Acute renal failure is characterised by a rapid fall in glomerular filtration rate, clinically manifest as an abrupt and sustained rise in urea and creatinine. Life threatening consequences include volume overload, hyperkalaemia, and metabolic acidosis. Acute renal failure is both common and costly and carries a high morbidity and mortality. As it is often preventable, identification of patients at risk and institution of appropriate preventive measures are crucial. In incipient or established acute renal failure rapid recognition and treatment may prevent irreversible loss of nephrons.
In most cases of acute renal failure initial management is by non-specialist clinicians, often comparatively junior ones. All clinicians should therefore be able to recognise the symptoms and signs of acute renal failure, request and interpret initial investigations, initiate appropriate treatment, and know when, and how urgently, to consult a more experienced colleague or specialist. This review highlights the common causes of acute renal failure, defines the population at risk, evaluates established and newer strategies for prevention and treatment, and identifies those patients who warrant early referral.
Who gets acute renal failure?
Acute renal failure is increasingly common, particularly in elderly people, although reported incidences vary according to the definition used and the population studied. In 1993 a community based study found an incidence of severe acute renal failure (serum creatinine > 500 μmol/l) of 172 per million adults per year, of whom 72% were over 70.1 Age related incidence rose from 17 per million per year in adults under 50 to 949 per million per year in the 80-89 age group. More recent prospective studies report an overall incidence of acute renal failure of almost 500 per million per year2,3 and an incidence of acute renal failure needing dialysis of more than 200 per million per year.4 This is double the UK incidence of end stage renal disease needing dialysis5 and places high demands on healthcare resources.
Acute renal failure accounts for 1% of hospital admissions and complicates more than 7% of inpatient episodes,6,7 mostly in patients with underlying chronic kidney disease. When the condition is severe enough to need dialysis in-hospital mortality is around 50%, and it may exceed 75% in the context of sepsis or in critically ill patients.3,4,8
Summary points
Acute renal failure is increasingly common, particularly in hospital inpatients, elderly people, and critically ill patients, and it carries a high mortality
The most common cause of in-hospital acute renal failure is acute tubular necrosis resulting from multiple nephrotoxic insults such as sepsis, hypotension, and use of nephrotoxic drugs or radiocontrast media
Patients at risk include elderly people; patients with diabetes, hypertension, or vascular disease; and those with pre-existing renal impairment
No drug treatment has been shown to limit the progression of, or speed up recovery from, acute renal failure
Advice from a nephrologist should be sought for all cases of acute renal failure
What causes acute renal failure?
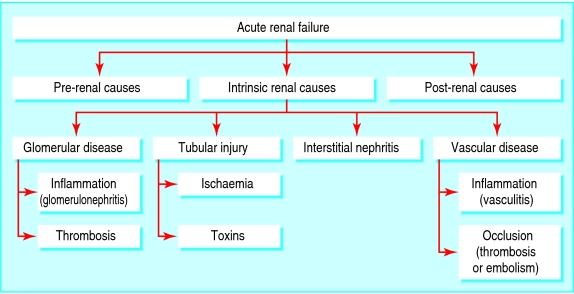
The causes of acute renal failure can be broadly grouped into three major categories (fig 1). These are decreased renal blood flow (pre-renal causes; 40-70% of cases6,9), direct renal parenchymal damage (intrinsic renal causes; 10-50% of cases6,10), and obstructed urine flow (post-renal or obstructive causes; 10% of cases10).
Fig 1.
Causes of acute renal failure
Sources and selection criteria
I searched PubMed with the terms “acute renal failure”, “prevalence”, “epidemiology”, “hospital acquired”, and “mortality”. I mainly selected publications from 2000 onwards but did not exclude earlier commonly referenced and highly regarded publications. I consulted the reference lists of articles and reviews identified by this strategy, and I also referred to my personal library of articles on acute renal failure accumulated from periodic PubMed searches. I used the Cochrane Library to identify relevant systematic reviews that evaluate the effectiveness of current interventions. I also used bmjlearning.com as a source for clinically relevant information on benefits and harms of treatments.
Box 1: Principal pre-renal causes of acute renal failure
Hypovolaemia
Haemorrhage
Volume depletion (for example, vomiting, diarrhoea, inappropriate diuresis, burns)
Renal hypoperfusion
Non-steroidal anti-inflammatory drugs/selective cyclo-oxygenase 2 inhibitors
Angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists
Abdominal aortic aneurysm
Renal artery stenosis/occlusion
Hepatorenal syndrome
Hypotension
Cardiogenic shock
Distributive shock (for example, sepsis, anaphylaxis)
Oedematous states
Cardiac failure
Hepatic cirrhosis
Nephrotic syndrome
Pre-renal failure (box 1)
Changes in pre-glomerular and post-glomerular arteriolar resistance enable renal blood flow and glomerular filtration rate to remain roughly constant across a wide range of mean arterial pressures. However, below a mean arterial pressure of 70 mm Hg autoregulation is impaired and glomerular filtration rate falls proportionately. Renal autoregulation chiefly depends on a combination of pre-glomerular arteriolar vasodilatation, mediated by prostaglandins and nitric oxide, and post-glomerular arteriolar vasoconstriction, mediated by angiotensin II. Drugs that interfere with these mediators—namely, non-steroidal anti-inflammatory drugs or selective cyclo-oxygenase 2 inhibitors, and angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists—may provoke pre-renal acute renal failure in particular clinical settings. People at high risk include elderly patients with atherosclerotic cardiovascular disease, patients with pre-existing chronic kidney disease, and patients with renal hypoperfusion, caused by volume depletion, hypotension, or renal artery stenosis, for example.
“Intrinsic” renal failure (box 2)
Intrinsic acute renal failure may be caused by diseases affecting the glomeruli, renal tubules, interstitium, or vasculature. Overall, the most common cause is acute tubular necrosis, resulting from continuation of the same pathophysiological processes that lead to pre-renal hypoperfusion. Intrinsic acute renal failure is often multifactorial; in intensive care the most common cause is sepsis, often accompanied by multi-organ failure.11 Postoperative acute tubular necrosis accounts for up to 25% of cases of hospital acquired acute renal failure, mostly resulting from prerenal causes.12 The third most common cause of hospital acquired acute renal failure is acute radiocontrast nephropathy.13
Post-renal failure (box 3)
Obstructive nephropathy presents as acute renal failure relatively infrequently but is important to recognise, as rapid diagnosis and prompt intervention can result in improvement or even complete recovery of renal function. At risk populations include older men with prostate disease and patients with intraabdominal, particularly pelvic, malignancy. An important clinical consequence is the substantial diuresis that generally occurs once obstruction is relieved, which needs careful monitoring and appropriate fluid replacement to avoid volume depletion.
Box 2: Principal causes of intrinsic renal acute renal failure
Glomerular disease
Inflammatory—post-infectious glomerulonephritis, cryoglobulinaemia, Henoch-Schonlein purpura, systemic lupus erythematosus, antineutrophil cytoplasmic antibody associated glomerulonephritis, anti-glomerular basement membrane disease
Thrombotic—disseminated intravascular coagulopathy, thrombotic microangiopathy
Interstitial nephritis
Drug induced—non-steroidal anti-inflammatory drugs, antibiotics
Infiltrative—lymphoma
Granulomatous—sarcoidosis, tuberculosis
Infection related—post-infective, pyelonephritis
Tubular injury
Ischaemia—prolonged renal hypoperfusion
Toxins—drugs (such as aminoglycosides), radiocontrast media, pigments (such as myoglobin), heavy metals (such as cisplatinum)
Metabolic—hypercalcaemia, immunoglobulin light chains
Crystals—urate, oxalate
Vascular
Vasculitis (usually associated with antineutrophil cytoplasmic antibody)
Cryoglobulinaemia
Polyarteritis nodosa
Thrombotic microangiopathy
Cholesterol emboli
Renal artery or renal vein thrombosis
Box 3: Principal post-renal causes of acute renal failure
Intrinsic
Intra-luminal—stone, blood clot, papillary necrosis
Intra-mural—urethral stricture, prostatic hypertrophy or malignancy, bladder tumour, radiation fibrosis
Extrinsic
Pelvic malignancy
Retroperitoneal fibrosis
Can acute renal failure be prevented?
The key preventive strategy is to identify people at risk. These include elderly patients; patients with diabetes, hypertension, or vascular disease; and those with pre-existing renal impairment. Appropriate preventive measures include maintaining adequate blood pressure and volume status and avoiding potentially nephrotoxic agents, particularly non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, or angiotensin II receptor blockers, as previously discussed.
Among the many causes of acute renal failure, radiocontrast nephropathy is potentially preventable.14 In high risk patients, alternative imaging methods should be considered where possible. Intravascular volume depletion, a key risk factor, should be corrected by appropriate volume expansion with intravenous saline. Oral use of the antioxidant N-acetylcysteine has been widely assessed with conflicting results,15,16 and its role remains uncertain. It is an inexpensive agent without significant side effects, however, and its use in clinical practice may not therefore be inappropriate.
How do I assess a patient with acute renal failure?
The diagnostic approach to a patient with acute renal failure requires a careful history, scrutiny of the case notes and drug charts, thorough physical examination, and interpretation of appropriate investigations, including laboratory tests and imaging (fig 2).
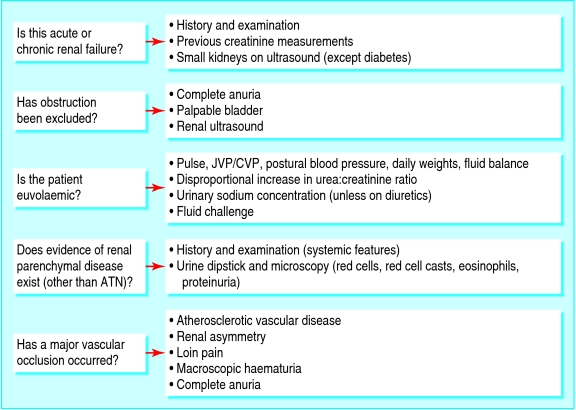
Fig 2.
Differential diagnosis of acute renal failure. ATN=acute tubular necrosis; JVP/CVP=jugular venous pressure/central venous pressure
Is this acute or chronic renal failure?
Distinguishing between acute and chronic renal failure is of paramount importance, as the approach to these patients differs greatly and this may, in addition, save a great deal of unnecessary investigation. Factors that suggest chronicity include long duration of symptoms, nocturia, absence of acute illness, anaemia, hyperphosphataemia, and hypocalcaemia, although similar laboratory findings may complicate acute renal failure. The most useful clue comes from previous creatinine measurements if these can be found. Reduced renal size and cortical thickness on ultrasonography is characteristic of chronic renal failure, although renal size is typically preserved in patients with diabetes.
Has obstruction been excluded?
Careful urological evaluation is mandatory if the cause of acute renal failure is not otherwise apparent. This includes inquiry about previous stones or symptoms of bladder outflow obstruction and palpation for a palpable bladder. Complete anuria suggests renal tract obstruction and is otherwise unusual in acute renal failure. Renal ultrasonography is the preferred method to detect dilatation of the renal pelvis and calyces, although obstruction may be present without dilatation,17 particularly in malignancy.
Is the patient euvolaemic?
Low venous pressure and a postural fall in blood pressure indicate intravascular volume depletion, whereas volume overload manifests as raised venous pressure and pulmonary crepitations. Clinical circumstances that lead to hypovolaemia are almost invariably associated with high concentrations of plasma antidiuretic hormone, causing increased tubular reabsorption of both water and urea and a disproportionate rise in the plasma urea:creatinine ratio. However, increased catabolism (caused, for example, by sepsis or corticosteroid treatment) also raises plasma urea concentrations, as does ingestion of protein (as a result of upper gastrointestinal bleeding, for example). Although in most pre-renal states avid retention of salt and water leads to low urinary sodium concentration, in clinical practice use of diuretics often renders urinary indices uninterpretable. If doubt remains, a cautious fluid challenge may be considered, but under continuous medical observation as life threatening pulmonary oedema may be induced, particularly in oliguric or anuric patients.
Is there evidence of renal parenchymal disease (other than acute tubular necrosis)?
Intrinsic renal disease other than acute tubular necrosis, although uncommon, must always be excluded as this has important management implications. The history and examination may reveal features of underlying systemic disease, such as rashes, arthralgia, or myalgia. Use of antibiotics and non-steroidal antiinflammatory drugs (widely available without prescription) should be specifically asked about, as these can cause acute interstitial nephritis. Urine dipstick and microscopy are mandatory to avoid missing a renal inflammatory process. Dipstick blood or protein, or dysmorphic red cells, red cell casts (suggestive of glomerulonephritis), or eosinophils (suggestive of acute interstitial nephritis) on microscopy, warrants prompt referral to a nephrologist.
Box 4: Management principles in acute renal failure
Identify and correct pre-renal and post-renal factors
Optimise cardiac output and renal blood flow
Review drugs: stop nephrotoxic agents; adjust doses and monitor concentrations where appropriate
Accurately monitor fluid balance and daily body weight
Identify and treat acute complications (hyperkalaemia, acidosis, pulmonary oedema)
Optimise nutritional support: adequate calories, minimal nitrogenous waste production, potassium restriction
Identify and aggressively treat infection; minimise indwelling lines; remove bladder catheter if anuric
Identify and treat bleeding tendency: prophylaxis with proton pump inhibitor or H2 antagonist, transfuse if required, avoid aspirin
Initiate dialysis before uraemic complications emerge
Has a major vascular occlusion occurred?
Acute renal failure is common in elderly people, as is coexisting atherosclerotic vascular disease, often involving the renal arteries. Indeed, renovascular disease has been found in 34% of elderly people with heart failure.18 Whereas occlusion of a normal renal artery results in loin pain and haematuria, occlusion of a previously stenosed renal artery may be asymptomatic, leaving the patient dependent on a single functioning kidney. In this setting, acute renal failure may be precipitated by occlusion (thrombotic or embolic) of the artery supplying the remaining kidney. An important clue is renal asymmetry on imaging, particularly in a patient with vascular disease elsewhere. Risk factors include use of angiotensin converting enzyme inhibitors and diuretics in the context of renal artery stenosis, hypotension (drug induced or resulting from volume depletion), or instrumentation of the renal artery or aorta. The diagnosis is supported by the presence of complete anuria. Occlusion of a previously normal renal artery is relatively rare, most commonly arising as a consequence of embolisation from a central source.
Cholesterol embolism is another important consideration in the differential diagnosis of acute renal failure in elderly patients after angiography or other intervention such as vascular surgery, thrombolysis, or anticoagulation.19 This is characterised by a classic triad of livedo reticularis, acute renal failure, and eosinophilia; its onset is typically one to four weeks after intervention, which may obscure the causative link. Morbidity and mortality in such patients are high, and death most often results from cardiovascular causes. Renal failure often progresses to dependence on dialysis.
What investigations are most useful in acute renal failure?
Table 1 shows a scheme of investigation, although clearly this should be tailored to individual circumstances. Requesting a full battery of immunological tests is unnecessary in a patient with postoperative acute renal failure or urinary tract obstruction, for example, but would be appropriate if the diagnosis is uncertain or a renal inflammatory condition is suspected.
Table 1.
Investigation of acute renal failure
| Test | Comment |
|---|---|
|
Urinalysis |
|
| Dipstick for blood, protein, or both |
Suggests a renal inflammatory process |
| Microscopy for cells, casts, crystals |
Red cell casts diagnostic in glomerulonephritis |
|
Biochemistry |
|
| Serial urea, creatinine, electrolytes |
Important metabolic consequences of ARF include hyperkalaemia, metabolic acidosis, hypocalcaemia, hyperphosphataemia |
| Blood gas analysis, serum bicarbonate | |
| Creatine kinase, myoglobinuria |
Markedly elevated creatine kinase and myoglobinuria suggests rhabdomyolysis |
| C reactive protein |
Non-specific marker of infection or inflammation |
| Serum immunoglobulins, serum protein electrophoresis, Bence Jones proteinuria |
Immune paresis, monoclonal band on serum protein electrophoresis, and Bence Jones proteinuria suggest myeloma |
|
Haematology |
|
| Full blood count, blood film |
Eosinophilia may be present in acute interstitial nephritis, cholesterol embolisation, or vasculitis |
| Thrombocytopenia and red cell fragments suggest thrombotic microangiopathy | |
| Coagulation studies |
Disseminated intravascular coagulation associated with sepsis |
|
Immunology |
|
| Antinuclear antibody (ANA) |
ANA positive in SLE and other autoimmune disorders; anti-dsDNA antibodies more specific for SLE |
| Anti-double stranded (ds) DNA antibodies | |
| Antineutrophil cytoplasmic antibody (ANCA) |
Associated with systemic vasculitis; c-ANCA and anti-PR3 antibodies associated with Wegener's granulomatosis; p-ANCA and anti-MPO antibodies present in microscopic polyangiitis |
| Antiproteinase 3 (PR3) antibodies | |
| Antimyeloperoxidase (MPO) antibodies | |
| Complement concentrations |
Low in SLE, acute postinfectious glomerulonephritis, cryoglobulinaemia |
| Antiglomerular basement membrane antibodies |
Present in Goodpasture's disease |
| Antistreptolysin O and anti-DNAse B titres |
High after streptococcal infection |
|
Virology |
|
| Hepatitis B and C; HIV |
Important implications for infection control within dialysis area |
|
Radiology |
|
| Renal ultrasonography | Renal size, symmetry, evidence of obstruction |
ARF=acute renal failure; SLE=systemic lupus erythematosus.
How do I manage a patient with acute renal failure?
Management of established acute renal failure encompasses general measures irrespective of cause (box 4) and specific treatments targeted to the particular cause (beyond the scope of this review). The most common cause of acute renal failure is acute tubular necrosis, for which the treatment is largely supportive; the goals are to maintain fluid and electrolyte balance, provide nutritional support, and prevent or treat complications such as infection. Table 2 summarises outcome data from randomised controlled trials of various established and newer agents to treat acute renal failure. In spite of much research, no drug treatment has as yet been shown to limit the progression of, or speed up recovery from, acute renal failure, and some drugs may be harmful.20 The use of furosemide warrants particular mention, as this is a commonly used and inexpensive intervention. A recent meta-analysis of randomised controlled trials showed that furosemide is ineffective in preventing and treating acute renal failure and that high doses may be associated with ototoxicity.21
Table 2.
Evidence for treatment of acute renal failure
| Treatment | Evidence of benefit | Comment |
|---|---|---|
| Loop diuretics versus placebo |
No difference in survival or renal recovery rate |
May promote diuresis, but can be ototoxic in high doses |
| Dopamine versus placebo |
No difference in mortality or need for dialysis |
Risks include tachycardia, extravasation necrosis, and peripheral gangrene |
| Natriuretic peptides versus placebo |
No difference in dialysis-free survival |
May cause hypotension |
| Renal replacement therapy: continuous versus intermittent haemodialysis |
No significant difference in survival or renal recovery |
Continuous venovenous haemodialysis less likely to provoke hypotension |
| Insulin-like growth factor-1 versus placebo |
No difference in renal recovery or need for dialysis |
|
| Thyroxine versus placebo | No difference in renal recovery or need for dialysis | Increased mortality in critically ill patients |
When do I need to speak to a nephrologist?
Substantial under-referral of patients with acute renal failure for specialist opinion remains. In a retrospective study of acute renal failure in an unselected population in Scotland, a nephrology opinion was sought for 22% of patients overall and 35% of those with advanced disease.22 In a more recent prospective study of patients with acute renal failure in Kent, initial assessment was often suboptimal and key features in investigation and initial management were often lacking.2 Advice from a nephrologist should therefore be sought for all cases of acute renal failure, as early consultation can improve outcomes.23 When the cause of acute renal failure is not apparent, and particularly if intrinsic renal disease other than acute tubular necrosis is suspected, early referral is mandatory as specialist treatment may be needed. Renal specialists are not necessary, however, for provision of renal replacement therapy, as this can be initiated promptly in most intensive care units by continuous venovenous haemofiltration.
Additional educational resources
Glynne PA, Allen A, Pusey CD, eds. Acute renal failure in practice. London: Imperial College Press, 2002 Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2006;2: 364-77
Firth JD. The clinical approach to the patient with acute renal failure. In: Davison AM, Cameron JS, Grünfeld J-P, Ponticelli C, Ritz E, Winearls CG, et al, eds. Oxford textbook of clinical nephrology. 3rd ed. Oxford: Oxford University Press, 2005:1465-93
Zacharias M, Gilmore ICS, Herbison GP, Sivalingam P, Walker RJ. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev 2005;(3): CD00359016034904
Information resources for patients
Renalinfo (www.renalinfo.com/uk/en/)—Offers help, advice, and support to people being treated for renal failure
Royal Infirmary of Edinburgh Renal Unit (renux.dmed.ed.ac.uk/EdREN/EdRenINFOhome.html)—Source of information about kidney diseases for patients and non-specialist doctors
National Kidney and Urologic Diseases Information Clearinghouse (kidney.niddk.nih.gov/index.htm)—US website with information about diseases of the kidneys and urological system for patients, families, healthcare professionals, and the general public
Conclusions
Acute renal failure is a life threatening illness with high mortality despite advances in supportive care. An additional cost exists in terms of morbidity and the high demands placed on healthcare resources. The pathophysiology is not well understood, therapeutic options are limited, and a considerable proportion of patients progress to dialysis dependent end stage renal disease. The priorities in management of acute renal failure include early recognition, institution of appropriate preventive measures, optimisation of fluid balance, identification and treatment of underlying causes, and timely initiation of renal replacement therapy where appropriate.
Competing interests: None declared.
References
- 1.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993;306: 481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens PE, Tamimi NA, Al-Hasani MK, Mikhail AI, Kearney E, Lapworth R, et al. Non-specialist management of acute renal failure. QJM 2001;94: 533-40. [DOI] [PubMed] [Google Scholar]
- 3.Hegarty J, Middleton RJ, Krebs M, Hussain H, Cheung C, Ledson T, et al. Severe acute renal failure in adults: place of care, incidence and outcomes. QJM 2005;98: 661-6. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM 2002;95: 579-83. [DOI] [PubMed] [Google Scholar]
- 5.Renal Association. UK renal registry: the eighth annual report, December 2005. Bristol: UK Renal Registry, 2005.
- 6.Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. Am J Kidney Dis 1991;17: 191-8. [DOI] [PubMed] [Google Scholar]
- 7.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39: 930-6. [DOI] [PubMed] [Google Scholar]
- 8.Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis 2005;46: 1038-48. [DOI] [PubMed] [Google Scholar]
- 9.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med 1983;74: 243-8. [DOI] [PubMed] [Google Scholar]
- 10.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int 1996;50: 811-8. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004;66: 1613-21. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ J Surg 2003;73: 144-53. [DOI] [PubMed] [Google Scholar]
- 13.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet 2005;365: 417-30. [DOI] [PubMed] [Google Scholar]
- 14.Lameire NH. Contrast-induced nephropathy—prevention and risk reduction. Nephrol Dial Transplant 2006;21: i11-23. [DOI] [PubMed] [Google Scholar]
- 15.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354: 2773-82. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu C, Belli AM, Oliveira DB. The role of N-acetylcysteine in the prevention of contrast-induced nephrotoxicity. Cardiovasc Intervent Radiol 2006;29: 344-7. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni S, Jayachandran M, Davies A, Mamoun W, Al-Akraa M. Non-dilated obstructed pelvicalyceal system. Int J Clin Pract 2005;59: 992-4. [DOI] [PubMed] [Google Scholar]
- 18.MacDowall P, Kalra PA, O'Donoghue DJ, Waldek S, Mamtora H, Brown K. Risk of morbidity from renovascular disease in elderly patients with congestive cardiac failure. Lancet 1998;352: 13-6. [DOI] [PubMed] [Google Scholar]
- 19.Dupont PJ, Lightstone L, Clutterbuck EJ, Gaskin G, Pusey CD, Cook T, et al. Lesson of the week: cholesterol emboli syndrome. BMJ 2000;321: 1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellum J, Leblanc M, Venkataraman R. Acute renal failure. www.clinicalevidence.com/ceweb/conditions/knd/2001/2001.jsp
- 21.Kwok MH, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 2006;333: 420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IH, Catto GR, Edward N, Macleod AM. Acute renal failure: factors influencing nephrology referral and outcome. QJM 1997;90: 781-5. [DOI] [PubMed] [Google Scholar]
- 23.Star RA. Treatment of acute renal failure. Kidney Int 1998;54: 1817-31. [DOI] [PubMed] [Google Scholar]