Abstract
To provide information about specific depositors, scent marks need to encode a stable signal of individual ownership. The highly polymorphic major histocompatibility complex (MHC) influences scents and contributes to the recognition of close kin and avoidance of inbreeding when MHC haplotypes are shared. MHC diversity between individuals has also been proposed as a primary source of scents used in individual recognition. We tested this in the context of scent owner recognition among male mice, which scent mark their territories and countermark scents from other males. We examined responses towards urine scent according to the scent owner's genetic difference to the territory owner (MHC, genetic background, both and neither) or genetic match to a familiar neighbour. While urine of a different genetic background from the subject always stimulated greater scent marking than own, regardless of familiarity, MHC-associated odours were neither necessary nor sufficient for scent owner recognition and failed to stimulate countermarking. Urine of a different MHC type to the subject stimulated increased investigation only when this matched both the MHC and genetic background of a familiar neighbour. We propose an associative model of scent owner recognition in which volatile scent profiles, contributed by both fixed genetic and varying non-genetic factors, are learnt in association with a stable involatile ownership signal provided by other highly polymorphic urine components.
Keywords: major histocompatibility complex, individual recognition, scent marks, major urinary proteins, mice
1. Introduction
The ability to recognize and respond to particular individuals is advantageous in many social contexts, particularly among higher animals that can learn information about specific individuals and use this to adjust their behaviour in subsequent interactions. While incidental cues might be used to recognize specific individuals, signals that allow an individual to be easily and reliably identified are likely to evolve when the signaller also benefits from being recognized. Among mammals, scents commonly play a role in recognition of species, sex and individual identity (reviewed by Brown & Macdonald 1985; Halpin 1986). Animals frequently use scent marks deposited in the environment to communicate information such as territory ownership when the owner is absent. In this case, the only information available is in the location and constitution of the scent. Scent marks can provide comprehensive information, including the owner's current social, reproductive, health and nutritional status (Brown & Macdonald 1985; Brown 1995; Ferkin et al. 1997; Penn & Potts 1998a), but can only provide such information about the specific owner if the scent also encodes a reliable signal of individual ownership. Scent marks thus need to communicate owner identity, which is a fixed characteristic of the individual, against a complex background of other scent information that reflects physiological and environmental factors. How can individual ownership be signalled reliably if an animal's scent changes according to current status and environment?
From a theoretical viewpoint, we might expect fixed identity to be signalled, and recognized, independently of variable information within the scent profile. Species and sex-specific components have frequently been identified in scents, but as yet we know little about the molecular basis of individual identity signatures. Signals that have evolved to reliably indicate the owner's identity are likely to be both genetically determined (Boyse et al. 1987) and involve components that are not susceptible to disruption by metabolic and environmental influences. Although many genes are likely to influence scents indirectly via influences on hormone levels and other metabolites, this will not result in a fixed identity signature if variable non-genetic factors cause fluctuations in the same scent components. To provide unambiguous information on individual identity, ownership signals also need to be sufficiently polymorphic so that each individual in the local population has essentially a unique signature, though in practice it may be adequate that there is a low probability of shared signatures.
In rodents, two highly polymorphic and polygenic complexes are known to contribute to individual differences in scents. The major histocompatibility complex (MHC) encodes highly polymorphic glycoproteins involved in self–nonself recognition in the immune system. MHC also influences the scents produced by a range of animals, including mice (Yamazaki et al. 1979), rats (Singh et al. 1987), fishes (Olsen et al. 1998; Reusch et al. 2001) and humans (Wedekind & Furi 1997). Both mice and rats are able to discriminate urinary odours from donors that differ genetically only at alleles within the MHC region (Yamazaki et al. 1999; Singh 2001; Carroll et al. 2002). The molecular basis of MHC-associated odours in mice is manifest through a complex mixture of volatile metabolites bound and released by urinary proteins or peptides (Singer et al. 1993, 1997). These MHC-associated odours are used in mate choice (Potts et al. 1991) and in kin recognition (Manning et al. 1992; Yamazaki et al. 2000). Mice imprint on the odours of their parents during rearing (D'Udine & Alleva 1983; Yamazaki et al. 1988; Penn & Potts 1998c). Given the high degree of diversity in MHC alleles together with additional variation in other genes that influence scents, only very close kin are likely to share the imprinted parental odour types. Once adult, females tend to avoid mating with males of the same MHC type as their parents (Egid & Brown 1989; Penn & Potts 1998c) but prefer to nest communally with other females of the same MHC type (Manning et al. 1992).
The high degree of allelic variation in the MHC means that most individuals will differ in MHC type in natural outbred populations, making MHC an excellent candidate for contributing to scents used for individual recognition. Indeed, it is widely assumed that MHC odours provide the main basis for individual recognition among mice and other mammals (Yamaguchi et al. 1981; Beauchamp et al. 1985; Brown et al. 1987; Yamazaki et al. 1992, 1999, 2000; Brown 1995; Singh 2001; Brennan & Peele 2003). However, while it has been demonstrated unequivocally that MHC odours can be discriminated, this does not necessarily mean that animals use these scents to recognize the individual scent owner. In humans, genes influence hair colour and texture but these are not primary cues for individual recognition. Individuals are still recognized when their hair is dyed a completely different colour and restyled because facial and vocal cues dominate. Of course, such hair changes are easily detected and are likely to lead to an increased duration of inspection from family and friends. Mice and rats can readily be trained to discriminate between MHC-associated odours (e.g. Yamaguchi 1981; Schellinck et al. 1991), but this applies to any odours that can be discriminated (social or non-social) and is not interpreted as a test of individual recognition (e.g. Yamazaki et al. 2002). Similarly, spontaneous discrimination between MHC odours in habituation–dishabituation tests (Penn & Potts 1998b; Carroll et al. 2002) indicates detection of a difference that induces investigation, but does not signify that the scent owners are two different individuals. Scents associated with non-genetic differences such as status or diet also induce increased investigation (e.g. Brown et al. 1987; Schellinck et al. 1997; Penn & Potts 1998b) as it is important to acquire information about a scent owner's status as well as its identity. Determining whether scents are used for individual recognition requires a different type of test that addresses the functional meaning of the information acquired rather than just the ability to detect a difference between two scents.
One functional test of individual recognition is the pregnancy block that results when recently mated female mice are exposed to the scent of an unfamiliar male in the absence of the familiar stud male (Bruce 1959; reviewed by Brown 1985; Brennan & Peele 2003). Females form an olfactory memory of the stud male in the accessory olfactory bulb shortly after mating (Brennan et al. 1990). If exposed to the scent of an unfamiliar male from a different strain within five days of mating, prolactin release is disrupted and the embryos fail to implant. This test has therefore been applied to assess the olfactory signature used to recognize the stud male (Yamazaki et al. 1983, 1989; Peele et al. 2003; Leinders-Zufall et al. 2004). Pregnancy block can be induced by scent from an unfamiliar strain that differs from the stud male only at the MHC (Yamazaki et al. 1983, 1986), but this response differs in two important ways from that demonstrated in other studies, suggesting a different mechanism of response to MHC-associated scents. First, many studies confirm that pregnancy block is a specific response to contact with androgen-dependent scents from an intact unfamiliar male (e.g. Bruce 1960; Dominic 1965; Hoppe 1975), but scent from an unfamiliar MHC congenic strain induces pregnancy block whether from males or from females (Yamazaki et al. 1983). Second, response to the scent of an unfamiliar male is mediated through the vomeronasal system (VNS) and requires contact with male urine odours (Brennan & Peele 2003); pregnancy is not normally blocked by exposure to airborne volatiles alone (Dominic 1966; Rajendren & Dominic 1984). However, airborne volatiles from MHC congenic urine are just as effective as direct contact in inducing pregnancy block (Yamazaki et al. 1983). Although pregnancy block can be a specific response to contact with unfamiliar male scents, mice also show a generalized implantation failure in response to a wide range of stressors (Chipman & Fox 1966). The unusual response to airborne volatiles from unfamiliar MHC congenic mice of both sexes may therefore be more consistent with a generalized stress response to unfamiliar mouse odours, rather than activation of the specific VNS pathway involved in individual recognition of the stud male. However, recently, Leinders-Zufall et al. (2004) have shown that peptides that serve as ligands for MHC class I molecules differentially stimulate a subset of vomeronasal sensory neurons. Peptides that bind to molecules coded by a different MHC haplotype to the mating male stimulate pregnancy block when subsequently added to mating male's urine, suggesting that MHC peptide ligands may play a direct role in the recognition of familiar mates through the VNS, although equivalent tests with female urine have not yet been conducted.
Competitive scent marking provides a specific behavioural test for the recognition of individual scent ownership (Humphries et al. 1999; Nevison et al. 2000, 2003; Hurst et al. 2001b). Many animals, including mice, use scent marks to advertise territory ownership (Gosling 1982). When territory owners recognize scent from other males within their territory, they increase their own rate of scent marking to countermark (Ralls 1971; Hurst 1990; Humphries et al. 1999). This response has important fitness consequences, as scent marks containing the owner's identity signature provide physical proof of territory ownership and the ability to overcome challenges from other males. It is unsurprising then that countermarking other male scents both increases the owner's attractiveness to females and reduces challenges from other males (reviewed by Hurst et al. 2001a). Territory owners therefore respond rapidly to scent marks from other males, even when these are from a close relative (Hurst et al. 2001b). Application of this test to wild-derived house mice suggests that a second, highly polymorphic complex expressed at high concentration in mouse urine may be largely responsible for the ownership signal in mouse scent marks: the major urinary proteins (MUPs) encoded by a family of genes on chromosome 4 (Hurst et al. 2005). MUPs have a central cavity that binds signalling pheromones (Robertson et al. 1993). Unlike MHC, their only known function is in scent signalling (Beynon et al. 2001; Beynon & Hurst 2004). The pattern of MUPs expressed in urine is extremely variable between individuals, even within relatively isolated populations (Payne et al. 2001; Beynon et al. 2002), while individual patterns remain constant regardless of changes in individual status or diet (Payne et al. 2001) and persist over many weeks in urinary scent marks (Beynon & Hurst 2004). Territory owners rapidly countermark scent from males expressing a MUP pattern different to their own but fail to respond to scent marks that share their own MUP type, even against the highly variable genetic background of wild-derived mice (Hurst et al. 2001b). This suggests that MUPs (or MUP–ligand complexes) are the primary signal of ownership in scent marks. Less clear is the contribution of MHC to scent owner recognition. A potentially important factor not investigated by Hurst et al. (2001b) is that animals might learn to recognize specific individual scent owners and may incorporate other aspects of scent cues in the template when identifying highly familiar individuals. Thus, individual scent owners may be recognized not only according to similarity or dissimilarity to own scent (allowing animals to broadly recognize any scent that is not their own regardless of familiarity) but also according to remembered scents from other familiar individuals.
To investigate the contribution of MHC (termed H-2 in mice) to individual scent ownership recognition in the context of territory scent marking, we used inbred laboratory strains of mice that differed only at H-2, at many different background genes or at both H-2 and background genes. By introducing different scents into the territories of individual males, we examined whether males recognized the scent of another male and countermarked according to the scent owner's genetic similarity to the territory owner. We also examined whether similarity of introduced scents to a familiar neighbour influenced the cues used for individual scent owner recognition. While H-2 variation should result in discriminable differences between scents which may be evidenced by differences in scent investigation according to familiarity (e.g. Penn & Potts 1998b), the recognition that scents are not those of the territory owner (i.e. they have a different individual ownership signature) should induce a countermarking response. Our results confirm that mice discriminate differences in scents according to H-2, which induced prolonged close-contact investigation, but only when the combination of both H-2 and genetic background corresponded to a familiar neighbour. However, we found no evidence that H-2 associated scents were used in the functional recognition of individual scent ownership, at least in the context of competitive scent marking among male mice.
2. Methods
(a) Subjects and enclosures
Subjects were adult male mice of the inbred strains BALB/c (H-2d), BALB.k (H-2k), B10 (C57BL/10, H-2b) and B10.D2 (H-2d), housed individually in laboratory enclosures (1.2×0.3×0.8 m3). BALB and B10 strains were selected because males of both lines show competitive aggression and scent marking if given sufficient appropriate social experience, similar to that shown by wild mice, but are derived from completely separate genetic lineages, including the expression of different urinary MUP types (Robertson et al. 1996, unpublished data). Enclosures were arranged such that each resident had olfactory contact with a neighbour male through two circular double mesh grilles (5 cm diameter, 7 mm square mesh) in the shared side wall of their enclosures. Neighbours were either of different H-2 haplotype but same genetic background (ΔH-2: 8 neighbour pairs of BALB/c and BALB.k; 8 neighbour pairs of B10 and B10.D2) or shared H-2 haplotype on a different genetic background (Δbkg: 15 neighbour pairs of BALB/c and B10.D2, both H-2d haplotype but derived from different strains). A polypropylene mouse cage (33×15×13 cm3, containing sawdust and shredded paper nest material), sited centrally in each enclosure with mesh lid propped open, acted as both nest site and food/water station. Males were introduced into enclosures at least 18 days prior to scent response tests. Since mice require social experience to respond competitively to the scents of other males (Hurst 1990; Humphries et al. 1999; Hurst et al. 2001b), male intruders from each of the four urine donor strains (BALB/c, BALB.k, B10, B10.D2) were introduced into each enclosure for a maximum of 10 min to stimulate territorial behaviour (intruders were removed if males showed sustained aggression) in a balanced order once residents were established (at least 7 days), with at least 24 h between each introduction. Residents attacked intruders in 73% of introductions (n=248), with no significant difference according to genetic similarity between resident and intruder (any male encountered was clearly an intruder regardless of identity), although own strain males that had a highly familiar scent tended to be attacked less frequently (Δnone, 61%; ΔH-2, 76%; Δbkg, 74%; Δall, 81%; χ2=6.85, 3 d.f., NS). A small quantity of soiled nest material from captive wild female house mice was added to each nest site at weekly intervals throughout the experiment to enhance competitive behaviour (Humphries et al. 1999; Hurst et al. 2001b). During tests, grilles were covered with opaque Perspex plates to prevent any neighbour interference, and cage lids were closed during each test to encourage mice to explore their enclosures.
(b) Scent countermarking tests
To check that subjects responded competitively when the urine of another male was introduced into their territory, 10 μl of urine from individual adult wild-caught males or 10 μl water was streaked in the centre of a 15×15 cm2 perspex and cork tile covered in absorbent paper and placed at one end of a subject's home enclosure in two separate preliminary tests, conducted in balanced order at least 48 h apart. Urine from wild-derived males was used in these preliminary tests to minimize any similarity to the laboratory strains used as donors in the main series of tests. After 2 h, tiles were scanned under ultraviolet light using a BioRad FluorS imager and the total scent coverage and number of separate (non-touching) scent marks assessed using Scionimage software (www.scioncorp.com), excluding marks less than 50 pixels (5.5 mm2) to avoid counting footprints. Scent marking was significantly greater in response to unfamiliar male urine than to water for both the number of scent marks deposited (urine: 62.5±3.3 marks, water: 49.0±3.3; z=−4.67, p<0.001) and area covered by scent (urine: 18.7±1.5 cm2, water: 15.4±1.7 cm2; z=−3.0, p<0.003). There were no differences in this response according to neighbour type or strain (Mann–Whitney tests of scent marking in response to urine minus water, NS).
In the main series of six tests, we introduced a urine stimulus or water control into a male's home territory, using urine from separately caged males from the same four strains as the subjects. Urine stimuli differed in similarity to the subject, coming from the subject itself (own), or from males of the same strain and thus genetically identical (Δnone), differing in H-2 (ΔH-2), differing in genetic background (Δbkg), or differing in H-2 and background (Δall). The balanced design ensured that urine from each strain represented a different type of stimulus when presented to different strains (e.g. B10.D2 urine was Δnone to B10.D2 subjects, ΔH-2 to B10, Δbkg to BALB/c and Δall to BALB.k), controlling for any qualitative or quantitative differences in urine between strains. In each test, two tiles covered in absorbent paper were introduced at opposite ends of a male's enclosure (balanced for location), one tile streaked with 10 μl urine and the other with 10 μl water. This allowed us to control for any differences in activity between tests by comparing investigatory behaviour towards urine relative to water within the same test. In the ‘water only’ control test, both tiles carried water streaks. Scent marking responses are less localized than direct investigation, and thus total scent marking on both tiles was compared between tests (Hurst et al. 2001b). A piece of 7 mm2 mesh (30×30 mm2) was stapled over the top of each stimulus so that mice could contact the stimulus by pushing their noses through the mesh. Scent investigation (time spent sniffing or chewing at the mesh) and total time on each tile was video recorded in the dark phase under dim red light for 30 min. Since mice showed very little investigation of the stimuli after the first few minutes, only the first 10 min of each test was transcribed from videos. Tiles were scanned after 2 h to assess scent marking. Six tests were carried out for each subject in a counterbalanced plan to eliminate order effects, at intervals of at least 48 h (except that urine of different genetic background but same H-2 (Δbkg) could not be tested for BALB.k and B10 subjects). Urine was collected from caged males prior to the introduction of subjects into enclosures by gently picking up donors by the scruff of the neck and, if urine was not voided voluntarily, the bladder region was gently massaged. Voided urine was collected directly into Eppendorf tubes and stored at −18 °C. Each urine stimulus comprised a pool of equivalent volumes from at least four donor males (or four separate samples of own urine) to reduce non-genetic sources of individual variation, with a unique mixture of individual donors for each subject. One BALB/c, one BALB.k, one B10 and five B10.D2 males failed to deposit any scent marks in most of their tests and were excluded from analyses.
(c) Data analysis
To take into account any individual or strain differences in behaviour, all responses were compared within individuals. We tested the prediction that males recognize urine stimuli from other males as ‘not own’ and increase scent marking (number and coverage) to countermark, using specific Wilcoxon matched pair tests of response to own urine compared with urine from other males. Non-parametric tests were used because of the large variation between individuals in marking rates and activity. We also examined whether the duration of investigation of own urine differed from other urine stimuli using non-specific Wilcoxon matched pair tests. In each case, we checked whether there was a difference in response according to neighbour type (Δbkg or ΔH-2) or strain type (BALB or B10) using Mann–Whitney tests of response to other male urine minus response to own urine.
3. Results
(a) Scent countermarking
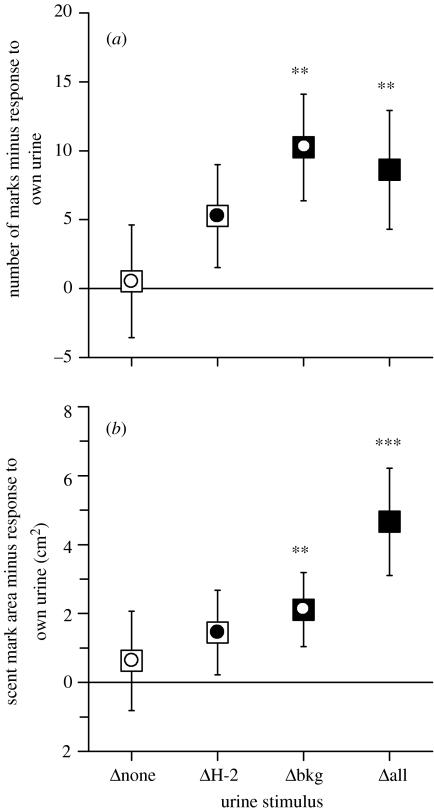
When territory owners encounter scent marks from another male, they countermark by increasing their own scent marks in the vicinity. Preliminary tests confirmed this response when urine from wild-derived males was introduced into each male's territory (see §2). Similarly, territory owners elevated scent marking in response to male urine from strains that were genetically different across the whole genome (Δall) relative to response to own urine, increasing both the number of scent marks deposited (figure 1a: Δall versus own; z=−2.36, p=0.009) and the total area of scent marks (figure 1b: Δall versus own; z=−3.11, p=0.001). A similar elevation of scent marking was elicited by Δbkg urine (figure 1: Δbkg versus own; frequency: z=−2.46, p=0.007; coverage: z=−2.51, p=0.006). These responses were consistent, regardless of neighbour type (neighbour Δbkg or ΔH-2, figure 2) or mouse strain (Mann–Whitney tests, NS).
Figure 1.
Scent marking response over 2 h to urine stimuli introduced into a male's home territory minus response to own urine (mean±s.e., all males combined). Responses greater than zero indicate an increased response relative to own urine for (a) number of scent marks deposited and (b) total area covered by scent marks. Specific Wilcoxon matched pair tests: **p<0.01; ***p<0.005. Squares indicate genetic background and circles show H‐2 haplotype (white, same as self; black, different from self). See text for further details of analysis.
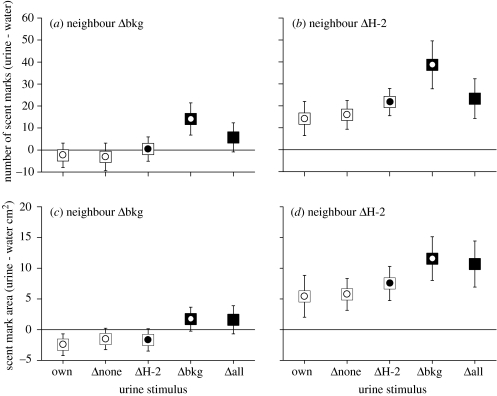
Figure 2.
Scent marking response over 2 h to urine stimuli introduced into a male's home territory according to neighbour type (mean±s.e., all strains combined). (a), (c) Response by males with a Δbkg neighbour (n=26); (b), (d) response by males with a ΔH-2 neighbour (n=27). To control for differences in the number and area of urine marks deposited by different strains and individuals within strains, data are expressed as the number (a), (c), or area (b), (d), of urine marks deposited on stimulus tiles in each urine stimulus test minus urine marking in the water control test.
By contrast, ΔH-2 urine did not stimulate a statistically significant increase in scent marking compared with own urine, even though data were collected for a large sample of animals (n=53) and analysed using a directional test for the predicted increase in scent marking (figure 1: ΔH-2 versus own; frequency: z=−1.45, p=0.073; coverage: z=−0.92, p=0.18). Urine from own strain mice also failed to stimulate a general increase in scent marking (figure 1: Δnone versus own; frequency: z=−0.01, p=0.50; coverage: z=−0.57, p=0.28), although the test strains differed in the direction of their response to own strain compared with own urine (frequency: z=−2.24, p=0.025; coverage: z=−2.89, p=0.004), as BALB strains tended to scent mark more while B10 strains tended to scent mark less. The scent marking response to each urine stimulus compared with own was not affected by neighbour type, although the amount of scent marking in all urine tests including own urine was greater among males with a ΔH-2 neighbour (figure 2a–d: neighbour ΔH-2 versus neighbour Δbkg, all stimuli). This was probably owing to a general difference in scent marking rates between the two batches of males that were tested with different neighbour types; this does not affect our results since responses were compared only relative to the response to own urine to take into account the wide variation in individual scent marking levels typically found between male mice. Alternatively, it is possible that a neighbour that shared the same genetic background as the subject stimulated a general increase in scent marking even in response to own and Δnone urine since, in this case, own and Δnone stimuli shared the same genetic background as the neighbour as well as the subject itself.
(b) Scent investigation
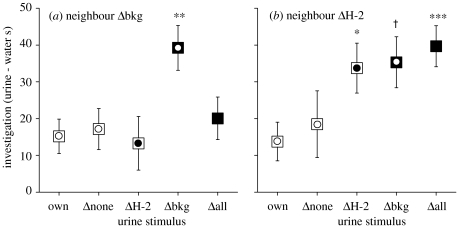
Although differences in H-2 clearly failed to stimulate a scent countermarking response, H-2 associated odours were discriminated as evidenced by the duration of close investigation to gather information from scent stimuli. This depended not on similarity to own scent but on similarity of the urine stimulus to the familiar neighbour's scent. All scents, including own, stimulated some initial close investigation (figure 3), but scents from males that were genetically identical to the familiar neighbour stimulated much more prolonged investigation than own (table 1). Thus, response to ΔH-2 urine differed according to neighbour type (table 1): when ΔH-2 urine matched the neighbour, investigation was prolonged (figure 3b: ΔH-2 versus own urine, neighbour ΔH-2; n=25, z=−2.22, p=0.026), but the same urine stimulated no greater investigation than own urine when the neighbour was Δbkg (figure 3a: ΔH-2 versus own urine, neighbour Δbkg; n=26, z=−0.55, p=0.60). Instead, Δbkg urine that matched the Δbkg neighbour strain stimulated prolonged investigation (figure 3a: Δbkg versus own, neighbour Δbkg; n=26, z=−2.76, p=0.005). Mice thus appeared to recognize a scent identical in genotype to a familiar neighbour (but originating from different donors) when this involved both H-2 and background genes. A match of only H-2 or genetic background was not sufficient to induce the same response (see figure 3a). Since a neighbour represents a socially important stimulus, particularly a neighbour that scent marks within a male's territory, prolonged investigation may be important to gain further information about the owner, despite the relative familiarity of the scent. Nonetheless, having investigated the scent closely, mice only countermarked urine that differed in genetic background from themselves (Δbkg, Δall and wild-derived).
Figure 3.
Close investigation of urine stimuli introduced into a male's home territory (mean±s.e., all strains combined). (a) Response by males with a Δbkg neighbour (n=26); (b) response by males with a ΔH-2 neighbour (n=27). To control for any differences in activity between tests, data are expressed as the difference in duration of sniffing at the mesh covering the urine minus water stimulus by each male over the first 10 min of each test. Zero difference would indicate equal sniffing at both urine and water. Non-specific Wilcoxon matched pair tests compared response to each urine stimulus versus own: *p<0.05, **p<0.01, ***p<0.005. † Because of the combination of strains available, only half the males with a ΔH-2 neighbour could be tested with Δbkg stimulus (n=13); this response only tended towards significance (p=0.10) but was of much lower statistical power than all other tests. See text for further details.
Table 1.
Effects of urine stimulus, neighbour type (neighbour ΔH-2 or Δbkg) and subject strain (subjects classified as BALB or B10) on duration of urine investigation relative to own urine.
| urine stimulus | difference between urine stimulus and own urine | difference in response according to neighbour typea | difference in response according to subject straina |
|---|---|---|---|
| Δnone | z=−0.83, p=0.41 | z=−0.82, p=0.43 | z=−2.00, p=0.046 |
| ΔH-2 | z=−1.32, p=0.19 | z=−2.00, p=0.046 | z=−1.34, p=0.19 |
| Δbkg | z=−3.15, p=0.001 | z=−0.30, p=0.78 | z=−2.37, p=0.017 |
| Δall | z=−2.75, p=0.005 | z=−2.42, p=0.015 | z=−1.67, p=0.10 |
| neighbour strain (ΔH-2 or Δbkg) | z=−3.53, p<0.0005 | z=−0.30, p=0.77 | z=−1.02, p=0.32 |
Mann–Whitney test of investigation response to urine stimulus minus own urine.
The genetic match of a scent to a familiar neighbour was not the only factor that provoked prolonged investigation. Novel scents typically induce much longer close investigation than very familiar scents, as animals need to gain all information de novo from the novel scent. All scents of unfamiliar genetic background stimulated prolonged investigation. Males with a ΔH-2 neighbour had only very brief pre-exposure to scents from mice of different genetic background prior to tests (when intruders were introduced briefly into each male's territory) while those with a Δbkg neighbour had substantial pre-exposure to scents associated with a different genetic background before and between tests. When tested with Δall urine (which was completely different from own), investigation depended significantly on neighbour type (table 1). When males were familiar with scents associated with a different genetic background through exposure to a Δbkg neighbour, Δall urine stimulated no more investigation than own (figure 3a: Δall versus own, neighbour Δbkg; n=26, z=−0.24, p=0.82). However, when genetic background was unfamiliar (differing from both own and neighbour), investigation of Δall urine was prolonged (figure 3b: Δall versus own, neighbour ΔH-2; n=27, z=−2.96, p=0.002). Similarly, Δbkg urine tended to stimulate prolonged investigation among mice unfamiliar with the background, although the sample size for this particular combination was much smaller so the power of the test was much weaker (figure 3b: Δbkg versus own, neighbour ΔH-2, n=13, z=−1.64, p=0.10).
Unfamiliar H-2 did not stimulate prolonged investigation. Males with a Δbkg neighbour had only very brief pre-exposure to scents of different H-2 prior to these tests. However, despite the apparent novelty, unfamiliar ΔH-2 urine stimulated no more investigation than own (figure 3a: ΔH-2 versus own, neighbour Δbkg; n=26, z=−0.55, p=0.60). Neither did mice investigate urine of unfamiliar H-2 but the same genetic background as a familiar neighbour more than own (figure 3a: Δall versus own, neighbour Δbkg; n=26, z=−0.24, p=0.82). Thus unfamiliar H-2 scents stimulated no significant responses, either with respect to investigation (figure 3) or scent marking (figure 1), while unfamiliar genetic background stimulated both close investigation and an increase in subsequent scent marking.
4. Discussion
H-2 haplotype influenced response only when an introduced scent matched the whole genome of a familiar neighbour, including both H-2 and genetic background. Animals were clearly stimulated to acquire further information from scents that were from males genetically identical to a familiar neighbour, as these induced prolonged close contact investigation despite their apparent familiarity. However, having gathered further information, H-2 associated odours (whether familiar or unfamiliar) did not then influence whether mice increased scent marking in the vicinity of the investigated scent; this depended entirely on whether the genetic background of the donor differed from own. This is consistent with results from wild mice (Hurst et al. 2001b; Hurst et al. 2005) and confirms that mice recognize scents from males of different genetic background as ‘not own’, and countermark them accordingly. This countermarking response occurs regardless of the familiarity of the scent owner; as scent marks are used to advertise territory ownership, it is essential that territory owners ensure that scents from competitors are countermarked, regardless of whether competitors are familiar or unfamiliar. This lack of response to urine of different H-2 cannot be explained as kin bias. Although males differing only in H-2 were genetically very similar, territory owners were just as aggressive towards intruders from a strain differing only at H-2 as they were towards those from a completely different lineage during direct interactions. Wild-derived males will also countermark scents from full siblings as strongly as those from unrelated individuals (Hurst et al. 2001b).
The lack of countermarking when scents differed in H-2 haplotype from the territory owner, in contrast to the countermarking of scents differing in genetic background, is consistent with the proposal that scent ownership recognition is based on the highly polymorphic pattern of MUPs or MUP–ligand complexes expressed in mouse urine (Hurst et al. 2001b). Individual MUP pattern is a fixed characteristic in adulthood (Payne et al. 2001) and contact investigation is required to stimulate a countermarking response, indicating that scent owner recognition involves an involatile signal (Nevison et al. 2003). It is likely that recognition occurs through the VNS, by pumping involatile proteins or protein–ligand complexes to the sensory epithelium. Neurons in the accessory olfactory bulb receiving input from the vomeronasal organ respond selectively according to the sex and strain of a stimulus animal, suggesting that the VNS is involved in sex and identity recognition (Dulac & Torello 2003; Luo et al. 2003; Brennan & Keverne 2004). Indeed, the countermarking response to other male scents is eliminated by the removal of the vomeronasal organ (Maruniak et al. 1986; Wysocki & Lepri 1991).
The H-2 type produces discriminable differences in urine scent, so why were these differences not enough to stimulate countermarking? While involatile MUP–ligand complexes are specialized signalling components in mouse urine, which are readily distinguishable from other urinary components (Beynon et al. 2001), this may not be the case for H-2 associated volatile odours. Although the molecular basis of H-2 odours and the mechanism underlying their production is not yet understood, these odours appear to be a complex mixture of volatile metabolites that are bound and released by urinary proteins (Singer et al. 1993, 1997). H-2 associated odours may not be detected independently of metabolic, dietary and bacterial flora effects that also influence volatile metabolites in urine. In natural populations, where the environment is considerably more variable than the laboratory, signatures based on patterns of volatile metabolites might be unstable. This may explain why MHC associated odours are masked when laboratory rats are fed different diets (Schellinck et al. 1997). Moreover, Eggert et al. (1996) failed to train rats to recognize familiar H-2 scents that were expressed on different genetic backgrounds. If many genes additional to H-2 contribute to the complex pattern of volatile urinary metabolites, this may explain why mice only recognized similarity to a familiar neighbour's scent when this involved the combination of both H-2 and background scents; familiar H-2 odours on a different background did not stimulate increased investigation.
This is not to suggest that the complex volatile profiles expressed by individuals, including those contributed by H-2, have no role to play in individual recognition. When animals encounter familiar volatile odours, they do not need to contact the scent source to recognize that it is familiar. Indeed, in semi-natural populations, competitors generally sniff towards each other from a distance, avoiding contact with a potentially dangerous competitor (Hurst 1993). However, the response on encountering a volatile odour that is unfamiliar, detected through the main olfactory system, is to approach and undertake more prolonged close contact investigation (Rowe & Redfern 1969; Hurst 1993; Humphries et al. 1999). Close investigation is provoked by scent from an unfamiliar individual, by a change in the odour of a familiar individual, or by scent that has not been encountered recently. Novelty and nasal contact stimulate the pumping mechanism required to deliver involatile scent components to the vomeronasal organ (Meredith 1994; Luo et al. 2003). An unfamiliar volatile profile, detected through the main olfactory system, thus controls the acquisition of further non-volatile information through the VNS. Close investigation of unfamiliar scents also provides an opportunity to associate in memory an involatile ownership signal (detected through the VNS) and volatile scents such as those associated with MHC haplotype (detected simultaneously through the main olfactory system; Guo et al. 1997; Hurst & Beynon 2004; Hurst et al. 2005). Although this is not yet established, it is possible that involatile MUPs bind and release H-2 volatiles (Singer et al. 1993; Beynon & Hurst 2004). While volatile scents may provide unstable information about identity, detection of airborne volatiles through the main olfactory system is much quicker than detection of involatile components through the VNS, which can take about 20 s to peak response even once the scent source is contacted (Luo et al. 2003). We suggest that mice learn to associate a familiar cocktail of volatiles with a stable and involatile individual ownership signal. This would provide the advantage of rapid recognition of familiar individuals of known status through familiar airborne scents without necessitating contact. If the airborne signal changes, close contact investigation could then provide unambiguous identification of the scent owner from involatile components such as MUPs and their ligands (Hurst & Beynon 2004). Notably, the ability to identify the sex of conspecifics from sex-specific volatiles also seems to depend on learning an association between volatile and involatile scent components by naive animals (Moncho-Bogani et al. 2002). Removal of vomeronasal input, and thus detection of involatile scents, causes a much greater deficit in sex recognition among naive animals that have not had the opportunity to learn this association compared with those with prior social experience (Wysocki & Lepri 1991). Most interestingly, vomeronasal sensory neurons respond differentially to involatile peptides that bind to H-2 molecules coded by different haplotypes even at extremely low concentrations, suggesting that H-2 specific ligands might contribute to such an involatile ownership signal (Leinders-Zufall et al. 2004). However, despite the ability to recognize H-2 associated peptides, these did not appear to contribute to the recognition of urine from different males in our study.
The prolonged investigation of unfamiliar background scents seen in this study was unsurprising as animals need to assess all information in novel scents de novo, although unfamiliar H-2 appeared to stimulate little if any interest. In dishabituation tests, unfamiliar H-2 only stimulates an increase in investigation of about 1 s relative to controls (Penn & Potts 1998b; Carroll et al. 2002). It is less clear why animals spent so long investigating scents that matched those of a familiar neighbour. We did not introduce urine collected from the neighbour itself but from males of the same inbred strain as the neighbour. There are discriminable differences in scents even from genetically identical individuals of the same inbred strain maintained under standardized conditions (Brown et al. 1987; Penn & Potts 1998b). This is presumed to be owing to physiological differences between animals from non-genetic sources. Urine from animals of the same strain as the neighbour would thus have been only partially familiar, and prolonged investigation may have reflected the need to assess any change in status or to check the identity of a familiar neighbour, which represented a socially important individual. Similar interest was not shown towards urine from males of the same strain as the territory owner, thus prolonged investigation was not due just to partial familiarity but to recognition of similarity to the familiar neighbour's scent. Nonetheless, investigated scents did not stimulate countermarking if these differed in H-2 but not genetic background from the territory owner, even when the scent was similar to that of a familiar neighbour and potentially important competitor. This implies that scents were only recognized as not own following the acquisition of scent information when the scent was of different genetic background, not of different H-2, since countermarking competitor scents is fundamental to maintaining territory scent marks. Scent components determined by genetic background therefore appear to be considerably more important than those determined by H-2 in signalling scent ownership. More significantly, odours associated with H-2 haplotype seem to be insufficient for identification of scent mark ownership, at least in the context of competitive scent marking. There is thus still no convincing empirical evidence that H-2 associated odours play a significant role in individual recognition, although these odours are likely to contribute to the recognition of familiar scents such as the recognition of a familiar mate or neighbour competitor.
Acknowledgments
This was supported by Biotechnology and Biological Sciences Research Council grants to J.L.H. and R.J.B. Authors C.M.N. and M.D.T. made an equal contribution to this work. We thank Linda Burgess, John Waters and Sue Jopson for technical help, Stuart Armstrong for checking the MUP patterns expressed by each mouse strain and members of the Animal Behaviour Group at Leahurst for helpful discussions.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Beauchamp G.K, Yamazaki K, Wysocki C.J, Slotnick B.M, Thomas L, Boyse E.A. Chemosensory recognition of mouse major histocompatibility types by another species. Proc. Natl Acad. Sci. USA. 1985;82:4186–4188. doi: 10.1073/pnas.82.12.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon R.J, Hurst J.L. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Beynon R.J, et al. Mice, MUPs and myths: structure–function relationships of the major urinary proteins. In: Marchelewska-Koj A, Muller-Schwarze D, Lepri J, editors. Chemical signals in vertebrates. vol. 9. Plenum Press; New York: 2001. pp. 149–156. [Google Scholar]
- Beynon R.J, Veggerby C, Payne C.E, Robertson D.H, Gaskell S.J, Humphries R.E, Hurst J.L. Polymorphism in major urinary proteins: molecular heterogeneity in a wild mouse population. J. Chem. Ecol. 2002;28:1429–1446. doi: 10.1023/a:1016252703836. [DOI] [PubMed] [Google Scholar]
- Boyse E.A, Beauchamp G.K, Yamazaki K. The genetics of body scent. Trends Genet. 1987;3:97–102. [Google Scholar]
- Brennan P.A, Keverne E.B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 2004;14:R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Brennan P.A, Peele P. Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem. Soc. Trans. 2003;31:152–155. doi: 10.1042/bst0310152. [DOI] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne E.B. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Brown R.E. The rodents I: effects of odours on reproductive physiology (primer effects) In: Brown R.E, Macdonald D.W, editors. Social odours in mammals. vol. 1. Clarendon Press; Oxford: 1985. pp. 245–344. [Google Scholar]
- Brown R.E. What is the role of the immune system in determining individually distinct body odours? Int. J. Immunopharmacol. 1995;17:655–661. doi: 10.1016/0192-0561(95)00052-4. [DOI] [PubMed] [Google Scholar]
- Brown R.E, McDonald D.W. Social odours in mammals. vol. 1 & 2. Clarendon Press; Oxford: 1985. [Google Scholar]
- Brown R.E, Singh P.B, Roser B. The major histocompatibility complex and the chemosensory recognition of individuality in rats. Physiol. Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- Bruce H.M. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Bruce H.M. A block to pregnancy in the mouse caused by proximity of strange males. J. Reprod. Fertil. 1960;1:96–103. doi: 10.1530/jrf.0.0010096. [DOI] [PubMed] [Google Scholar]
- Carroll L.S, Penn D.J, Potts W.K. Discrimination of MHC-derived odors by untrained mice is consistent with divergence in peptide-binding region residues. Proc. Natl Acad. Sci. USA. 2002;99:2187–2192. doi: 10.1073/pnas.042244899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman R.K, Fox K.A. Oestrus synchronization and pregnancy blocking in wild house mice (Mus musculus) J. Reprod. Fertil. 1966;12:233–236. doi: 10.1530/jrf.0.0120233. [DOI] [PubMed] [Google Scholar]
- Dominic C.J. The origin of the pheromones causing pregnancy block in mice. J. Reprod. Fertil. 1965;10:469–472. doi: 10.1530/jrf.0.0100469. [DOI] [PubMed] [Google Scholar]
- Dominic C.J. Observations on the reproductive pheromones of mice I. Source. J. Reprod. Fertil. 1966;11:407–414. doi: 10.1530/jrf.0.0110407. [DOI] [PubMed] [Google Scholar]
- D'Udine B, Alleva E. Early experience and sexual preferences in rodents. In: Bateson P, editor. Mate choice. Cambridge University Press; Cambridge, UK: 1983. pp. 311–327. [Google Scholar]
- Dulac C, Torello A.T. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat. Rev. Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Eggert F, Holler C, Luszyk D, Muller-Ruchholtz W, Ferstl R. MHC-associated and MHC-independent urinary chemosignals in mice. Physiol. Behav. 1996;59:57–62. doi: 10.1016/0031-9384(95)02029-2. [DOI] [PubMed] [Google Scholar]
- Egid K, Brown J.L. The major histocompatibility complex and female mating preferences in mice. Anim. Behav. 1989;38:548–550. [Google Scholar]
- Ferkin M.H, Sorokin E.S, Johnston R.E, Lee C.J. Attractiveness of scents varies with protein content of the diet in meadow voles. Anim. Behav. 1997;53:133–141. [Google Scholar]
- Gosling L.M. A reassessment of the function of scent marking in territories. Z. Tierpsychol. 1982;60:89–118. [Google Scholar]
- Guo J, Zhou A, Moss R.L. Urine and urine-derived compounds induce c-fos mRNA expression in accessory olfactory bulb. Neuroreport. 1997;8:1679–1683. doi: 10.1097/00001756-199705060-00024. [DOI] [PubMed] [Google Scholar]
- Halpin Z.T. Individual odors among mammals: origins and functions. Adv. Stud. Behav. 1986;16:39–70. [Google Scholar]
- Hoppe P.C. Genetic and endocrine studies of the pregnancy-blocking pheromone of mice. J. Reprod. Fertil. 1975;45:109–115. doi: 10.1530/jrf.0.0450109. [DOI] [PubMed] [Google Scholar]
- Humphries R.E, Robertson D.H.L, Beynon R.J, Hurst J.L. Unravelling the chemical basis of competitive scent marking in house mice. Anim. Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- Hurst J.L. Urine marking in populations of wild house mice Mus domesticus Rutty 1. Communication between males. Anim. Behav. 1990;40:209–222. [Google Scholar]
- Hurst J.L. The priming effects of urine substrate marks on interactions between male house mice Mus musculus domesticus Schwarz and Schwarz. Anim. Behav. 1993;45:55–81. [Google Scholar]
- Hurst J.L, Beynon R.J. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst J.L, Beynon R.J, Humphries R.E, Malone N, Nevison C.M, Payne C.E, Robertson D.H.L, Veggerby C. Information in scent signals of competitive social status: the interface between behaviour and chemistry. In: Marchelewska-Koj A, Muller-Schwarze D, Lepri J, editors. Chemical signals in vertebrates. vol. 9. Plenum Press; New York: pp. 43–52. [Google Scholar]
- Hurst J.L, Payne C.E, Nevison C.M, Marie A.D, Humphries R.E, Robertson D.H, Cavaggioni A, Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Hurst J.L, Thom M.D, Nevison C.M, Humphries R.E, Beynon R.J. The “scents” of ownership. In: Mason R, Muller-Schwarze D, editors. Chemical signals in vertebrates. vol. 10. Kluwer; Dordrecht: In press. [Google Scholar]
- Leinders-Zufall T, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee M.S, Katz L.C. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Manning C.J, Wakeland E.K, Potts W.K. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature. 1992;360:581–583. doi: 10.1038/360581a0. [DOI] [PubMed] [Google Scholar]
- Maruniak J.A, Wysocki C.J, Taylor J.A. Mediation of male mouse urine marking and aggression by the vomeronasal organ. Physiol. Behav. 1986;37:655–657. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol. Behav. 1994;56:345–354. doi: 10.1016/0031-9384(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Hernandez A, Novejarque A, Martinez-Garcia F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol. Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- Nevison C.M, Barnard C.J, Hurst J.L, Beynon R.J. The consequences of inbreeding for recognising competitors. Proc. R. Soc. B. 2000;267:687–694. doi: 10.1098/rspb.2000.1057. 10.1098/rspb.2000.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison C.M, Armstrong S, Beynon R.J, Humphries R.E, Hurst J.L. The ownership signature in mouse scent marks is involatile. Proc. R. Soc. B. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. 10.1098/rspb.2003.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K.H, Grahn M, Lohm J, Langefors A. MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.) Anim. Behav. 1998;56:319–327. doi: 10.1006/anbe.1998.0837. [DOI] [PubMed] [Google Scholar]
- Payne C.E, Malone N, Humphries R.E, Bradbrook C, Veggerby C, Beynon R.J, Hurst J.L. Heterogeneity of major urinary proteins in house mice: population and sex differences. In: Marchelewska-Koj A, Muller-Schwarze D, Lepri J, editors. Chemical signals in vertebrates. vol. 9. Plenum Press; New York: 2001. pp. 233–240. [Google Scholar]
- Peele P, Salazar I, Mimmack M, Keverne E.B, Brennan P.A. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur. J. Neurosci. 2003;18:622–628. doi: 10.1046/j.1460-9568.2003.02790.x. [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W.K. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 1998a;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W.K. Untrained mice discriminate MHC-determined odors. Physiol. Behav. 1998b;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W. MHC-disassortative mating preferences reversed by cross-fostering. Proc. R. Soc. B. 1998c;265:1299–1306. doi: 10.1098/rspb.1998.0433. 10.1098/rspb.1998.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts W.K, Manning C.J, Wakeland E.K. Mating patterns in seminatural populations of mice influenced By MHC genotype. Nature. 1991;352:619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dominic C.J. Involvement of contact stimuli in the male-induced implantation block (the Bruce effect) in mice. Anim. Reprod. Sci. 1984;7:377–383. [Google Scholar]
- Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- Reusch T.B, Haberli M.A, Aeschlimann P.B, Milinski M. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 2001;414:300–302. doi: 10.1038/35104547. [DOI] [PubMed] [Google Scholar]
- Robertson D.H.L, Beynon R.J, Evershed R.P. Extraction, characterization and binding analysis of two pheromonally active ligands associated with major urinary protein of house mouse (Mus musculus) J. Chem. Ecol. 1993;19:1405–1416. doi: 10.1007/BF00984885. [DOI] [PubMed] [Google Scholar]
- Robertson D.H, Cox K.A, Gaskell S.J, Evershed R.P, Beynon R.J. Molecular heterogeneity in the major urinary proteins of the house mouse Mus musculus. Biochem. J. 1996;316:265–272. doi: 10.1042/bj3160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.F, Redfern R. Aggressive behaviour in related and unrelated wild house mice (Mus musculus L.) Ann. Appl. Biol. 1969;64:425–431. [Google Scholar]
- Schellinck H.M, Brown R.E, Slotnick B.M. Training rats to discriminate between the odors of individual conspecifics. Anim. Learn. Behav. 1991;19:223–233. [Google Scholar]
- Schellinck H.M, Slotnick B.M, Brown R.E. Odors of individuality originating from the major histocompatibility complex are masked by diet cues in the urine of rats. Anim. Learn. Behav. 1997;25:193–199. [Google Scholar]
- Singer A.G, Tsuchiya H, Wellington J.L, Beauchamp G.K, Yamazaki K. Chemistry of odortypes in mice—fractionation and bioassay. J. Chem. Ecol. 1993;19:569–579. doi: 10.1007/BF00994326. [DOI] [PubMed] [Google Scholar]
- Singer A.G, Beauchamp G.K, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc. Natl Acad. Sci. USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.B. Chemosensation and genetic individuality. Reproduction. 2001;121:529–539. doi: 10.1530/rep.0.1210529. [DOI] [PubMed] [Google Scholar]
- Singh P.B, Brown R.E, Roser B. MHC antigens in urine as olfactory recognition cues. Nature. 1987;327:161–164. doi: 10.1038/327161a0. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Furi S. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc. R. Soc. B. 1997;264:1471–1479. doi: 10.1098/rspb.1997.0204. 10.1098/rspb.1997.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki C.J, Lepri J.J. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991;39:661–669. doi: 10.1016/0960-0760(91)90265-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yamazaki K, Beauchamp G.K, Bard J, Thomas L, Boyse E.A. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc. Natl Acad. Sci. USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Yamaguchi M, Baranoski L, Bard J, Boyse E.A, Thomas L. Recognition among mice. Evidence from the use of a Y-maze differentially scented by congenic mice of different major histocompatibility types. J. Exp. Med. 1979;150:755–760. doi: 10.1084/jem.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Wysocki C.J, Bard J, Thomas L, Boyse E.A. Recognition of H-2 types in relation to the blocking of pregnancy in mice. Science. 1983;221:186–188. doi: 10.1126/science.6857281. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Matsuzaki O, Kupniewski D, Bard J, Thomas L, Boyse E.A. Influence of a genetic difference confined to mutation of H-2K on the incidence of pregnancy block in mice. Proc. Natl Acad. Sci. USA. 1986;83:740–741. doi: 10.1073/pnas.83.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Kupniewski D, Bard J, Thomas L, Boyse E.A. Familial imprinting determines H-2 selective mating preferences. Science. 1988;240:1331–1332. doi: 10.1126/science.3375818. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Bard J, Boyse E.A. Sex-chromosomal odor types influence the maintenance of early pregnancy in mice. Proc. Natl Acad. Sci. USA. 1989;86:9399–9401. doi: 10.1073/pnas.86.23.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Imai Y, Bard J, Thomas L, Boyse E.A. MHC control of odortypes in the mouse. In: Doty R.L, Muller-Schwarze D, editors. Chemical signals in vertebrates VI. Plenum Press; New York: 1992. pp. 189–196. [Google Scholar]
- Yamazaki K, Beauchamp G.K, Singer A, Bard J, Boyse E.A. Odortypes: their origin and composition. Proc. Natl Acad. Sci. USA. 1999;96:1522–1525. doi: 10.1073/pnas.96.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp G.K, Curran M, Bard J, Boyse E.A. Parent-progeny recognition as a function of MHC odortype identity. Proc. Natl Acad. Sci. USA. 2000;97:10 500–10 502. doi: 10.1073/pnas.180320997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse E.A, Bard J, Curran M, Kim D, Ross S.R, Beauchamp G.K. Presence of mouse mammary tumor virus specifically alters the body odor of mice. Proc. Natl Acad. Sci. USA. 2002;99:5612–5615. doi: 10.1073/pnas.082093099. [DOI] [PMC free article] [PubMed] [Google Scholar]