Abstract
Streptococcus pyogenes can be efficiently internalized by a variety of human epithelial cells. β-lactam antibiotics, commonly used to treat S. pyogenes infections, do not readily permeate mammalian cells. There is growing evidence that the ability of streptococci to enter host cells contributes to the frequent failure of antibiotics to eradicate the organism from infected individuals. Recent studies have suggested that host cell entry requires the formation of a complex of a bacterial fibronectin (Fn) binding protein (e.g., M1 protein or protein F1/SfbI), human Fn, and the epithelial cell Fn receptor, integrin α5β1. We report here that a low molecular weight, nonpeptide antagonist of integrin α5β1, SJ755, can inhibit internalization of streptococci by primary human tonsillar epithelial cells and immortalized human epithelial (A549) cells, thus increasing the extent of bacterial killing by antibiotics. SJ755 blocked Fn binding by human tonsillar epithelial and A549 cells, suggesting that integrin α5β1 is the major Fn receptor expressed by both cell types. SJ755 did not affect Fn binding by purified M1 protein or M1+ bacteria. Purified M1 protein failed to associate with integrin α5β1 unless the integrin had been prebound by Fn. Also, SJ755 blocked formation of α5β1-Fn-M1 complexes in vitro. These results support the previous proposal that Fn functions as a molecular bridge between M1 protein and integrin α5β1. Furthermore, these results suggest that integrin antagonists may enhance the efficacy of antibiotics in treatment of S. pyogenes infections.
The Gram-positive bacterial pathogen Streptococcus pyogenes is a common cause of acute pharyngitis and superficial skin infections in humans. During the past decade, S. pyogenes was increasingly associated with life-threatening, invasive disease including necrotizing fasciitis, toxic shock, scarlet fever, and bacteremia (1–4). β-lactam antibiotics are commonly used for treatment of S. pyogenes infections. Although most infections respond well to antibiotic therapy, from 30 to 40% of children continue to carry the organism after treatment, despite the fact that S. pyogenes has not acquired penicillin resistance (5). Furthermore, epidemiological studies suggest that asymptomatic carriers can serve as a reservoir for outbreaks of invasive disease (6, 7). Therefore, new therapies that reduce the persistence or carriage of streptococci could result in decreased incidence of both invasive and uncomplicated infections.
Mammalian cells are poorly permeable to β-lactam antibiotics, and there is considerable evidence that the presence of intracellular streptococci in vivo contributes to the frequent failure of β-lactams to eradicate the organism from infected persons (8–10). Collectively, these results suggest that the ability of S. pyogenes to enter host cells may, in effect, confer β-lactam resistance on the bacterium. LaPenta et al. (11) first demonstrated that S. pyogenes can efficiently enter immortalized human cells in vitro. Subsequent studies have revealed much about the mechanisms whereby the bacterium gains entry to host cells. At least two types of streptococcal cell surface proteins, M proteins and high-affinity fibronectin (Fn) binding proteins, can facilitate entry into mammalian cells (12–18). For a number of strains, host cell entry requires bacterial binding of serum Fn. In these cases, bacterial-bound Fn appears to function as a bridging molecule, promoting bacterial interaction with host Fn receptors (13, 16, 17, 19).
Recent results (13, 16) indicated a role for the epithelial cell Fn receptor, integrin α5β1, in cellular invasion by streptococci. Integrins are a family of heterodimeric, transmembrane, cell adhesion molecules that mediate cell-cell and cell-extracellular matrix binding as well as bi-directional signal transduction (20, 21). Integrin ligands frequently possess the tripeptide sequence RGD, which serves as the integrin recognition/binding site. Because integrins are involved in diverse biological processes, integrin antagonists have been developed as potential therapeutic agents. Antagonists, which include monoclonal antibodies (mAbs), RGD-containing peptides, and peptidomimetic compounds, have been successfully used for treatment of thromboembolic, chronic inflammatory, and autoimmune disorders and as anti-neoplastic and anti-angiogenic agents (22–24).
Numerous bacterial, fungal, and viral pathogens exploit integrins to gain entry into eukaryotic cells (25–27). Thus, compounds capable of disrupting a pathogen's ability to engage integrins have the potential to affect the outcome of microbial or viral infections. In this work, we demonstrate that antagonists of integrin α5β1 can inhibit the entry of streptococci into human epithelial cells, thus increasing bacterial killing by antibiotics.
Materials and Methods
Bacterial Strains.
The M1 serotype strain, S. pyogenes 90-226, and its isogenic M1− derivative have been described (12). The serotype M6 strain, JRS4, was provided by M. Caparon (Washington University, St. Louis) (19). Escherichia coli BL21/pM42-382 was previously described (13).
Cell Culture.
A549 human lung epithelial cells (American Type Culture Collection CCL 185) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Life Technologies) as described (13). Human tonsillar epithelial cells were cultured from palatine tonsil tissue obtained from otherwise normal healthy individuals undergoing routine tonsillectomy in the Department of Surgery at the University of Minnesota. Segments of tonsil were rinsed in growth medium (RPMI medium containing 10% heat-inactivated fetal calf serum, penicillin, streptomycin, and fungizone), and then the tissue was mechanically disrupted. Single-cell, mixed populations of cells were cultured in standard tissue culture flasks (≈2–5 × 107cells per T25 flask), and microcolonies of adherent cells were first visible within 4–8 days. Proliferating populations of large elongated cells spread throughout the flasks to establish confluent monolayers within 20–30 days. The adherent cell populations were readily released by trypsinization and were further expanded and maintained by routine passaging procedures for adherent cells. All experiments were performed with cells that were cultured for no more than 6–12 weeks from the time of tissue disruption. The expanded primary cell populations were determined to be predominantly epithelial from strong positive immunocytochemical staining with antibodies directed against polycytokeratin, E-cadherin, and β1 and α5β1 integrins. (P.J.S., unpublished work).
Proteins, Antibodies, and Integrin Antagonists.
Human plasma fibronectin (Fn) and the 120-kDa α-chymotryptic Fn fragment were purchased from Life Technologies. Monoclonal antibodies (mAbs) were the same as those previously described (13). Anti-integrin β1 and β2 mAbs were of the same isotype (IgG1). Recombinant M1 protein was purified from E. coli BL21/pM42-382 as described (13). Integrin α5β1 was purified from human placenta as described (23).
The integrin antagonists SJ755 and SJ749 (active against integrin α5β1), SQ885 (active against RGD peptide-binding integrins), XJ754 (active against integrin GPIIbIIIa), and XT199 (active against integrin αvβ3) were synthesized and characterized at Dupont Pharmaceutical Company (Wilmington, DE) (23, 24, 28).
Specificity of Integrin Antagonists.
Enzyme-linked immunoabsorbent (ELISA)-based assays were employed to determine the specificity of SJ755 for inhibition of ligand binding by integrins (23, 24). Integrin-mediated cell adhesion assays were performed to determine the effects of various integrin antagonists on ligand binding by human cell lines. Cells were labeled with Calcein-AM (Molecular Probes) as described (23, 24), were incubated with an integrin antagonist, then were added to wells of microtiter plates, previously coated with an integrin ligand. Concentrations of SJ755 required for 50% inhibition of ligand binding (IC50) were determined by performing binding assays in the presence of varying concentrations of the antagonist.
Epithelial Cell Adherence and Invasion Assays.
Assays of bacterial adherence to and invasion of A549 cells were performed as described (13). Unless stated otherwise, assays were performed in RPMI medium supplemented with 10% FBS. Percent internalization was calculated as the percentage of colony-forming units (cfu) in the inoculum that survived antibiotics (100 μg of gentamicin and 5 μg of penicillin per ml). Percent adherence was calculated as the percentage of total cfu that remained associated with monolayers after three successive washes with Hank's balanced salt solution (Life Technologies).
To assay inhibition of adherence and invasion by the integrin antagonists SJ755 and SJ749, the antagonists (1 mM in 50% DMSO, 50% H2O), or DMSO as a control, were mixed with RPMI medium containing ≈5 × 105 S. pyogenes cfu per ml, just before infection of monolayers. Percent inhibition was calculated as [cfu recovered from control wells (containing DMSO) − cfu recovered from SJ755 containing wells]/(cfu from control wells) × 100. Inhibition of invasion by other integrin antagonists or mAbs was determined similarly, except DMSO was omitted.
Fn Binding Assays.
Binding of epithelial cells to a 120-kDa α-chymotryptic Fn fragment was assayed as described (23). Fn binding to immobilized M1 protein was determined as follows. Wells of microtiter plates (Maxisorp Immuno-plates, Nunc) were coated with 200 ng of purified recombinant M1 protein or BSA (Sigma), as a negative control, in 100 μl of 50 mM carbonate buffer (pH 9.6) overnight at 5°C. After removal of unbound protein and blocking with 0.5% BSA, 100 μl of 50 μg/ml Fn in wash buffer [Dulbecco's PBS (Life Technologies)/0.05% Tween 20/0.5% BSA] was added and incubated at ambient temperature for 2 hr. Inhibitors were included in this incubation as appropriate. Bound Fn was detected with anti-human Fn Ab (ICN) under standard conditions (13). Percent inhibition of Fn binding was calculated as [Abs of control wells (lacking an inhibitor) − Abs of wells containing inhibitor]/(Abs of control wells) × 100. All data are the means ± SEM from three independent experiments. Fn binding to immobilized bacteria was performed similarly, except wells were coated with a suspension (OD560 = 0.05 in carbonate buffer) of gentamicin-killed S. pyogenes 90-226 cells.
Formation of α5β1-Fn-M1 Complexes in Vitro.
To assay M1 binding to immobilized integrin α5β1, microtiter wells were coated with 50 ng of integrin α5β1 in 100 μl of 0.1 M Tris⋅Cl (pH 8.0) and 2 mM CaCl2 overnight at 4°C. As controls, some wells were coated with 200 ng of ovalbumin (Sigma) or buffer. After washing to remove unbound integrin and blocking with 0.5% ovalbumin, 100 μl of 50 μg/ml Fn in wash buffer (0.1 M Tris⋅Cl, pH 7.5/0.1M NaCl/2 mM CaCl2/1 mM MgCl2/1 mM MnCl2/0.5% ovalbumin), was added and the plate was incubated at room temperature for 90′. In some wells, the Fn solution contained 10 μM SJ755, DMSO, or anti-α5β1 Ab. After washing to remove unbound Fn, 100 μl of wash buffer containing 4 μg/ml of M1 protein was added. As before, some wells contained SJ755 or DMSO in addition to M1. To detect M1 binding to α5β1 or α5β1-Fn complexes, wells were successively incubated with rabbit anti-M1 serum and mouse anti-rabbit-alkaline phosphatase (Sigma) for 60′ at room temperature.
Results
Integrin α5β1 Antagonists Can Inhibit Streptococcal Invasion of A549 (Human Lung Epithelial) Cells.
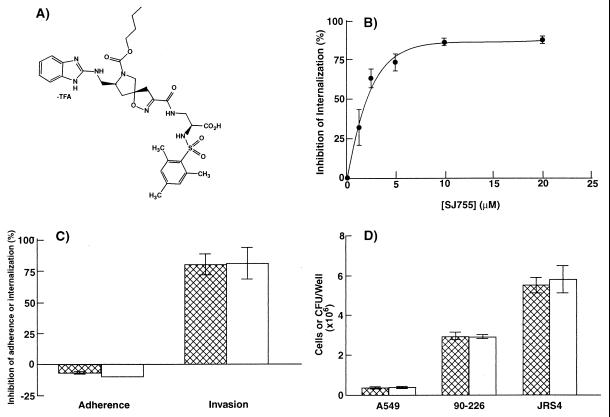
The integrin antagonist SJ755 is a novel spiroisoxazoline that competitively inhibits Fn binding by integrin α5β1 (Fig. 1A) (22, 28). The antagonist demonstrates both high affinity and specificity for this integrin (Table 1). The ability of SJ755 to inhibit Fn binding by integrin α5β1 led us to propose that SJ755 may also be effective in inhibiting bacterial interaction with host cells in cases in which the interaction involves direct or indirect binding of integrin α5β1. Intracellular invasion by S. pyogenes strains that express M1 protein (e.g., S. pyogenes 90-226) apparently requires binding of M1 protein-Fn complexes by integrin α5β1 (13). Streptococcal invasion of a human lung epithelial cell line (A549 cells) was inhibited over 80% in the presence of 10 μM SJ755 (Fig. 1B). SJ755 slightly stimulated adherence of 90-226 to host cells (Fig. 1C) and did not affect bacterial growth or the integrity of A549 monolayers (Fig. 1D). Thus, SJ755 specifically blocks internalization of streptococci by epithelial cells.
Figure 1.
Inhibition of streptococcal invasion by the integrin α5β1 antagonist, SJ755. (A) Chemical structure of SJ755 ((S,S,S,)-2-[(2, 4, 6-trimethylphenyl) sulfonylamino-3-[[7-n-butoxycarbonyl-8- [(benzimidazol-2-ylamino)methyl]-1-oxa-2,7-diazaspiro- [4,4]-non-2-en-3-yl]carbonylamino] propionic acid, trifluoroacetate). (B) Internalization of S. pyogenes 90-226 by A549 cells vs. SJ755 concentration. (C) Effects of 10 μM SJ755 on bacterial adherence to and internalization by A549 human lung epithelial cells. Hatched bars, strain 90-226 (M1+, PrtF1−); open bars, JRS4 (M6+, PrtF1+). (D) Effect of SJ755 on bacterial viability and adherence of A549 cells to the substratum. Hatched bars, medium contained 0.5% DMSO; open bars, medium contained 10 μm SJ755, 0.5% DMSO. All data are the means ± SEM of three independent experiments, in which each assay was performed in triplicate (i.e., n = 9). In the absence of SJ755, percent internalization was 33.1 ± 5.4 for strain 90-226 and 11.5 ± 1.0 for strain JRS4.
Table 1.
Specificity of antiintegrin SJ755
| Integrin | IC50, nM* | Ligand |
|---|---|---|
| α5β1 | 0.39 | Fibronectin |
| αvβ3 | 87 | Fibrinogen |
| αvβ5 | 2,200 | Vitronectin |
| GPIIbIIIa | 100,000 | Fibrinogen |
Data are the means from three to five independent experiments; variation was typically <10%.
The concentration of SJ755 required for 50% inhibition of ligand binding by purified integrins.
Other integrin antagonists were tested for their ability to inhibit streptococcal invasion. Of these, only antagonists capable of inhibiting Fn binding by integrin α5β1 were effective invasion inhibitors (Table 2). The integrin antagonist SJ749 is chemically related to SJ755 and demonstrated a comparable ability to inhibit binding of Jurkat cells to Fn and inhibit invasion of A549 cells. SQ885 is an isoxaline that can inhibit ligand binding by all integrins that recognize the tripeptide sequence, RGD, in their respective ligands. Although SQ885 lacks the specificity of the spiroisoxazolines, it is a potent invasion inhibitor. Similarly, the broad-specificity hexapeptide, GRGDTP, inhibited invasion by ≈50% when present at a concentration of 1.5 mM. In contrast, XT199 and XJ754, which specifically inhibit vitronectin binding by integrin αvβ3 and fibrinogen binding by integrin GPIIbIIIa, respectively, did not appreciably affect bacterial invasion even when present at 200 μM concentrations. These results support our previous conclusion (13) that streptococcal entry into A549 cells is primarily mediated by integrin α5β1.
Table 2.
Activities of integrin antagonists on ligand binding by human cells and invasion of A549 cells by S. pyogenes 90-226
| Integrin antagonist | Target integrin | IC50, μM | Concentration required for 80% inhibition of invasion, μM |
|---|---|---|---|
| SJ755 | α5β1 | 0.25 | 10 |
| SJ749 | α5β1 | 0.34 | 5 |
| SQ885 | Multiple | 0.025 | 0.5 |
| XT199 | αvβ3 | 0.02 | >200 |
| XJ754 | GPIIbIIIa | 0.02 | >200 |
| GRGDTP | Multiple | 10–100 | >1500 |
Concentrations of antagonists required for 50% inhibition of Jurkat (human T cells) cell binding to Fn (via integrin α5β1) and vitronectin (via integrin αvβ3) were determined as described (23, 24). Assay of fibrinogen binding by human platelets (via integrin GPIIbIIIa) has been described (23, 24). The isoxaline SQ885 and the hexapeptide GRGDTP are bound by multiple integrins, including α5β1. Binding data are the means from three to five independent experiments.
Approximately 90% of S. pyogenes clinical isolates carry a gene encoding a high-affinity Fn receptor [i.e., protein F1 (PrtF1) or SfbI]. The genes encoding these proteins, prtF1 and sfbI, are considered variants of the same gene (15, 16). Recent results suggest that PrtF1/SfbI contribute significantly to intracellular invasion by streptococci and their persistence in patients treated with antibiotics (10). Because strain 90-226 does not carry prtF1 (13), it was of interest to determine whether SJ755 could inhibit invasion by a PrtF1+ strain. S. pyogenes JRS4 is a well characterized, serotype M6 strain that constitutively expresses PrtF1. Jadoun et al. (14) reported that invasion by JRS4 is mediated by both PrtF1 and M6 protein. SJ755 was found to effectively inhibit invasion of A549 cells by JRS4 (Fig. 1C). Thus, SJ755 appears to be equally effective at inhibiting invasion by M1 protein- or PrtF1-expressing strains of S. pyogenes.
Streptococcal Invasion of Primary Human Tonsillar Epithelial Cells.
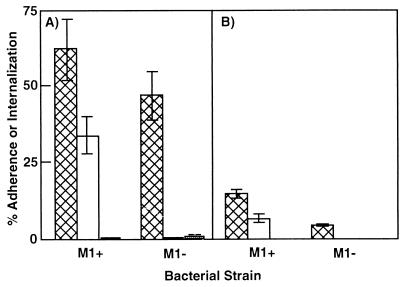
Although several recent reports suggest that streptococci invade epithelial cells in vivo (9, 10), bacterial or host factors required for invasion of primary cells have not been reported. Invasion of immortalized epithelial cells by S. pyogenes 90-226 requires expression of M1 protein and the presence of serum or serum Fn (ref. 13; Fig. 2A). To determine whether the same is true for invasion of primary cells, we studied the interaction of strain 90-226 and an isogenic M1− strain, with primary human tonsillar epithelial (HTE) cells. HTE cell adherence was 3-fold higher for M1+ vs. M1− bacteria, and invasion was over 40-fold more efficient with M1+ bacteria. Also, invasion by the M1+ strain was found to depend on exogenous serum (Fig. 2B). Thus, the major difference between bacterial interaction with HTE and A549 cells is that, overall, the efficiency of bacterial adherence and internalization was lower for HTE cells. We have not investigated what may account for the lower efficiency; however, assays were performed under conditions optimal for invasion of A549 cells, conditions that may not be optimal for HTE cells.
Figure 2.
Interaction of M1+ and M1− streptococci with A549 (A) and human tonsillar epithelial (HTE) (B) cells. S. pyogenes 90-226 (M1+) and 90-226 emm1∷Km (M1−) were suspended in RPMI 1640 medium or medium supplemented with 10% FBS, just before infection of monolayers. Hatched bars, percent of bacterial cfu that adhered to host cells; open bars, percent of bacteria internalized in the presence of 10% FBS; solid bars, percent of bacteria internalized in the absence of FBS. Some bars are too short to be visible in the figure. Data are the means ± SEM of two (A) or three (B) experiments in which each assay was performed in triplicate. HTE cell experiments were performed with cells obtained from three different donors.
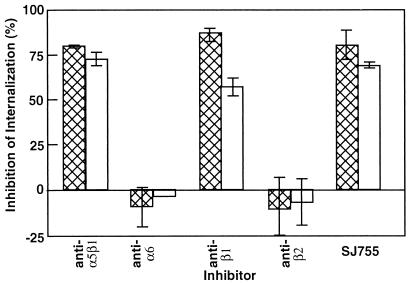
We next determined whether integrin α5β1 was involved in uptake of S. pyogenes by HTE cells. SJ755 and monoclonal antibodies (mAb) against β1 or α5β1 integrins inhibited invasion of HTE cells, although the level of inhibition was slightly lower for HTE cells than for A549 cells (Fig. 3). mAbs recognizing the α6 or β2 integrin subunits did not inhibit invasion of either cell type. These results validate the relevance of earlier studies performed with immortalized cell lines: invasion of HTE cells is M1 protein- and Fn-dependent and is primarily mediated by integrin α5β1.
Figure 3.
Effect of integrin antagonists on internalization of M1+ bacteria by HTE cells. Monolayers of A549 (hatched bars) and HTE (open bars) were infected with S. pyogenes 90-226 in medium containing either 0.5% DMSO, 10 μM SJ755, 0.5% DMSO, or mAb reactive against the indicated integrin. Inhibition was determined as described in Materials and Methods. The effect of anti-integrin α6 mAb on bacterial internalization by HTE cells was determined from a single experiment; all other data are the means ± SEM from three independent experiments (n = 9).
SJ755 Inhibits Invasion by Blocking Fn Binding by Integrin α5β1.
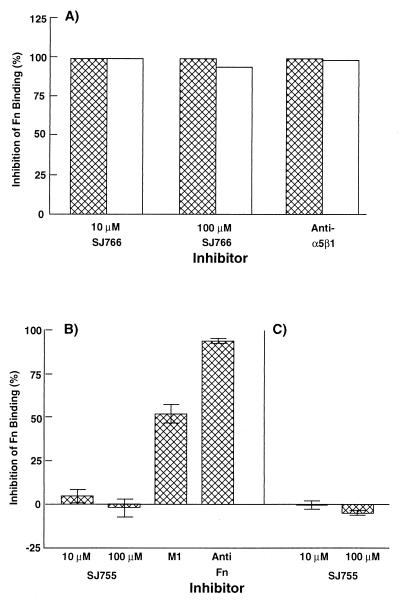
Available evidence suggests that streptococcal invasion requires the formation of a trimeric complex of a bacterial Fn binding protein (e.g., M1 or PrtF1), Fn, and integrin α5β1 (13, 16). The invasion antagonist SJ755 was presumed to exert its inhibitory effect by competing with streptococcal-bound Fn for binding to α5β1. To confirm this, the effect of SJ755 on epithelial cell binding of immobilized Fn was determined. 10 μM SJ755 or anti-integrin-α5β1 mAb caused nearly complete blocking of Fn binding by HTE and A549 cells (Fig. 4A). Thus, α5β1 appears to be the major Fn receptor of both cell types. Purified M1 protein did not inhibit binding of either cell type to Fn, a result consistent with Fn serving as a molecular bridge between M1 and epithelial cells.
Figure 4.
Effects of integrin antagonists on Fn binding by epithelial cells (A), M1 protein (B), and M1+ bacteria (C). (A) Effect of SJ755 and anti-integrin α5β1 mAb on binding of A549 (hatched bars) and HTE (open bars) cells to a 120-kDa α-chymotryptic Fn fragment. (B) Effects of SJ755, soluble M1, and anti-Fn Ab on binding of Fn to immobilized M1 protein. (C) Effect of SJ755 on Fn binding to immobilized M1+ bacteria. Percent inhibition of Fn binding was calculated as [absorbance (Abs) of control wells (lacking an inhibitor) − Abs of wells containing inhibitor]/(Abs of control wells) × 100. Data in A are the means from experiments performed in triplicate. Data in B and C are the means ± SEM from three independent experiments (n = 9).
Similar experiments were performed to determine whether SJ755 could influence Fn binding to M1 protein (Fig. 4B). SJ755 was found to have no effect on Fn binding even when present at a concentration of 100 μM. In control experiments, soluble M1 and anti-Fn Ab effectively competed with immobilized M1 for Fn binding. Similarly, SJ755 was ineffective at inhibiting Fn binding to immobilized M1+ bacteria (Fig. 4C). These results indicate that SJ755 inhibits invasion by blocking Fn binding to integrin α5β1.
As independent confirmation of this conclusion, we determined whether SJ755 could inhibit the interaction of purified integrin α5β1 with M1 protein. In these experiments (Table 3), Fn was first incubated with immobilized α5β1, and then, after removal of unbound Fn, M1 protein was added. Binding of M1 protein to immobilized integrin α5β1 depended on prior incubation of Fn with the receptor, suggesting that M1 protein can bind to integrin α5β1-Fn complexes, but not directly to integrin α5β1. Our data do not exclude the possibility, however, that direct M1-integrin α5β1 interactions occur when M1-Fn complexes are bound by the integrin. SJ755 strongly inhibited formation of integrin α5β1-Fn-M1 complexes, if the antagonist was present during the integrin α5β1-Fn binding step. In contrast, only weak inhibition was observed if SJ755 was added during the Fn-M1 binding step. These results support the conclusion that SJ755 exerts its inhibitory effect by blocking Fn binding by integrin α5β1. Moreover, these results provide direct evidence that Fn forms a molecular bridge between M1 protein and integrin α5β1.
Table 3.
Effect of SJ755 on the formation of α5β1-Fn-M1 protein complexes in vitro
| Solid phase | First
incubation
|
Second incubation
|
Absorbance at 405 nm | |||
|---|---|---|---|---|---|---|
| Fn | SJ755 | Anti-α5β1 | M1 | SJ755 | ||
| No protein | + | − | − | + | − | 0 |
| Ovalbumin | + | − | − | + | − | 0 |
| Integrin α5β1 | + | − | − | + | − | 0.49 ± 0.10 |
| " | − | − | − | + | − | 0.03 ± 0.01 |
| " | + | − | − | − | − | 0.02 ± 0.01 |
| " | + | + | − | + | − | 0.04 ± 0.01 |
| " | + | − | + | + | 0.31 ± 0.08 | |
| " | + | − | + | + | − | 0.04 ± 0.01 |
Assays to detect binding of M1 protein to α5β1 or α5β1-Fn complexes were performed as described in Materials and Methods. Data are the means ± SEM from two independent experiments in which each assay was performed in triplicate. Plus and minus signs indicate the presence or absence of a given reactant in an incubation step.
Discussion
A number of recent studies have established that S. pyogenes, long considered a model extracellular pathogen, can be ingested by and survive within a variety of human cell types. Investigations into the mechanisms whereby streptococci enter host cells have revealed that at least two types of streptococcal cell-surface proteins can facilitate intracellular invasion. The Fn binding proteins PrtF1 and SfbI mediate Fn-dependent entry into HEp2, HeLa, and G25 cells (14–17). Similarly, several serotypes of M protein promote Fn-dependent entry into human epithelial cell lines (12–14, 18).
Previous results suggest that invasion involves at least two separate interactions between S. pyogenes 90-226 and epithelial cells because adherence only partially depends on M1 protein (Fig. 5). The second adhesin of strain 90-226 and its cellular receptor have not been identified. It has been proposed that the functions of M1 protein and PrtF1 in the invasion process are solely to bind Fn to the surface of the bacterial cell. In turn, the bound Fn is bound by integrins on the surface of a host cell. Fn binding is apparently sufficient for bacterial uptake because latex beads coated with SfbI or M1 protein are ingested by epithelial cells (12, 15). Fn-coated beads are also efficiently ingested by HEp2 cells (16). Ozeri et al. (16) reported that only intact Fn is capable of supporting streptococcal invasion and that PrtF1 does not alter the affinity of Fn to HeLa cells. These results are all consistent with Fn functioning as a bridging molecule for intracellular invasion by streptococci. Additional support for this model is presented here. A549 and HTE calls can bind serum Fn in the absence of bacteria, and binding is unaffected by the presence of M1 protein. Moreover, purified M1 protein was found to associate with integrin α5β1 only if the integrin had been preincubated with Fn. Inhibition of Fn binding to integrin α5β1 by either mAb or SJ755 blocked subsequent interaction of M1 with the integrin.
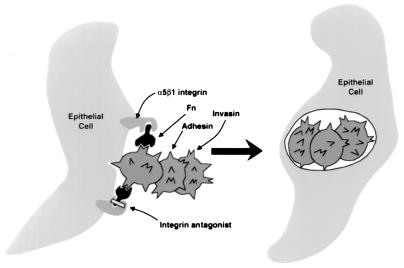
Figure 5.
Model for ingestion of streptococci by epithelial cells and mechanism of inhibition by integrin antagonists. Ingestion of streptococci requires the formation of a trimeric complex of a bacterial invasin, either M1 protein or protein F1/Sfbl, Fn, and the epithelial cell Fn receptor, integrin α5β1. Because antibiotics such as penicillin do not readily permeate eukaryotic cells, intracellular streptococci are resistant to killing by the antibiotic. Antagonists of integrin α5β1 can inhibit bacterial ingestion by blocking Fn binding by the integrin, thus increasing the susceptibility of a bacterial population to antibiotics.
Streptococcal invasion appears to occur in vivo as well as in vitro, and there is increasing evidence that intracellular invasion contributes to the severity and frequency of S. pyogenes disease. Recent studies have suggested that intracellular streptococci constitute an in vivo reservoir of bacteria that could, in part, account for the failure of β-lactam antibiotics to eradicate bacteria from infected individuals, recurrent cases of pharyngotonsillitis, and localized outbreaks of invasive streptococcal disease (6–10). For example, Österlund et al. (9) demonstrated the presence of intracellular streptococci within pharyngeal epithelial cells obtained from tonsillitis patients. The same group reported intracellular streptococci within macrophage-like and epithelial cells obtained from asymptomatic carriers. Neeman et al. (10) compared two types of clinical isolates, those that were cleared from patients by amoxicillin therapy and isolates that persisted despite therapy, for the presence of prtF1. Ninety percent of the persisting strains carried prtF1, compared with 29.6% of the strains that did not persist. This result suggests that PrtF1-mediated intracellular invasion contributes to bacterial survival in patients treated with β-lactam antibiotics.
S. pyogenes appears incapable of true intracellular growth, and the number of viable, intracellular bacteria declines steadily over time (9, 30). Bacterial growth can resume, however, even after several days' exposure to antibiotics, after removal of antibiotics from infected monolayers (8). These findings suggest that there is a relatively steady, low rate of endosomal and cellular escape by intracellular streptococci. Cellular escape likely results in bacterial death when antibiotics are present in the extracellular environment or, alternatively, in recolonization and recurrent disease if antibiotics are absent. Thus, maintenance of an intracellular reservoir seems most likely to occur by a cycling process, whereby intracellular bacteria escape from host cells and are then internalized by neighboring cells. In this context, disruption of bacterial entry or reentry into cells would increase the sensitivity of a bacterial population to antibiotics, thus increasing the likelihood of bacterial clearance. Results presented here establish that integrin antagonists can inhibit internalization of streptococci, resulting in increased killing by antibiotics. Specifically, antagonists of integrin α5β1 can inhibit internalization of M1 protein and PrtF1-expressing streptococci. Moreover, α5β1 antagonists were found effective at inhibiting bacterial entry into primary tonsillar epithelial cells as well as immortalized epithelial cells.
The use of integrin antagonists to inhibit intracellular invasion is a novel and potentially important approach to the treatment of infectious disease. To date, however, we have not directly tested the feasibility of using integrin antagonists to enhance the efficacy of antibiotics in treatment of S. pyogenes infections. Numerous factors, including the solubility of an antagonist, its tissue distribution, stability, and toxicity, will ultimately dictate its clinical application. For microorganisms like S. pyogenes, for which interaction with the oral mucosa or skin is the initial step in host colonization, achieving an effective dose of an antagonist could likely be accomplished by topical administration of the drug. We hope that our results will help spur the development of high potency, low toxicity integrin antagonists that can be administered by multiple routes as exemplified by the step-wise development of orally administered, platelet GPIIbIIIa antagonists (22–24).
Numerous bacterial, fungal, viral, and protozoal pathogens exploit integrins for adherence to or invasion of eukaryotic cells (25–27). Recent advances in the development of cell adhesion antagonists have yielded an array of compounds with great potential for treatment of physiological, immunological, and oncological disorders (22). Our results suggest that such compounds may, in conjunction with other antimicrobials, also find beneficial application in treatment of infectious diseases.
Acknowledgments
We thank H. Lam for technical assistance, T. Leonard for help in figure preparation, and Dr. J. Dale for anti-M1 serum. We thank Chuck Sieber for invaluable assistance in the acquisition and delivery of tonsil tissue. This work was supported by Public Health Service Grant AI34503 and a grant from Dupont Pharmaceutical (P.P.C.).
Abbreviations
- Fn
fibronectin
- HTE cells
human tonsillar epithelial cells
- cfu
colony-forming unit
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050587897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050587897
References
- 1.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Lancet. 1992;321:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 2.Schlievert P M, Assimacopoulos A P, Cleary P P. J Lab Clin Med. 1996;127:13–22. doi: 10.1016/s0022-2143(96)90161-4. [DOI] [PubMed] [Google Scholar]
- 3.Musser J M, Kapur V, Szeto J, Pan X, Swanson D S, Martin D R. Infect Immun. 1995;63:994–1003. doi: 10.1128/iai.63.3.994-1003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens D L. Emerg Infect Dis. 1995;1:69–78. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber M A. Pediatrics. 1996;97:971–975. [PubMed] [Google Scholar]
- 6.Cockerill F R, MacDonald K L, Thompson R L, Roberson F, Kohner P C, Besser-Wiek J, Manahan J M, Musser J M, Schlievert P M, Talbot J, et al. J Am Med Assoc. 1997;277:38–43. [PubMed] [Google Scholar]
- 7.Kiska D L, Thiede B, Caracciolo J, Jordan M, Johnson D, Kaplan E L, Gruninger R P, Lohr J A, Gilligan P H, Denny F W., Jr J Infect Dis. 1997;176:992–1000. doi: 10.1086/516540. [DOI] [PubMed] [Google Scholar]
- 8.Österlund A, Engstrand L. Acta Otolaryngol. 1995;115:685–688. doi: 10.3109/00016489509139387. [DOI] [PubMed] [Google Scholar]
- 9.Österlund A, Popa R, Nikkila T, Scheynius A, Engstrand L. Laryngoscope. 1997;107:640–646. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Neeman R, Keller N, Barzilai A, Korenman Z, Sela S. Lancet. 1998;352:1974–1977. doi: 10.1016/S0140-6736(97)12452-7. [DOI] [PubMed] [Google Scholar]
- 11.LaPenta D, Rubens C, Chi E, Cleary P P. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombek P E, Cue D, Sedgewick J, Lam H, Ruschkowski S, Finlay B B, Cleary P P. Mol Microbiol. 1999;31:859–870. doi: 10.1046/j.1365-2958.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 13.Cue D, Dombek P E, Lam H, Cleary P P. Infect Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadoun J, V, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 15.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozeri V, Rosenshine I, Mosher D F, Fassler R, Hanski E. Mol Microbiol. 1998;30:629–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 17.Okada N, Tatsuno I, Hanski E, Caparon M, Sasakawa C. Microbiology. 1998;144:3079–3086. doi: 10.1099/00221287-144-11-3171. [DOI] [PubMed] [Google Scholar]
- 18.Berkower C, Ravins M, Moses A E, Hanski E. Mol Microbiol. 1999;31:1463–1475. doi: 10.1046/j.1365-2958.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 19.Okada N, Pentland P A, Falk P, Caparon M G. J Clin Invest. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz M A, Schaller M D, Ginsberg M. Annu Rev Cell Dev Biol. 1995;11:549–559. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 22.Mousa S A. Drugs of the Future. 1996;21:283–289. doi: 10.1358/dof.2010.035.01.1452012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mousa S A, Bozarth J, Youssef A, Levine B. Thromb Res. 1998;89:217–225. doi: 10.1016/s0049-3848(98)00007-3. [DOI] [PubMed] [Google Scholar]
- 24.Mousa S A, Forsythe M, Lorelli W, Bozarth J, Xue C-B, Wityak J, Sielecki T M, Olson R E, DeGrado W, Kapil R, et al. Coron Artery Dis. 1996;7:767–774. doi: 10.1097/00019501-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Finlay B B, Falkow S. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patti J M, Allen B L, McGavin M J, Hook M. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 27.van Putten J P, Duensing T D, Cole R L. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 28.Cleary P P, Cue D R, Mousa S A. International Patent PCT/US98/12010. 1998. [Google Scholar]
- 29.Molinari G, Chhatwal G S. J Infect Dis. 1998;177:1600–1607. doi: 10.1086/515310. [DOI] [PubMed] [Google Scholar]
- 30.Schrager H M, Rheinwald J G, Wessels M R. J Clin Invest. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]