autoimmune reactions and contact sensitivity reactions such as that to poison ivy) immune responses.
Stress is term that defines a constellation of events, which begins with a stimulus (stressor), which results in the recognition of threat or danger (stress perception), which then activates physiologic systems within the body (stress response) (1, 2). A stress response involves the release of neurotransmitters and hormones that serve as the brain's signaling molecules to the body. The consequences of this response are generally adaptive in the short run (1, 3) but can be damaging when stress is chronic (1, 4).
Stress is suspected to play a role in the etiology of many diseases, and numerous elegant studies have shown that stress can be immunosuppressive and, hence, may be detrimental to health (5–12). However, we have suggested that, under certain conditions, a stress response may enhance immune function and prepare the immune system for challenges (e.g., wounding or infection) that may be imposed by a stressor, just as a stress response prepares the nervous, cardiovascular, musculoskeletal, and neuroendocrine systems for fight or flight (1, 3, 13–15). Recent studies have shown that, in contrast to chronic stress, which is immunosuppressive (1), acute stress (1–4 h duration) can significantly enhance skin immune function (1–3). A stress-induced trafficking of leukocytes from the blood to the skin has been identified as one of the factors mediating this immunoenhancement (3).
Based on these data, we propose a model positing cellular and molecular mechanisms by which acute stress may enhance skin immunity (Fig. 1) (1). According to this model, acute stress increases immune surveillance by inducing circulating leukocytes to marginate within the vasculature of organs, such as the skin. That is, without “knowing” exactly where an immune challenge may occur, a stress response induces a selective retention of leukocytes within the vasculature of compartments (e.g., skin and gastrointestinal and urogenital tracts), which may be compromised by a stressor (e.g., wounding, eating, or mating). If the stress signal is accompanied by an immune challenge (e.g., wounding or infection), the stressed organism is poised to mount a more robust immune response by virtue of having higher numbers of leukocytes at the site of challenge. Thus, stress hormones and changes in leukocyte distribution within the body are global/systemic mediators of a stress-induced enhancement of immune function. However, to mount an effective immune response, marginated leukocytes must be recruited from the luminal surface of the vascular endothelium into the site of antigen entry. Inflammatory mediators, such as cytokines and chemokines released at the site of antigen entry, recruit and activate leukocytes. These molecules may serve as local mediators of a stress-induced enhancement of immune function.
Figure 1.
Mechanisms by which acute stress may enhance skin immunity. Stage 1: The brain detects the presence of a stressor and informs/warns the body by releasing stress hormones. Stage 2: Stress hormones increase the affinity/expression of adhesion molecules on leukocytes and/or endothelial cells. Stage 3: This results in a selective margination of leukocytes within the vasculature of organs such as the skin. Stage 4a: Upon cessation of stress, and in the absence of immunologic challenge at the site of leukocyte margination, leukocytes demarginate and join the circulating leukocyte pool (14). Stage 4b: However, if the stressor is accompanied by an immune challenge at the site of leukocyte margination, leukocytes are recruited by chemokines and cytokines released at the site of antigen entry. Thus, a stressed organism, by virtue of having higher numbers of leukocytes available for recruitment at potential sites of challenge, may mount a more robust immune response than an unstressed organism. Stage 5: In addition to affecting leukocyte distribution, acute stress may increase immunopreparedness by increasing inflammatory mediator production/function and by enhancing antigen presentation, leukocyte recruitment, leukocyte activation, and effector cell function within the site of an immune reaction. Thus, stress hormones and changes in leukocyte distribution within the body may be systemic mediators, and cytokines and chemokines local mediators, of a stress-induced enhancement of immune function.
Delayed-type hypersensitivity (DTH) falls under the type IV category in the Coombs and Gell classification of hypersensitivity. Skin-specific DTH reactions are also known as contact sensitivity reactions and involve two phases: The first phase is the sensitization or immunization phase, which involves a primary response resulting in the formation of memory T cells. The second phase is the challenge or elicitation phase, which involves a secondary response involving antigen-presenting cells, T cells, neutrophils, and macrophages. We have observed an immunoenhancement when acute stress or adrenal stress hormones are administered before either sensitization (16) or challenge (1–3).
Interferon (IFN)γ plays an important role in the development of different stages of DTH. It increases the efficiency of antigen presentation by inducing the expression of antigen-presenting molecules on endothelial cells, macrophages, and keratinocytes. IFNγ also enhances leukocyte recruitment and activation by increasing adhesion molecule expression on leukocytes, endothelial cells, and keratinocytes. Moreover, IFNγ is a potent activator of macrophages, which are the critical effector cells in a DTH response (17). Therefore, the studies described here were designed to examine IFNγ as a local mediator of a stress-induced enhancement of skin DTH.
Materials and Methods
Animals.
Wild-type (B6×129) and IFNγ receptor-deficient (IFNγR−/−) mice (Hoffmann–La Roche) were bred and housed in plastic cages in the accredited (American Association for Accreditation of Laboratory Animal Care) animal facilities of The Rockefeller University (New York). The generation of IFNγR−/− mice has been described previously (18). Young adult male mice (20–30 g) were maintained on a 12-h light-dark cycle (lights on 7 a.m.) and given mouse chow and water ad libitum.
Induction of DTH.
On day 1, animals were anesthetized with the inhalant methoxyflurane (Metofane; Pitman–Moore, Washington Crossing, NJ). An area of approximately 2 × 3 cm was shaved on the dorsum. Animals were numbered (tail marking) and weighed, and the thickness of both ears was recorded with a constant-loading dial micrometer (Mitutoyo, Tokyo). Animals were sensitized on days 1 and 2, by administration of 25 μl of 2,4-dinitro-1-fluorobenzene (DNFB; 0.5% in 4:1 acetone/olive oil) (Research Organics) to the shaved skin. On day 5, baseline thickness of both pinnae was measured. On day 6, experimental animals were subjected to restraint for 2.5 h. Control animals were left undisturbed in their home cages. The dorsal surface of the right pinnae of all animals was challenged with 20 μl of DNFB (0.2% in 4:1 acetone/olive oil), whereas left pinnae were treated as controls. The thickness of both pinnae was measured at the times shown, and data were plotted as a percent increase in pinna thickness relative to baseline. Measurements were made gently and rapidly and on the same relative region of the pinna across different animals and treatment groups. The immune reaction thus induced is characterized by swelling at the site of challenge, and by an infiltration of macrophages and lymphocytes into the epidermis and dermis (19, 20). A positive correlation between the increase in pinna thickness and the ongoing immune response has been reported (21, 22), and this method has been widely used to monitor cell-mediated immune responses in vivo (19, 23).
Stress.
Animals were placed in adequately ventilated wire mesh restrainers for 2.5 h, while ensuring that they were not squeezed or compressed. Restraint stress is thought to be largely psychological in nature, resulting from the perception of confinement on part of the animal (24, 25). Restraint activates the autonomic nervous system (26) and the hypothalamic–pituitary–adrenal axis (27, 28) and results in the activation of adrenal steroid receptors in tissues throughout the body (28).
Leukocyte Analysis.
Blood samples for all leukocyte analyses were obtained from the same group of animals. White blood cell counts and lymphocyte–neutrophil differentials were obtained on a hematology analyzer (F800; Sysmex, McGraw Park, IL). Specific leukocyte subtypes were identified by immunofluorescent antibody staining by using three-color flow cytometry (FACScan; Becton Dickinson). Neutrophils and monocytes were identified by using forward vs. side scatter and anti-CD11b-FITC-positive staining (clone M1/70; PharMingen). L-selectin-positive cells were identified by using anti-CD62L-PE (MEL-14; PharMingen; PE, phycoerythrin). Briefly, cell suspensions were incubated with antibody for 20 min at room temperature, washed with PBS, and read on the FACScan with 3,000–5,000 events being acquired from each preparation. Appropriate isotype controls were used to set negative staining criteria. Data were analyzed with cellquest software (Becton Dickinson).
Immunoneutralization.
Monoclonal rat anti-mouse IFNγ antibody (clone R4-6A2, courtesy of A. Satoskar, Harvard School of Public Health) was administered by i.p. injection (360 μg per mouse, 200 μl of PBS/1% mouse serum) 1.5 h before the beginning of the stress session. Vehicle-treated animals were injected with an equivalent quantity of rat IgG (Sigma). Animals were returned to their home cages after anti-body administration.
Recombinant IFNγ Administration.
Recombinant murine IFNγ (BioSource International, Camarillo, CA) was administered to each pinna (0.1 μg in 10 μl of saline, per ear). A 1-mm2 area at the periphery of the pinna was gently but rapidly pierced 20 times with a 26-gauge needle, and the IFNγ solution was applied to the pierced area. Control animals were treated with saline.
Corticosterone Radioimmunoassay.
Blood was collected in heparinized Microfuge tubes by the tail-clip method (14, 15, 27). Plasma was obtained by centrifugation and stored at −70°C. Corticosterone was measured by radioimmunoassay (sensitivity 5.7 ng/ml) by using an RIA kit (Diagnostic Products, Los Angeles). The antiserum is highly specific for rat corticosterone and has very low crossreactivity to other steroids.
Data Analysis.
Each experiment was repeated at least twice. Significant differences between groups were determined by using the Student's t test. Data are expressed as mean ± SEM in all figures. A computer statistics program was used for analyses (systat v5.2.1; Systat, Evanston, IL).
Results
The studies described here were conducted to examine the role of IFNγ as a local mediator of a stress-induced enhancement of skin DTH. These studies examined the effects of stress on skin DTH during states of chronic or acute IFNγ deficiency. They also examined the effects of stress on the magnitude of inflammation after direct administration of IFNγ to the skin.
The first series of experiments examined the effects of acute stress on skin DTH in wild-type and IFNγR−/− mice. We hypothesized that if IFNγ were an important mediator of a stress-induced enhancement of skin DTH, the immunoenhancing effect of stress would be significantly diminished in IFNγR−/− mice. Fig. 2 demonstrates that wild-type mice showed a significant stress-induced enhancement of skin DTH (n = 5; ∗, P < 0.05; ∗∗, P < 0.005; independent t test). The DTH response of stressed animals occurred at a faster rate, attained a higher peak, and remained significantly elevated for 6 days after antigen challenge. In contrast, IFNγR−/− mice failed to show the immunoenhancing effects of acute stress. These results suggested that IFNγ was a potential mediator of an acute stress-induced enhancement of skin DTH.
Figure 2.
Effects of stress on the DTH response of wild-type (A) and IFNγR−/− (B) mice. Control animals were left undisturbed while stressed animals were restrained for 2.5 h. The right pinnae of all animals were then challenged with DNFB (0.2%). A time course of changes in the thickness of challenged pinnae is shown. Acute stress significantly enhanced the DTH response of wild-type mice (n = 5, *, P < 0.05; **, P < 0.005, independent t test). In contrast, IFNγR−/− mice failed to show a stress-induced enhancement of skin DTH.
We have previously shown that acute stress induces an initial increase followed by a decrease in blood leukocyte numbers (29) and that these changes represent a significant redistribution of leukocytes within the body (3, 14, 30). We have also identified a stress-induced increase in plasma corticosterone, and a stress-induced redistribution of blood leukocytes, as systemic mediators of an acute stress-induced enhancement of skin DTH (1–3). Therefore, it was important to compare stress-induced changes in plasma corticosterone and blood leukocyte numbers between wild-type and IFNγR−/− mice.
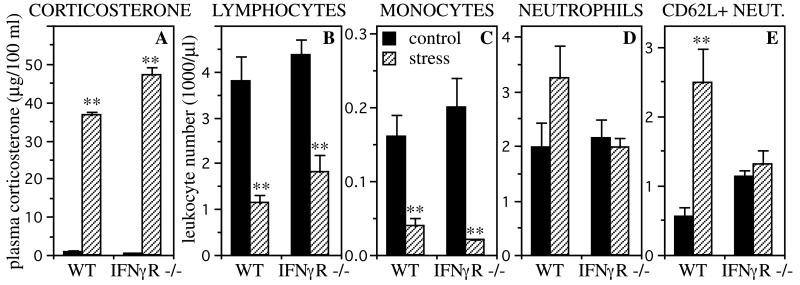
Fig. 3 shows that wild-type and IFNγR−/− mice showed a robust corticosterone stress response and a robust stress-induced decrease of blood lymphocytes (70% and 58%, respectively; P < 0.005, independent t test) and monocytes (76% and 88%, respectively; P < 0.005, independent t test). Wild-type mice also showed a stress-induced increase (65% higher than baseline) in blood neutrophil numbers, but IFNγR−/− mice failed to show this response. Moreover, the stress-induced neutrophilia in wild-type mice was largely the result of an increase in l-selectin (CD62-L)-positive neutrophils (P < 0.005, independent t test).
Figure 3.
Stress-induced changes in plasma corticosterone levels (A) and blood leukocyte numbers (B–E). Control animals were left undisturbed while stressed animals were restrained for 2.5 h. Wild-type (WT) and IFNγR−/− mice showed a robust stress-induced increase in plasma corticosterone (n = 3, A), and a robust stress-induced decrease in blood lymphocyte (B) and monocyte (C) numbers (n = 5). Wild-type mice also showed a stress-induced increase in blood neutrophil numbers (n = 5, D), which was particularly prominent for CD62L+ neutrophils (E). IFNγR−/− mice failed to show this stress-induced neutrophilia (n = 5). Statistically significant differences are indicated (**, P < 0.005, independent t test).
The experiments described above suggested that IFNγ was an important mediator of an acute stress-induced enhancement of skin DTH. However, it is necessary to exercise caution when using gene knockout mice for examining protein function in vivo. This is because both the developmental and permissive functions of the “knocked out” gene are eliminated in these animals, and this may significantly alter the overall physiology of the animal.
Therefore, our next set of studies examined the effects of short-term immunoneutralization of IFNγ on the magnitude of a stress-induced enhancement of skin DTH in wild-type animals. Fig. 4 shows the effects of acute stress on skin DTH responses of wild-type mice injected with either vehicle or anti-IFNγ antibody. Vehicle-injected animals showed a significant stress-induced enhancement of skin DTH (n = 5, P < 0.05, independent t test). In contrast, animals injected with anti-IFNγ failed to show this enhancement (n = 3).
Figure 4.
Effects of immunoneutralization of IFNγ on a stress-induced enhancement of skin DTH in wild-type mice. Animals were injected with rat IgG (A) or anti-IFNγ (B). Then, 1.5 h after antibody administration, control animals were left undisturbed, while stressed animals were restrained for 2.5 h. The right pinnae of all animals were then challenged with DNFB (0.2%). A time course of changes in the thickness of challenged pinnae is shown. Vehicle-injected wild-type mice showed a significant stress-induced enhancement of skin DTH (n = 5; *, P < 0.05, independent t test). In contrast, wild-type mice injected with anti-IFNγ antibody failed to show this enhancement (n = 3).
Figs. 2 and 4 showed that the magnitude of a stress-induced enhancement of skin DTH was virtually eliminated in the absence of IFNγ. These findings led us to predict that if IFNγ was a mediator of the stress-induced enhancement of skin immunity, acute stress may enhance the magnitude of an inflammatory response after direct administration of IFNγ to the skin of wild-type animals. Fig. 5 shows that acute stress did indeed enhance an inflammatory response induced by direct administration of recombinant IFNγ to the skin (n = 9, P < 0.05, independent t test). Taken together, these results identify IFNγ as an important local mediator of a stress-induced enhancement of skin immunity.
Figure 5.
Effects of acute stress on an inflammatory response to direct administration of IFNγ to the skin of wild-type animals. Animals were not sensitized for this experiment. Control animals were left undisturbed, while stressed animals were restrained for 2.5 h. Mouse recombinant IFNγ was then administered to the right pinnae of all animals. A time course of changes in pinna thickness is shown. Stressed animals showed a significant enhancement of inflammation at the site of IFNγ administration (n = 9; *, P < 0.05). Stressed animals also showed an enhancement of inflammation after saline administration, although the overall response to saline was lower than that observed following IFNγ administration (n = 9; *, P < 0.05).
Discussion
Contrary to the widespread belief that stress is necessarily immunosuppressive, increasing evidence suggests that stress and stress hormones have important immunomodulatory effects (31, 32) and in some cases may even enhance immune function (2, 33). For example, stress or stress hormones have been shown to enhance cell-mediated (1–3, 34, 35) humoral (36–38) and innate (39, 40) immunity. As summarized in Fig. 1, our studies have shown that the stress hormones epinephrine and corticosterone (2) and changes in blood leukocyte distribution (1, 3) are systemic or global mediators of a stress-induced enhancement of immune function. The studies described here show that IFNγ is an important local mediator of an acute stress-induced enhancement of skin immunity. While the roles played by adrenal stress hormones (2) and by changes in leukocyte distribution have been discussed previously (1–3, 14), here we have focused on the role of IFNγ in mediating a stress-induced enhancement of skin DTH.
Given the importance of IFNγ as a mediator of DTH, we initially expected a dampened baseline DTH response in IFNγR−/− mice. Surprisingly, we observed few differences in baseline DTH responses between wild-type mice and mice that were either chronically (IFNγR−/−) or acutely (immunoneutralized) deficient in IFNγ. Other studies have similarly reported that baseline DTH responses are unaffected by deficiencies of either the IFNγR (41) or IFNγ (42, 43). Thus, the elimination of IFNγ function had no effect on baseline DTH responses but specifically resulted in an elimination of the stress-induced enhancement of skin DTH. This suggests that, although other immune mechanisms may compensate for IFNγ deficiency with respect to mounting a baseline DTH response, IFNγ is critically involved in mediating an acute stress-induced enhancement of skin DTH.
How might stress hormones and IFNγ interact to mediate an enhancement of skin immunity? Numerous studies support the idea that, in addition to directing leukocyte traffic to the skin, acute stress may enhance skin DTH by potentiating the actions of IFNγ locally at the site of an immune response. IFNγ mRNA (44) and protein (45) are up-regulated in skin DTH reactions, and stress hormones have been shown to enhance the biological effects of various cytokines, including IFNγ (31, 32). Moreover, acute stress has been shown to induce IL-18 gene expression (46), and because IL-18 is a potent inducer of IFNγ, this finding suggests that acute stress may enhance immune function by increasing the production of IFNγ itself. Importantly, glucocorticoid treatment has been shown to induce an increase in IFNγ receptors on human monocytes (47), and this may account for some of the potentiating effects of stress on different aspects of IFNγ function.
Stress hormones may thus potentiate IFNγ induction of immune processes, such as antigen presentation, leukocyte recruitment, leukocyte activation or effector cell function. With respect to antigen presentation, catecholamine stress hormones have been shown to up-regulate MHC class I and potentiate IFNγ-induced up-regulation of MHC class II molecules on endothelial cells (48). Thus, antigen presentation may be one stage of DTH during which stress hormones and IFNγ interact to enhance immune function.
However, because inflammation after direct IFNγ administration to the skin of nonsensitized, wild-type animals is not likely to involve antigen presentation, the results described in Fig. 5 suggest that IFNγ may also mediate a stress-induced enhancement of skin DTH by enhancing the recruitment, activation, and/or effector function of leukocytes. With respect to leukocyte recruitment, numerous studies have shown that intradermal administration of IFNγ to normal human skin (in doses similar to those applied in our studies) induces a significant increase in the expression of cell adhesion molecules, such as L-selectin (CD62-L), lymphocyte function-associated antigen (LFA-1), and intercellular adhesion molecule (ICAM-1), on endothelial cells (49, 50), leukocytes (51, 52), and keratinocytes (53), with ICAM-1 expression occurring first on endothelial cells and later on keratinocytes (54). With respect to leukocyte activation, low levels of corticosterone have been shown to potently enhance the early stages of T cell activation and T lymphocyte proliferation (55). With respect to effector cell function, stress hormones have been shown to enhance macrophage production of acute phase proteins (56), complement factors (57), macrophage migration inhibitory factor (MIF) (58), and sialoadhesin (59). Moreover, glucocorticoid hormones have been shown to act synergistically with IFNγ to induce Fcγ receptors on human monocytic cell lines (60, 61) and peritoneal macrophages (62) and glucocorticoid induction of high-affinity Fcγ receptor expression on human monocytes and mouse macrophages is correlated with increased phagocytosis (61). Thus, stress hormones may enhance skin immunity by potentiating IFNγ actions at different stages of a DTH response.
The time course of our experiments and the kinetics of the observed results suggest that some effects of acute stress may occur within the first few hours after the beginning of a DTH response. This is because our experiments involve a single 2.5-h stress session followed immediately by a single antigen challenge, and most stress-induced changes in physiologic parameters return to baseline within 3 h after the cessation of stress (14). Interestingly, neutrophils constitute the early wave of leukocyte entry into the site of a DTH response (17, 63) and IFNγR−/− mice, which fail to show a stress-induced neutrophilia in the blood, also fail to show a stress-induced enhancement of skin DTH. We suggest that the delayed neutrophilia observed toward the end of an acute stress session may increase circulating neutrophil numbers and make more cells available at sites of immune activation. The lack of a stress-induced neutrophilia in IFNγR−/− mice may contribute to their failure to show an enhancement of skin DTH. However, our studies using wild-type mice demonstrated that immunoneutralization of IFNγ also eliminates the stress-induced enhancement of DTH (Fig. 4), and that acute stress enhances the magnitude of inflammation after direct administration of IFNγ to the skin (Fig. 5). Because wild-type mice show a robust stress-induced neutrophilia, the results described in Figs. 4 and 5 suggest that a stress-induced neutrophilia alone is not sufficient for mediating a stress-induced enhancement of skin DTH.
Taken together, the preceding discussion shows that stress hormones and IFNγ may act synergistically to enhance different immune reactions, such as antigen presentation, leukocyte recruitment, leukocyte activation, and effector cell function, ultimately resulting in an enhanced immune response.
Studies showing bidirectional effects of stress on immune function (1, 2) help address two paradoxical issues: First, it is paradoxical that organisms would have evolved to suppress immune function at a time when an active immune response may be critical for survival, for example, under conditions of stress when an organism may be injured or infected by the actions of the stress-inducing agent (e.g., an attacking predator). Second, on the one hand, stress is thought to suppress immunity and increase susceptibility to infections and cancer (7, 9, 64–66), while on the other, it is thought to exacerbate inflammatory diseases like psoriasis, asthma, and arthritis (which should be ameliorated by a suppression of immune function) (67–71). The finding that depending on specific physiologic conditions stress can either enhance or suppress immune function may help reconcile these paradoxical observations (1, 2).
These studies have important clinical implications because DTH reactions are antigen-specific, cell-mediated immune responses, which, depending on the antigen, mediate protective (e.g., resistance to viral, bacterial, parasitic, and fungal infections) or pathological (e.g., autoimmune reactions, poison ivy sensitivity, contact sensitivities to metals and chemicals) immune responses. Thus, a stress-induced enhancement of DTH would be beneficial to the host if the response were directed against a pathogen or a vaccine antigen, and harmful if it were directed against innocuous (poison ivy, metal allergies) or self (in case of autoimmune reactions) antigens. Moreover, intradermal IFNγ administration has been used for the treatment of diseases such as leprosy (72, 73) and leishmaniasis (74). Our experiments showing a stress-induced enhancement of inflammation after IFNγ administration to the skin suggest that the stress status of the patient may significantly affect the efficacy of treatment, with acute stress perhaps enhancing, and chronic stress reducing, treatment effectiveness.
In conclusion, these studies suggest that IFNγ is an important local mediator of an acute stress-induced enhancement of skin DTH. Because stress plays a role in the etiology of many diseases, a determination of the mechanisms by which the nervous, endocrine, and immune systems interact to enhance or suppress immune responses may help alleviate diseases thought to be affected by stress. It is hoped that further elucidation of these mechanisms will lead to the development of treatments designed to harness an individual's physiology to selectively enhance (during vaccination, wounding, infections, or cancer) or suppress (during autoimmune or inflammatory disorders) an immune response, depending on what would be most beneficial for the patient.
Acknowledgments
We thank John Sheridan, Phil Marucha, David Padgett, and Ning Quan for their helpful suggestions during the preparation of this manuscript. This work was supported by The John D. and Catherine T. MacArthur Foundation.
Abbreviations
- DNFB
2,4-dinitro-1-fluorobenzene
- DTH
delayed-type hypersensitivity
- IFNγ
γ interferon
- IFNγR
IFNγ receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050569397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050569397
References
- 1.Dhabhar F S, McEwen B S. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 2.Dhabhar F S, McEwen B S. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhabhar F S, McEwen B S. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 4.McEwen B S. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 5.Khansari D N, Murgo A J, Faith R E. Immunol Today. 1990;11:170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 6.Maier S F, Watkins L R, Fleshner M. Am Psychol. 1994;49:1004–1017. doi: 10.1037//0003-066x.49.12.1004. [DOI] [PubMed] [Google Scholar]
- 7.Kiecolt-Glaser J K, Glaser R, Gravenstein S, Malarkey W B, Sheridan J. Proc Natl Acad Sci USA. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marucha P T, Kiecolt-Glaser J K, Favagehi M. Psychosomat Med. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan J F. Brain Behav Immun. 1998;12:1–6. doi: 10.1006/brbi.1998.0521. [DOI] [PubMed] [Google Scholar]
- 10.Padgett D A, Marucha P T, Sheridan J F. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Herbert R B. Annu Rev Psychol. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- 12.Irwin M, Patterson T, Smith T L, Caldwell C, Brown S A, Gillin C J, Grant I. Biol Psychiatry. 1990;27:22–30. doi: 10.1016/0006-3223(90)90016-u. [DOI] [PubMed] [Google Scholar]
- 13.Dhabhar F S, Miller A H, Stein M, McEwen B S, Spencer R L. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- 14.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- 15.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 16.Dhabhar F S, McEwen B S. Neuroimmunomodulation. 1999;6:213. [Google Scholar]
- 17.Abbas A K, Lichtman A H, Pober J S. Cellular and Molecular Immunology. Philadelphia: Saunders; 1991. [Google Scholar]
- 18.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 19.Turk J L. Delayed Hypersensitivity. Amsterdam: Elsevier; 1980. [Google Scholar]
- 20.Malorny U, Goebeler M, Gutwald J, Roth J, Sorg C. Int Arch Allergy Appl Immunol. 1990;92:356–360. doi: 10.1159/000235164. [DOI] [PubMed] [Google Scholar]
- 21.Phanuphak P, Moorhead J W, Claman H N. J Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- 22.Kimber I, Dearman R. J Pharmacol Toxicol Methods. 1993;29:11–16. doi: 10.1016/1056-8719(93)90045-g. [DOI] [PubMed] [Google Scholar]
- 23.Thorne P S, Hawk C, Kaliszweski S D, Guiney P D. Fundam Appl Toxicol. 1991;17:790–806. doi: 10.1016/0272-0590(91)90186-8. [DOI] [PubMed] [Google Scholar]
- 24.Berkenbosch F, Wolvers D A, Derijk R. J Steroid Biochem Mol Biol. 1991;40:639–647. doi: 10.1016/0960-0760(91)90286-e. [DOI] [PubMed] [Google Scholar]
- 25.Glavin G B, Paré W P, Sandbak T, Bakke H-K, Murison R. Neurosci Biobehav Rev. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 26.Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein D S, Kopin I J. Endocrinology. 1993;133:1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- 27.Dhabhar F S, McEwen B S, Spencer R L. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- 28.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Neuroimmunol. 1995;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- 29.Dhabhar F S, McEwen B S. Neuroimmunomodulation. 1999;6:213. [Google Scholar]
- 30.Dhabhar F S. In: Neuroimmunomodulation: Molecular, Integrative Systems, and Clinical Advances. McCann S M, Lipton J M, Sternberg E M, Chrousos G P, Gold P W, Smith C C, editors. Vol. 840. New York: New York Acad. Sci.; 1998. pp. 359–372. [Google Scholar]
- 31.Wilckens T, DeRijk R. Immunol Today. 1997;18:418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 32.Wiegers G J, Reul J M H M. Trends Pharmacol Sci. 1998;19:317–321. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 33.Spencer R L, Kalman B A, Dhabhar F S. In: Handbook of Physiology: Coping with the Environment. McEwen B S, editor. Oxford: Oxford Univ. Press; 2000. , in press. [Google Scholar]
- 34.Blecha F, Barry R A, Kelley K W. Proc Soc Exp Biol Med. 1982;169:239–246. doi: 10.3181/00379727-169-41338. [DOI] [PubMed] [Google Scholar]
- 35.Wood P G, Karol M H, Kusnecov A W, Rabin B S. Brain Behav Immun. 1993;7:121–134. doi: 10.1006/brbi.1993.1014. [DOI] [PubMed] [Google Scholar]
- 36.Persoons J H A, Berkenbosch F, Schornagel K, Thepen T, Kraal G. J Allergy Clin Immunol. 1995;95:765–770. doi: 10.1016/s0091-6749(95)70184-2. [DOI] [PubMed] [Google Scholar]
- 37.Solomon G F. Int Arch Allergy. 1969;35:97–104. doi: 10.1159/000230163. [DOI] [PubMed] [Google Scholar]
- 38.Cocke R, Moynihan J A, Cohen N, Grota L J, Ader R. Brain Behav Immun. 1993;7:36–46. doi: 10.1006/brbi.1993.1004. [DOI] [PubMed] [Google Scholar]
- 39.Lyte M, Nelson S G, Baissa B. Physiol Behav. 1990;48:685–691. doi: 10.1016/0031-9384(90)90212-m. [DOI] [PubMed] [Google Scholar]
- 40.Shurin M R, Kusnecov A, Hamill E, Kaplan S, Rabin B S. Brain Behav Immun. 1994;8:163–169. doi: 10.1006/brbi.1994.1015. [DOI] [PubMed] [Google Scholar]
- 41.Saulnier M, Huang S, Aguet M, Ryffel B. Toxicology. 1995;102:301–312. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- 42.Emery D L, Davey R J. Immunol Cell Biol. 1995;73:146–152. doi: 10.1038/icb.1995.23. [DOI] [PubMed] [Google Scholar]
- 43.Thomson J A, Troutt A B, Kelso A. Immunology. 1993;78:185–192. [PMC free article] [PubMed] [Google Scholar]
- 44.Szepietowski J C, McKenzie R C, Keohane S G, Aldridge R D, Hunter J A. Br J Dermatol. 1997;137:195–200. doi: 10.1046/j.1365-2133.1997.18051888.x. [DOI] [PubMed] [Google Scholar]
- 45.Chu C Q, Field M, Andrew E, Haskard D, Feldmann M, Maini R N. Clin Exp Immunol. 1992;90:522–529. doi: 10.1111/j.1365-2249.1992.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conti B, Jahng J W, Tinti C, Son J H, Joh T H. J Biol Chem. 1997;272:2035–2037. doi: 10.1074/jbc.272.4.2035. [DOI] [PubMed] [Google Scholar]
- 47.Strickland R W, Wahl L M, Finbloom D S. J Immunol. 1986;137:1577–1580. [PubMed] [Google Scholar]
- 48.Bourdoulous S, Durieu-Trautmann O, Strosberg A D, Couraud P O. J Immunol. 1993;150:1486–1495. [PubMed] [Google Scholar]
- 49.Groves R W, Allen M H, Barker J N, Haskard D O, MacDonald D M. Br J Dermatol. 1991;124:117–123. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 50.Spertini O, Luscinskas F W, Kansas G S, Munro J M, Griffin J D, Gimbrone M A, Jr, Tedder T F. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- 51.Gilhar A, Aizen E, Pillar T, Eidelman S. J Am Acad Dermatol. 1992;27:710–716. doi: 10.1016/0190-9622(92)70243-9. [DOI] [PubMed] [Google Scholar]
- 52.Momose T, Okubo Y, Horie S, Takashi S, Tsukadaira A, Suzuki J, Isobe M, Sekiguchi M. Int Arch Allergy Immunol. 1999;120:30–33. doi: 10.1159/000053590. [DOI] [PubMed] [Google Scholar]
- 53.Barker J N, Allen M H, MacDonald D M. J Invest Dermatol. 1989;93:439–442. doi: 10.1111/1523-1747.ep12284016. [DOI] [PubMed] [Google Scholar]
- 54.Buchsbaum M E, Kupper T S, Murphy G F. J Cutan Pathol. 1993;20:21–27. doi: 10.1111/j.1600-0560.1993.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 55.Wiegers G J, Labeur M S, Stec I E, Klinkert W E, Holsboe R F, Reul J M. J Immunol. 1995;155:1893–1902. [PubMed] [Google Scholar]
- 56.Baumann H, Firestone G L, Burgess T L, Gross K W, Yamamoto K R, Held W A. J Biol Chem. 1983;258:563–570. [PubMed] [Google Scholar]
- 57.Lappin D F, Whaley K. Biochem J. 1991;280:117–123. doi: 10.1042/bj2800117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelley T, Cerami A, Bucala R. Nature (London) 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 59.van den Berg T K, van Die I, de Lavalette C R, Dopp E A, Smit L D, van der Meide P H, Tilders F J H, Crocker P R, Dijkstra C D. J Immunol. 1996;157:3130–3138. [PubMed] [Google Scholar]
- 60.Girard M T, Hjaltadottir S, Fejes-Toth A N, Guyre P M. J Immunol. 1987;138:3235–3241. [PubMed] [Google Scholar]
- 61.Warren M K, Vogel S N. J Immunol. 1985;134:2462–2469. [PubMed] [Google Scholar]
- 62.Kizaki T, Oh-Ishi S, Ookawara T, Yamamoto M, Izawa T, Ohno H. Endocrinology. 1996;137:4260–4267. doi: 10.1210/endo.137.10.8828485. [DOI] [PubMed] [Google Scholar]
- 63.Buchanan K L, Murphy J W. Immunology. 1997;90:189–197. doi: 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Eliyahu S, Yirmiya R, Liebeskind J C, Taylor A N, Gale R P. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 65.Cohen S, Tyrrell D A J, Smith A P. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 66.Glaser R, Pearl D K, Kiecolt-Glaser J K, Malarkey W B. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 67.Solomon G F, Moos R H. Arch Gen Psychiatry. 1964;11:657–669. doi: 10.1001/archpsyc.1964.01720300087011. [DOI] [PubMed] [Google Scholar]
- 68.Mei-Tal V, Meyerowitz S, Engel G. Psychosom Med. 1970;32:67–86. doi: 10.1097/00006842-197001000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Thomason B T, Brantley P J, Jones G N, Dyer H R, Morris J L. J Behav Med. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- 70.Pawlak C, Heiker H, Witte T, Wiese B, Heijnen C J, Schmidt R E, Schedlowski M. Neuroimmunomodulation. 1999;6:241. [Google Scholar]
- 71.Al'Abadie M S, Kent G G, Gawkrodger D J. Br J Dermatol. 1994;130:199–203. doi: 10.1111/j.1365-2133.1994.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 72.Nathan C F, Kaplan G, Levis W R, Nusrat A, Witmer M D, Sherwin S A, Job C K, Horowitz C R, Steinman R M, Cohn Z A. N Engl J Med. 1986;315:6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- 73.Kaplan G, Mathur N K, Job C K, Nath I, Cohn Z A. Proc Natl Acad Sci USA. 1989;86:8073–8077. doi: 10.1073/pnas.86.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harms G, Zwingenberger K, Chehade A K, Talhari S, Racz P, Mouakeh A, Douba M, Nakel L, Naiff R D, Kremsner P G, et al. Lancet. 1989;i:1287–1292. doi: 10.1016/s0140-6736(89)92686-x. [DOI] [PubMed] [Google Scholar]