Abstract
Metacestodes of Hymenolepis diminuta secrete a molecule that decreases vitellogenin (Vg) synthesis in the beetle host, Tenebrio molitor. The 5608 bp T. molitor Vg cDNA represents a single-copy gene encoding a single open reading frame of 1821 amino acids with a predicted molecular mass of 206 kDa. Northern blot analysis revealed detectable levels of transcripts only in adult females. In vivo, Vg mRNA abundance was significantly higher in fat bodies from infected females compared with control females at all but the earliest time point. In vitro, Vg mRNA abundance was significantly increased in fat bodies incubated with live stage I–II parasites. The apparent conflict between increased Vg mRNA abundance and decreased Vg protein in fat bodies from infected females is discussed.
Keywords: Tenebrio molitor, Hymenolepis diminuta, vitellogenin, mRNA abundance
Introduction
A decrease in reproductive output is commonly found in insect intermediate hosts that are parasitized (Hurd, 2001). We are using the relationship between the rat tapeworm, Hymenolepis diminuta and its intermediate beetle host, Tenebrio molitor, to explore a parasite strategy to manipulate intermediate-host resource management in a way that decreases the resources devoted to host reproduction. Changes in both fecundity and fertility have long been observed in female T. molitor parasitized by H. diminuta. Reductions in both egg production and egg viability are associated with two major events. First, there is a decrease in synthesis of vitellogenin (Vg) by the fat body and secondly, the uptake of circulating Vg by the ovaries is diminished (Webb & Hurd, 1996). Developing parasites produce a manipulator molecule that directly affects insect vitellogenesis by decreasing Vg synthesis in the fat body of female insects both in vivo and in vitro (Webb & Hurd, 1996, 1999). The parasite molecule is stage-specific in that significant decreases in Vg synthesis are typically associated only with early (stage I–II) metacestodes (Webb & Hurd, 1999). We have shown that this molecule is secreted by incubating fat body tissue from female beetles in medium conditioned by parasites (Hurd, unpublished). Briefly, fat body incubation medium was incubated with early (stage I–II) metacestodes (100 μl per 100 parasites) at 26 °C for 4 h. When fat body tissue was incubated in this conditioned medium, Vg production was reduced by 61% compared with fat body tissue incubated in control medium, reflecting a significant difference (P = 0.04). Although we have been unable to purify this manipulator molecule to date, reverse phase high-performance liquid chromatography of an acid-extract of stage II metacestodes has revealed the presence of a biologically active peak containing two compounds. Our data suggest that one of these compounds is a short peptide of about 200–250 Da. Despite extensive investigations, we have been unable to sequence this peptide, which appears to be N-terminally blocked (Hurd, unpublished).
We investigated previously the possible role of apoptosis (programmed cell death) in the beetle fat body as a mechanism by which Vg synthesis might be reduced (Warr et al., 2004). Our data provided evidence that H. diminuta infection causes apoptosis in a proportion of the fat body cells. While this may contribute to the reduction in Vg synthesis in vivo, our in vitro studies showed that apoptosis was not rapidly induced by the presence of live parasites. We therefore concluded that apoptosis was not directly induced by the manipulator molecule that down-regulates Vg synthesis. Consequently, we investigated whether the decrease was associated with transcriptional regulation through reduced abundance of Vg mRNA. In mosquitoes, infection with malaria parasites causes a significant decrease in Vg mRNA abundance, changes in the titre of Vg circulating in the haemolymph and a decrease in Vg content in the ovaries (Ahmed et al., 2001). The hypothesis to be tested in the present study was that H. diminuta infection in T. molitor would similarly affect Vg mRNA abundance. As there was no information available on Vg gene(s) in T. molitor, we first cloned and characterized the complete Vg cDNA so that our hypothesis could be tested.
Over the past decade, there has been a steady increase in the number of insect Vg genes that have been cloned and sequenced (Chen et al., 1994; Romans et al., 1995; Lee et al., 2000; Tufail & Takeda, 2002). Analysis of their deduced amino acid sequences has identified a number of common characteristics allowing them to be aligned confidently along their entire length. These include a highly conserved GL/ICG motif and a series of cysteine residues at conserved locations towards the C-terminus of the protein (Romans et al., 1995; Lee et al., 2000). A similar motif (CGLCG) is also common in the C-terminus of Vg proteins in a number of vertebrate and nematode species and can be aligned with the GL/ICG motif of insects (Hagedorn et al., 1998).
Although some of the molecular events involved in Vg gene activation have been studied in insects, most work has focused on hormonal control by 20-hydroxyecdysone and little extends beyond this to investigate the specific mechanisms whereby Vg genes are regulated. One exception is the Vg gene from the mosquito, Aedes aegypti, where transcriptional regulation has been studied in detail, revealing three distinct promoter domains. A proximal region, responsible for tissue and stage specificity, carries binding sites for EcR/USP, GATA, C/EBP and HNF3/fkh. Upstream of this, a median domain, carrying binding sites for ecdysone response factors E74 and E75, is required for hormonal enhancement. Further upstream, a distal domain containing binding sites for multiple GATA factors is required for the high levels of Vg expression seen in vivo (Kokoza et al., 2001). More recently, the A. aegypti Vg gene promoter has also been shown to carry multiple binding sites for iso-forms of the Broad gene that encode C2H2-type zinc-finger DNA binding proteins, acting as transcriptional regulators of the 20-hydroxyecdysone cascade (Chen et al., 2004). In stark contrast, nothing is known of the regulation of the T. molitor Vg gene.
We have previously shown that Vg synthesis in T. molitor is decreased both in vivo and in vitro by a molecule secreted by H. diminuta (Webb & Hurd, 1996, 1999; Hurd, unpublished). Here, we study Vg mRNA abundance to determine whether the parasite manipulator molecule can act to alter the expression of the Vg gene. This was accomplished by comparing Vg mRNA abundance profiles between infected and non-infected beetles at various time-points post-emergence.
Results
Characterization of the Vg precursor cDNA from Tenebrio molitor
The 3′ end of the Vg gene from T. molitor was isolated by 3′-RACE from a cDNA template (Lee et al., 2000; Piulachs et al., 2003). Reactions were carried out with both GLCG and GICG degenerate forward primers but only the former produced a band of the expected size (600–700 bp; results not shown). This was re-amplified in a nested reaction to improve yield and specificity and the product cloned and sequenced. The resulting 637 bp sequence corresponded to the 3′ end of the T. molitor Vg precursor gene. To obtain the remaining 5′ portion of the cDNA we used 5′-RACE and the complete 5608 bp Vg cDNA sequence was deposited in the DDBJ/EMBL/GenBank databases (accession no. AY714212). The cDNA included a 5′-UTR of 30 bp, 3′-UTR of 98 bp, two putative polyadenylation signals (AATAAA) located either 74 or 78 bp downstream of the stop codon (TGA, nucleotides 5494–5496) and a poly(A) tract either 12 or 16 nucleotides downstream of the polyadenylation signal.
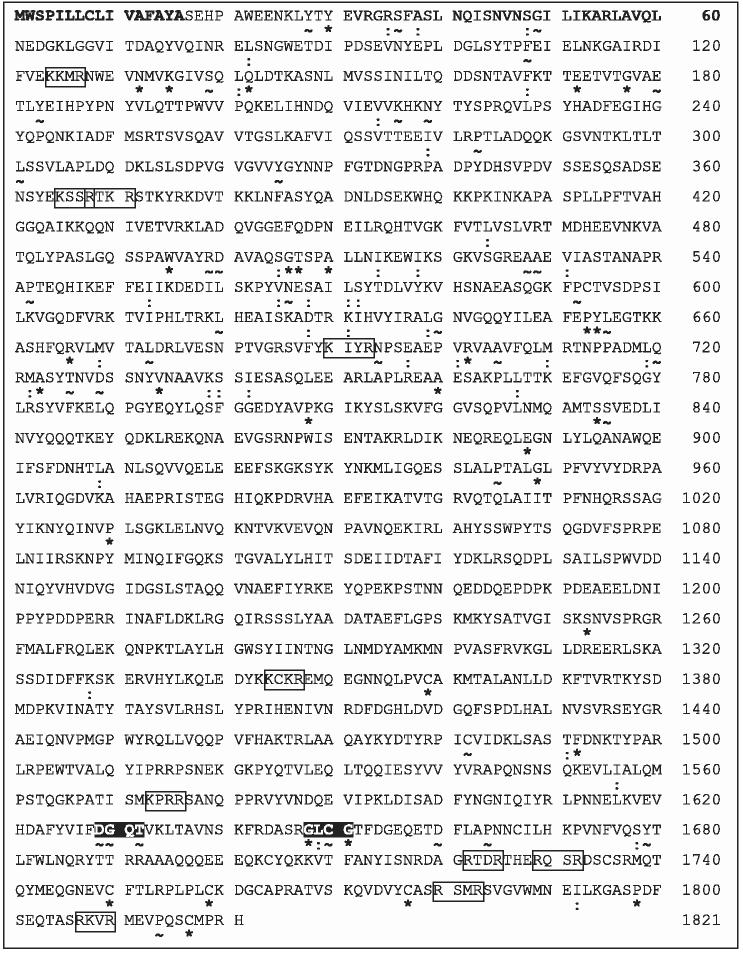
Conceptual translation of the cDNA identified a single open reading frame of 1821 amino acids with the first 16 residues corresponding to a signal peptide (Fig. 1). The translated sequence was aligned (Clustal W) with all 31 complete insect Vgs available to date to reveal highly conserved residues and motifs (Fig. 1). Phylogenetic analysis (data not shown) supported previous reports that Vg relationships reflect the accepted classification of insects (Piulachs et al., 2003). Notable features of the T. molitor Vg include the anticipated GL/ICG motif (residues 1648–1651) and 32 residues that are identical across all 31 insect Vgs (Fig. 1), including four cysteines at the C-terminus that have been described previously (Romans et al., 1995; Hagedorn et al., 1998; Lee et al., 2000). The closest match (identity 34%, similarity 54%) was with the Vg protein of the boll weevil, Anthonomus grandis (Trewitt et al., 1992), the only other complete Coleopteran sequence available. In a comparison of insect, vertebrate and nematode Vgs, Chen et al. (1997) described five subdomains that could be aligned with confidence. For T. molitor Vg, these subdomains I–V correspond to residues 22–339, 427–846, 883–1072, 1480–1687 and 1743–1821, respectively. We note that they reflect the majority of conserved regions in our analysis of insect Vgs but that they omit three residues conserved among all 31 sequences, including an identical serine [S(1253)] and cysteine [C(1359)].
Figure 1.
Conceptual translation of the Tenebrio molitor Vg cDNA showing predicted signal sequence (bold), putative R/KXXR cleavage motifs (open boxes) and conserved DGXR and GL/ICG motifs (reverse highlight). See text for details. Highly conserved residues (100% identity = *, > 90% identity = −; high similarity as defined by Clustal W = :) across all 31 available insect Vgs are indicated below each line. Sequences included were: Aedes aegypti VgA1, Q16927; Aedes aegypti VgB, AAQ92367; Aedes aegypti VgC, AAQ92366; Anopheles gambiae G3 Vg1, AAF82131; Anopheles gambiae PEST Vg1, XM_313104.2; Antheraea pernyi, BAB16412; Antheraea yamamai, BAB32640; Anthonomus grandis, 283605; Apis mellifera, CAD56944; Athalia rosae, 7522163; Blattella germanica, CAA06379; Bombyx mandarina, BAB32642; Bombyx mori, Q27309; Encarsia formosa, AAT48601; Graptopsaltria nigrofuscata, BAA85987; Leucophaea maderae, BAD72597; Lymantria dispar, 11277187; Periplaneta americana Vg1, Q9U8M0; Periplaneta americana Vg2, Q9BPS0; Pimpla nipponica, AAC32024; Plautia stali Vg1, BAA88075; Plautia stali Vg2, BAA88076; Plautia stali Vg3, BAA88077; Riptortus clavatus, AAB72001; Samia cynthia pryeri, BAD91196; Samia cynthia ricini, BAB32641; Saturnia japonica, BAD91195; Solenopsis invicta Vg1 AAP47155; Solenopsis invicta Vg2 AAY22960; Solenopsis invicta Vg3 AAY22961 and Tenebrio molitor, AY714212.
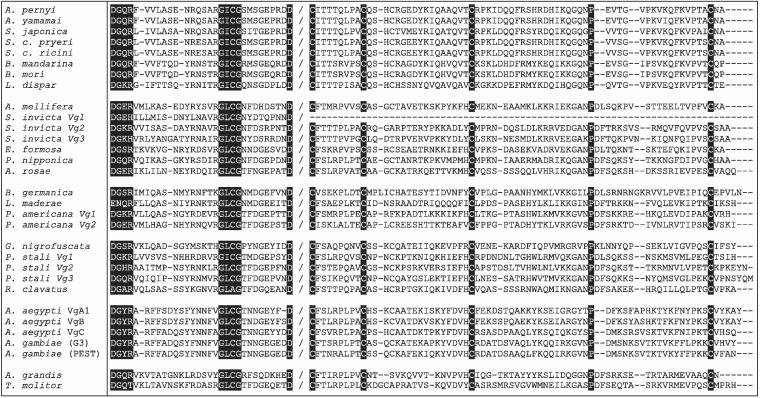
Tufail et al. (2000) identified a conserved DGXR motif, which was described as being located 18 residues upstream of GL/ICG in all insects except the cockroach, Lucophaea maderae, where it was reportedly absent. Using all 31 complete insect Vgs and a partial Vg sequence (U63328) from the spruce terminal weevil, Pissodes strobi (Leal et al., 1997) we noted instead that, with one exception, this motif is located 17 residues upstream of GL/ICG in the Lepidoptera, 18 residues upstream of GL/ICG in the Hymenoptera, Orthoptera, Hemiptera and Diptera and 19 residues upstream of GL/ICG in the Coleoptera (Fig. 2). The one exception is the Hymenopteran Solenopsis invicta Vg2, where the motifs are separated by 19 residues. The final residue of the motif is [R] in all sequences except S. invicta Vg1 and Vg3 [H] and T. molitor [T] and a divergent motif is clearly recognizable in L. maderae where [EN] replaces [DG]. The reasons for this variation in sequence and location are unclear. The C-terminal alignment also revealed a series of identical residues, including an aspartic acid [D] nine residues downstream of GL/ICG, four cysteines and a proline (Fig. 2). We note that the GL/ICG motif itself is not invariant, with a divergent GLAG motif evident in Riptortus clavatus (Fig. 2).
Figure 2.
C-terminal alignment across all 31 available insect Vgs as identified in the legend to Fig. 1. Note that the S. invicta Vg1 sequence appears to be C-terminally truncated. Protein sequences are arranged in six blocks that represent the Lepidoptera, Hymenoptera, Orthoptera, Hemiptera, Diptera and Coleoptera, respectively. The alignment shows the conserved DGXR and GL/ICG motifs and identical aspartic acid (D), cysteine (C) and proline (P) residues in reverse highlight. With one exception, the DGXR and GL/ICG motifs are separated by 17 residues in the Lepidoptera, 19 residues in the Coleoptera and 18 residues in all other insects. For clarity of presentation, only the most proximal and distal parts of the C-terminus are shown, with the sequence break identified by a forward slash. For orientation purposes, the residue immediately preceding the break in T. molitor is D[1660] and that immediately following the break is C[1750].
Genomic organization of the Tenebrio molitor Vg gene
The copy number of Vg genes varies from one to three among the 23 species for which data exist and duplication is an apparently common event (Scott et al., 2005). Southern blot analysis of pooled genomic DNA from T. molitor larvae indicated that the Vg gene was present as a single copy per haploid genome. A 666 bp fragment corresponding to nucleotides 2129–2794 of the Vg cDNA hybridized at high stringency to single fragments of approximately 3.6 kb, 2.4 kb, 5.0 kb and 1.9 kb in genomic DNA digested with XmnI, BamHI, SalI and HindIII, respectively (Fig. 3). Phosphorimages of the membrane taken after a series of lower stringency washes failed to detect any other hybridizing fragments that might have indicated additional Vg genes in the T. molitor genome (data not shown). In a separate Southern blot analysis, a 637 bp fragment corresponding to nucleotides 4972–5608 at the 3′ end of the Vg cDNA also hybridized to single fragments of 2.6 kb and 3.5 kb in genomic DNA digested with BamHI and XmnI, respectively (data not shown), consistent with the interpretation of a single-copy Vg gene in T. molitor.
Figure 3.
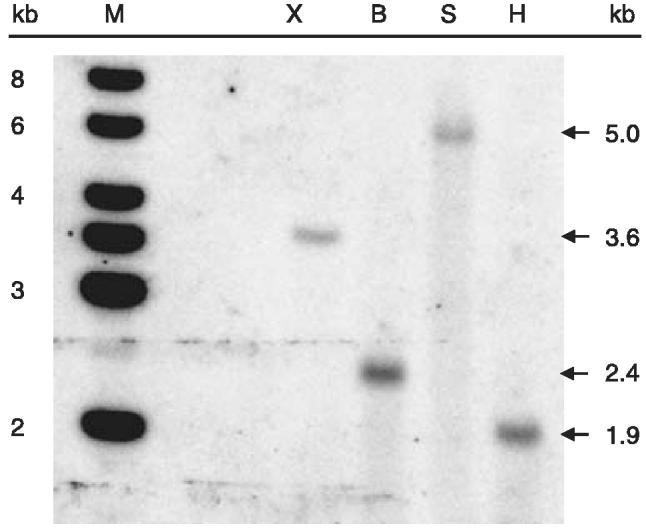
Southern blot analysis of pooled genomic DNA from T. molitor larvae. Ten micrograms of genomic DNA was digested with either XmnI (X), BamHI (B), SalI (S) or HindIII (H), separated on 1% agarose and transferred onto Hybond-N+ membrane. The membrane was probed with a 666 bp fragment corresponding to nucleotides 2129–2794 of the Vg cDNA, washed at high stringency (1 × SSC; 0.1% SDS; 68 °C) and exposed to a phosphor screen for 40 h. Positively hybridizing fragments (arrows) were calibrated against the Generuler™ 1 kb Ladder (M).
Transcriptional profile of the Tenebrio molitor vitellogenin gene
Northern blot analysis of T. molitor mRNA abundance revealed no detectable expression of Vg transcripts in either larvae, male or female pupae, or male adults (Fig. 4A). The profile of Vg mRNA abundance indicated that Vg gene expression was continuous throughout the development of the female adult beetle, up to day 15 post-emergence after which no further measurements were taken (Fig. 4A,B). Following normalization of Vg mRNA abundance to an internal 18S rRNA control, anova revealed no significant differences in Vg mRNA abundance across the female adult profile (F6,19 = 1.88; P = 0.137).
Figure 4.
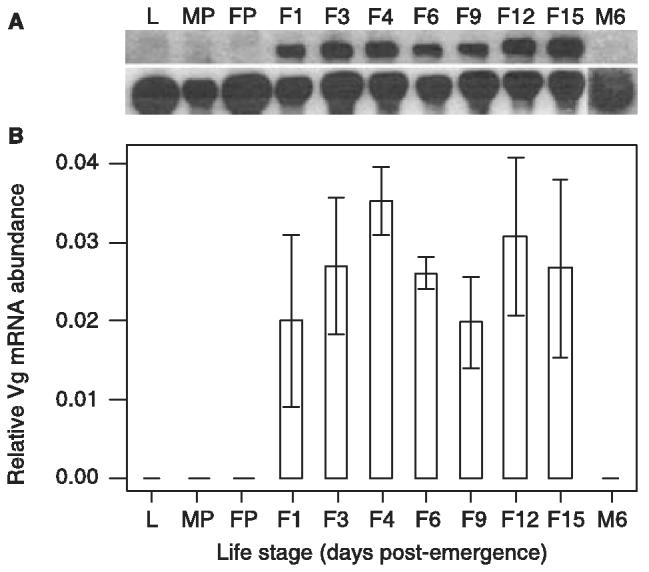
Profile of Vg mRNA abundance at various life stages of T. molitor. (A) Northern blot analysis of total RNA from larvae (L), male pupae (MP), female pupae (FP), fat body tissue from non-infected females at various days post-emergence (F1–F15) and fat body tissue from males at 6 days post-emergence (M6). Ten micrograms of total RNA was fractionated on denaturing 1% agarose-formaldehyde gels and transferred onto Hybond-N+ membrane. The membrane was probed with a 3′ Vg cDNA fragment (upper panel) and 18S internal control (lower panel). (B) Relative abundance of Vg mRNA following normalization to the 18S internal control for the life stages described above. For F4–F15, Vg mRNA abundance was determined from the fat bodies of six individuals and for other samples, fat bodies from two individuals were used. Bars represent the mean ± standard error.
Impact of parasite infection of Vg mRNA abundance in vivo
The abundance of Vg mRNA was compared in both control and infected females at various time points post-emergence. As the effect of the parasite manipulator molecule is seen early in infection (Webb & Hurd, 1996, 1999), no mRNA samples were analyzed beyond 15 days post-emergence. Northern blot analysis revealed that Vg mRNA abundance was higher in the fat body tissue from infected beetles at all time points post-emergence, compared with the fat body tissue of the control beetles (Fig. 5A). These differences were significant (F9,30 = 2.74; P < 0.05) for all time points except day 4 post-emergence.
Figure 5.
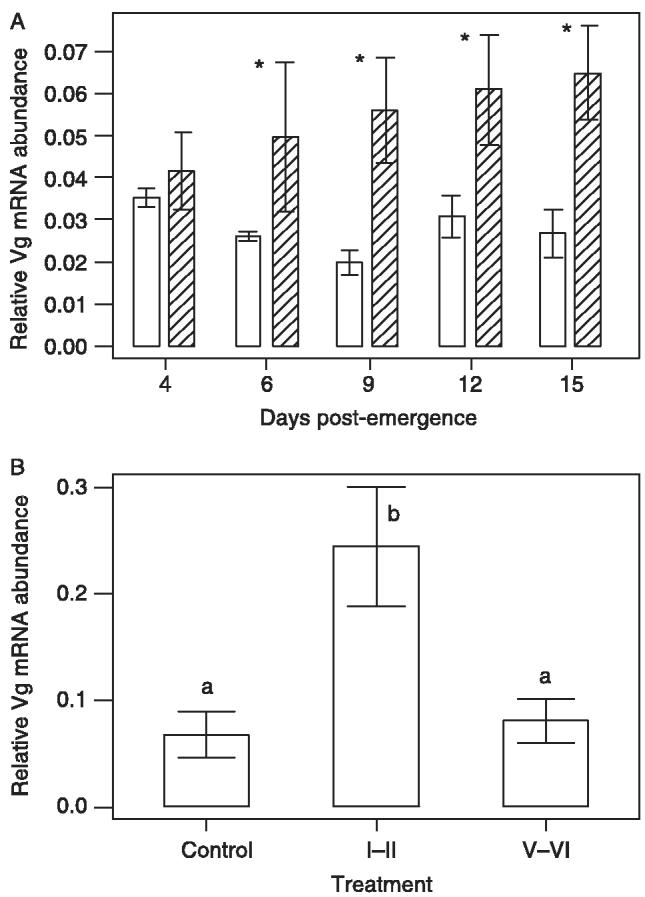
Effect of Hymenolepis diminuta infection in intact females or fat-body culture with live H. diminuta parasites on Vg mRNA abundance in T. molitor. (A) Relative abundance of Vg mRNA for non-infected (open bars) and infected (hatched bars) T. molitor at various days post-emergence. Vg mRNA abundance was determined from the fat bodies of four individuals and significant differences between control and infected beetles are identified with an asterisk. (B) Relative Vg mRNA abundance in fat body tissue from non-infected T. molitor incubated in vitro with either 10 stage I–II parasites, 10 stage V–VI parasites or incubation medium alone (control). Vg mRNA abundance was determined independently from the fat bodies of six individuals and the bars represent the mean abundance ± standard error. Significant differences are represented by different letters, such that a vs. b corresponds to a probability of P < 0.05.
Impact of live parasites on Vg mRNA abundance in vitro
The abundance of Vg mRNA in fat body tissue that had been incubated in the presence or absence of live parasites was investigated (Fig. 5B). In comparison with control fat body incubations or fat bodies incubated with mature parasites (developmental stage V–VI), there was a significantly higher (F2,15 = 28.75; P < 0.001) abundance of Vg mRNA transcripts in fat body tissue incubated with live stage I–II parasites (Fig. 5B). We conclude from this that the manipulatory molecule is secreted by live, early stage parasites.
Discussion
We consider here the manipulation of Vg gene expression in T. molitor by a secreted molecule from the tapeworm H. diminuta. Initially, a 637 bp fragment of the T. molitor Vg gene was cloned by 3′-RACE using gene-specific degenerate primers targeted at the GL/ICG motif conserved within the C-terminus of all insect Vg precursors (Lee et al., 2000; Piulachs et al., 2003). Such primers are very short (11 bp) but appear to work effectively in this context because of the high abundance of Vg transcripts in the fat body tissue of insects. cDNA sequencing was completed by 5′-RACE using reverse primers designed to the 3′ sequence. Vg genes appear to be commonly duplicated within insect genomes (Scott et al., 2005) though this is not universal. Our data indicate that the T. molitor Vg gene is present as a single copy per haploid genome. Following conceptual translation of the T. molitor Vg cDNA, blastp analysis confidently aligned the protein sequence with other insect Vgs through sequence conservation at numerous sub-domains. The closest match was with the Vg protein of the boll weevil, A. grandis (Trewitt et al., 1992), which is consistent with the phylogenetic analysis of Piulachs et al. (2003).
Insect Vgs are generally encoded by mRNAs of 5.5–6.5 kb and translated as polypeptides of about 200 kDa (Wyatt & Davey, 1996). The 5608 bp mRNA of the T. molitor Vg encodes a polypeptide with a predicted molecular mass of 206 kDa. Most insect Vg precursors are cleaved once (within the fat body) by subtilisin-like proprotein endoproteases to produce two subunits. Cleavage immediately follows an R/KXXR motif, which may in some cases be flanked by polyserine tracts (Barr, 1991; Sappington & Raikhel, 1998) but which may not be followed immediately by L, I or V (Denault & Leduc, 1996). The T. molitor Vg has 10 putative cleavage sites (Fig. 1), although only two of these would be supported by published observations of cleavage products. Both Harnish & White (1982) and Webb & Hurd (1995) detected large subunits of 154 and 145 kDa and small subunits of 56 and 45 kDa, while Michalik et al. (1996) detected a single large subunit of 158 kDa and two small subunits of 56 and 45 kDa. We note that both T. molitor and A. grandis have recognizable R/KXXR motifs in almost identical locations (A. grandis, RRFR[359–362] or RFRR[360–363]; T. molitor, KSSR[365–368] or RTKR[368–371]). These motifs are not flanked by obvious polyserine tracts, although there is some evidence of serine richness in the residues immediately preceding each site. Cleavage of the A. grandis Vg at this site has been determined experimentally (Heilmann et al., 1993) yielding subunits of about 47 and 160 kDa, which might be compared with in silico predictions for molecular weight (ExPASy) following cleavage at this site of about 40 and 165 kDa. Cleavage of the T. molitor Vg at KSSR[365–368] or RTKR[368–371] would also yield predicted subunits of about 40 and 165 kDa. It is possible that the variant large and small subunits might result from post-translational modifications, such as glycosylation, phosphorylation or sulphation, all of which have been observed in A. aegypti Vg, or as artefacts of cleavage by proteases during sample preparation (Sappington & Raikhel, 1998).
Alternatively, cleavage of the T. molitor Vg at the C-terminal motif KCKR[1344–1347] would yield fragments of 54 and 152 kDa, which are also consistent with the experimentally determined subunits. In this case, further cleavage of the small subunit at one of the four distal C-terminal motifs (Fig. 1) could generate the 45 kDa peptide and further cleavage of the large subunit at KKMR[124–127] (Fig. 1) could generate the 145 kDa peptide. Cleavage at a C-terminal R/KXXR motif is unusual among insects, having been reported only for the gypsy moth, Lymantria dispar (Hiremath & Lehtoma, 1997). Interestingly, Lymantria Vg is cleaved at KAKR[1459–1462] and the authors highlight the absence of flanking serine-rich regions, a property shared by the KCKR[1344–1347] motif in T. molitor.
Expression of the T. molitor Vg gene is restricted to adult females with no evidence of significant variation in Vg mRNA abundance across females of different ages. This correlates with egg production in this species, which is continuous and characterized by asynchronous development of oocytes in adjacent ovarioles (Ullmann, 1973; Gerber, 1975). It was previously reported that in vitro incubation of fat body tissue from male T. molitor yielded a small amount of Vg, approximating to 10% of that produced by fat body tissue from females (Webb & Hurd, 1996). Although rare, a number of other male insects have been reported to produce Vg in the absence of exogenous hormonal stimulation (Webb & Hurd, 1996). No Vg mRNA was detected by Northern blot analysis in male fat bodies in this study, despite the use of sensitive phosphorimaging, although low-abundance mRNA might be detected by more sensitive technologies such as real-time quantitative PCR.
Contrary to our expectations, Vg mRNA abundance in the fat body of infected females was significantly higher than that from uninfected females at all but the earliest time point tested. An in vitro assay had previously been established to test the direct action of the secreted parasite molecule of fat body Vg synthesis (Webb & Hurd, 1999). An incubation time of 4 h was established as sufficient to demonstrate significant reductions in Vg synthesis, either by the addition of live parasites or a parasite extract (Webb & Hurd, 1996, 1999). In the present study, incubation with early (stage I–II) parasites resulted in a significant increase in Vg mRNA abundance. Thus, in contrast to malaria-infected mosquitoes, H. diminuta does not cause a reduction in Vg mRNA abundance in T. molitor. Clearly, the observed decrease in Vg protein in infected females is not the result of a decrease in Vg mRNA abundance. These data present an apparent contradiction. The manipulator molecule from early stage parasites causes both a significant increase in Vg mRNA abundance in the fat body and a significant decrease in the levels of Vg protein both in vivo and in vitro. One possible interpretation is that the parasite molecule alters the storage of Vg mRNA, with more transcripts being stored than translated. In locusts, adipokinetic hormone (AKH) is involved in the negative control of Vg synthesis and is known to act at the protein level (Moshitzky & Applebaum, 1990; Glinka et al., 1994). There has been a suggestion that AKH is involved in the shift between mRNA fates, with transcripts either being sequestered into storage particles or translated into protein (Wyatt et al., 1995). So, in terms of a possible post-transcriptional mode of action, it is conceivable that the H. diminuta molecule could be mimicking an endogenous insect hormone or neuropeptide such as AKH, which normally regulates the production of Vg (Webb & Hurd, 1999). In this context, it has been shown that certain parasites, for example Spirometra mansonoides, can synthesize and release host-like molecules (Halton et al., 1994). More relevant to this study is the detection of peptides that exhibit AKH-like bioactivity in the nematodes Panagrellus redivivus, Ascaridia galli and Ascaris suum (Davenport et al., 1991, 1994). We speculate that the increase in Vg mRNA abundance coupled to a decrease in Vg protein synthesis might result from a positive feedback mechanism that recognizes falling Vg synthesis and attempts to compensate by increasing Vg gene transcription.
Reduction in the reproductive output of insect hosts is a life history trait that is commonly associated with parasitic infections. There has been much debate in the literature about whether such changes are simply by-products of infection or adaptive manipulation by the host or parasite (Hurd, 1998; Poulin & Combes, 1999). In our host–parasite association, H. diminuta is able directly to manipulate the synthesis of Vg in the fat bodies of female T. molitor. It is of particular interest that the parasite specifically reduces the production of Vg but not of other proteins in the fat body (Webb & Hurd, 1996). The survival of female insects does not rely on the production of eggs, which places a heavy demand on the resource allocation of amino acids. Thus, there may be an advantage to reducing egg production such that nutrients may be redistributed and better used elsewhere. An infected female beetle producing a normal quantity of eggs may not be viable, so a shift in resource allocation away from egg production may provide a better environment for both host and parasite (Webb & Hurd, 1996). In addition to these reductions in egg production, it has been demonstrated that infection with H. diminuta extends the life-span of female T. molitor by about 50%. This increase in host longevity may provide a selective advantage to the parasite by providing more opportunity for the host to be predated and therefore parasite transmission to occur (Hurd, 2001; Hurd et al., 2001).
Experimental procedures
Maintenance of parasite and host
Eggs from adult H. diminuta (ARME strain) were obtained from faecal pellets of male Wistar rats by salt flotation (Hundley & Berntzen, 1969) and stored at 4 °C in distilled water for a maximum of 2 weeks. T. molitor (Rice University strain) were kept in plastic tanks at 26 °C, 60–70% relative humidity on a 12 : 12 h light/dark cycle and fed wheat bran ad libitum with a weekly potato supplement to provide moisture. Pupae from the stock colony were sexed (Bhattacharya et al., 1970) and maintained at 26–27 °C, 60–70% relative humidity until emergence. Adults were placed in Petri dishes at a ratio of 10 females: three males and starved for 48 h. On day 3 post-emergence, control populations were fed apple pulp alone and infected populations were fed apple pulp mixed with parasite eggs, such that the age of the beetle is 3 days more than that of the parasite. Apple pulp was removed after 24 h and all insects fed subsequently on wheat bran (ad libitum) supplemented with apple slices every third day.
Characterization of the Vg precursor cDNA from T. molitor mRNA was purified from total RNA (TRIZOL reagent, Invitrogen, Paisley, UK; Nucleotrap, Takava Bio Inc, France) and subjected to 3′-RACE (GeneRacer; Invitrogen). Briefly, mRNA was reverse-transcribed (SuperScript II) with an oligo dT primer to create first strand cDNA with known primer binding sites at the 3′ end. This was amplified using gene-specific, degenerate forward primers and a generic reverse primer. The forward primers (Lee et al., 2000) were based on GLCG (5′-GGICTSTGYGG-3′) or GICG (5′-GGIATYTGYGG-3′) and the reverse primer was the GeneRacer 3′ primer (5′-GCTGTCAACGATACGCTACGTAACG-3′). PCR reactions [50 μl; 0.2 μm primers; 0.2 mm dNTPs; 2 mm MgSO4; 2.5 U high fidelity Platinum Taq DNA polymerase (Invitrogen)] were cycled [94 °C, 2 min; (94 °C, 30 s; 55 °C, 1 min; 68 °C, 2 min) × 30; 68 °C, 10 min] in a PTC-200 cycler (MJ Research, Bio-Rad Laboratorier, MA, USA). Reaction products were reamplified with a gene-specific nested reverse primer to increase sensitivity and specificity. PCR products were excised from 1% agarose TAE gels, purified (GeneClean, Q-BIOgene, MP Biomedical, CA, USA) and cloned into pCR4-TOPO (Invitrogen) for sequencing. 5′-RACE used reverse primers designed to the 3′ sequence. mRNA was reverse-transcribed with Vit5race (5′-GCAACGGACGAAGAGTGAAGCAAACCT-3′) and amplified with the gene-specific Vit5Nrace (5′-CAGCAGCGCGTCTCGTCGTGTAAC-3′) and the generic GeneRacer 5′ primer (5′-CGACTGGAGCACGAGGACACTGA-3′) using the conditions described above but following the manufacturer's cycling parameters with an annealing temperature of 65 °C and extension time of 7 min. The purified product (≈ 5 kb) was cloned into pCR 2.1 TOPO (Invitrogen) after a 20 μl overnight ligation using undiluted salt solution and subsequent cleanup with Pellet Paint co-precipitant (Novagen Merck Biosciences, Darmstadt, Germany). The insert was sequenced on both strands using M13 primers followed by the internal primers Vitfwd1 (5′-GTCAACACTAAACTCACCCTAACA-3′), Vitfwd2 (5′-ATAGTAATGCAGAAGCCTCAC-3′), Vitrevl (5′-ATCTCAGCTCGACCATACTC-3′) and Vitrev2 (5′-GAGCGGATCTTGACTACGAA-3′).
Southern blot analysis of the Tenebrio molitor genome
Genomic DNA was isolated using a salting out procedure (Miller et al., 1988). Briefly, four larvae were crushed in liquid nitrogen and resuspended in lysis buffer (500 mm Tris–HCl, pH 9.0; 20 mm EDTA; 10 mm NaCl) containing 0.5 mg/ml proteinase-K and 1% SDS. Preparations were incubated at 48 °C for 2 h and polypeptides were salted out of solution using saturated NaCl. Genomic DNA was ethanol precipitated, washed repeatedly with 75% ethanol and resuspended in 300 μl water. Following digestion with either XmnI, BamHI, SalI or HindIII, approximately 10 μg of genomic DNA was separated on 1% agarose in TAE buffer and blotted on to Hybond-N+ (Amersham Biosciences GE Healthcare, Bockinghamshire, UK). Membranes were probed with a 666 bp fragment corresponding to nucleotides 2129–2794 of the Vg cDNA, radiolabelled to high specific activity with 32P by random priming. Membranes were washed at increasing stringency from [5 × SSC; 0.1% SDS; room temperature] to a final stringency of [1 × SSC; 0.1% SDS; 68 °C] in order to detect weakly hybridizing homologous sequences. All membranes were exposed to phosphor screens (Cyclone, Perkin Elmer) for between 2 and 48 h and the resulting images interpreted using Optiquant software (Perkin Elmer, MA, USA).
Northern blot analysis of Tenebrio molitor Vg mRNA abundance
Fat body tissue from individual infected and non-infected female beetles was dissected at the required age (days 1, 3, 4, 6, 9, 12 or 15 post-emergence) and total RNA isolated with TRIZOL reagent (Invitrogen). To measure the effect of live parasites in vitro, fat body tissue from individual uninfected females at 6 days post-emergence was dissected and incubated for 4 h at 26 °C with 10 live parasites (early stage I–II or late-stage V–VI) or with incubation medium alone, prior to isolation of total RNA. Total RNA was fractionated on denaturing 1% agarose-formaldehyde gels and transferred to Hybond-N+ membranes (Amersham Biosciences). Membranes were probed with a 637 bp fragment corresponding to nucleotides 4972–5608 at the 3′ end of the Vg cDNA, radiolabelled to high specific activity with 32P by random priming. Membranes were washed at increasing stringency and exposed to phosphor screens (Cyclone, Perkin Elmer) for between 2 and 48 h, with the resulting images interpreted using Optiquant software (Perkin Elmer). For normalization, membranes were stripped by washing in 0.1% SDS at 80 °C for 20 min and re-probed with a 200 bp 18S rRNA control fragment generated by RT–PCR using generic eukaryotic 18S primers (Applied Biosystems CA, USA). Final washing stringencies for the Vg and 18S probes were (2 × SSC; 0.1% SDS; 42 °C) and (0.1 × SSC; 0.1% SDS; 47 °C), respectively.
Statistical analysis
Data sets were tested for normality (Anderson–Darling) prior to one-way anova (Minitab). Where significant differences (P < 0.05) were observed, individual means were subjected to unplanned comparisons (Tukey's method; family error rate = 0.05).
Acknowledgements
This work was supported by BBSRC Project Grant S14125 (to HH and PE) and by Wellcome Trust Equipment Grants 061081 and 069162 (to PE and HH).
References
- Ahmed AM, Maingon R, Romans P, Hurd H. Effects of malaria infection on vitellogenesis in Anopheles gambiae during two gonotrophic cycles. Insect Mol Biol. 2001;10:347–356. doi: 10.1046/j.0962-1075.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- Barr PJ. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991;66:1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bhattacharya AK, Ameel JJ, Waldbauer GP. A method of sexing living pupal and adult yellow mealworms. Ann Entomol Soc Am. 1970;63B:1783–1785. [Google Scholar]
- Chen J-S, Cho W-L, Raikhel AS. Analysis of mosquito vitellogenin cDNA. Similarity with vertebrate phosvitins and arthropod serum proteins. J Mol Biol. 1994;237:641–647. doi: 10.1006/jmbi.1994.1261. [DOI] [PubMed] [Google Scholar]
- Chen J-S, Sappington TW, Raikhel AS. Extensive sequence conservation among insect, nematode and vertebrate vitellogenins reveals ancient common ancestry. J Mol Evol. 1997;44:440–451. doi: 10.1007/pl00006164. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu J, Sun G, Raikhel AS. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. J Mol Endocrinol. 2004;33:743–761. doi: 10.1677/jme.1.01531. [DOI] [PubMed] [Google Scholar]
- Davenport TR, Isaac RE, Lee DL. The presence of peptides related to the adipokinetic hormone/red pigment concentrating hormone family in the nematode, Panagrellus redivivus. Gen Comp Endocrinol. 1991;81:419–425. doi: 10.1016/0016-6480(91)90169-7. [DOI] [PubMed] [Google Scholar]
- Davenport TR, Eaves LA, Hayes TK, Lee DL, Isaac RE. The detection of AKH/HrTH-like peptides in Ascaridia galli and Ascaris suum using an insect hyperglycaemic bioassay. Parasitology. 1994;108:479–485. doi: 10.1017/s0031182000076046. [DOI] [PubMed] [Google Scholar]
- Denault JB, Leduc R. Furin/PACE/SPC1: a convertase involved in exocytic and endocytic processing of precursor proteins. FEBS Lett. 1996;379(2):113–116. doi: 10.1016/0014-5793(95)01487-x. [DOI] [PubMed] [Google Scholar]
- Gerber GH. Reproductive behaviour and physiology of Tenebrio molitor (Coleoptera: Tenebrionidae). II. Egg development and oviposition in young females and the effects of mating. Can Entomol. 1975;107:551–559. [Google Scholar]
- Glinka AV, Kleiman AM, Wyatt GR. Roles of juvenile hormone, brain-derived factor, and adipokinetic hormone I in regulation of biosynthesis of vitellogenin in the migratory locust, Locusta migratoria. Biochemistry (Moscow) 1994;59:695–700. [PubMed] [Google Scholar]
- Hagedorn HH, Maddison DR, Tu Z. The evolution of vitellogenins, cyclorrhaphan yolk proteins and related molecules. Adv Insect Physiol. 1998;27:335–384. [Google Scholar]
- Halton DW, Shaw C, Maule AG, Smart D. Regulatory peptides in helminth parasites. Adv Parasitol. 1994;34:163–227. doi: 10.1016/s0065-308x(08)60139-6. [DOI] [PubMed] [Google Scholar]
- Harnish DG, White BN. Insect vitellins: identification, purification and characterisation from eight orders. J Exp Zool. 1982;220:1–10. [Google Scholar]
- Heilmann LJ, Trewitt PM, Kumaran AK. Proteolytic processing of the vitellogenin precursor in the boll weevil, Anthonomus grandis. Arch Insect Biochem Physiol. 1993;23:125–134. doi: 10.1002/arch.940230304. [DOI] [PubMed] [Google Scholar]
- Hiremath S, Lehtoma K. Complete nucleotide sequence of the vitellogenin mRNA from the gypsy moth: novel arrangement of the subunit encoding regions. Insect Biochem Mol Biol. 1997;27:27–35. doi: 10.1016/s0965-1748(96)00067-7. [DOI] [PubMed] [Google Scholar]
- Hundley DF, Berntzen AK. Collection, sterilization and storage of Hymenolepis diminuta eggs. J Parasitol. 1969;55:1095–1096. [Google Scholar]
- Hurd H. Parasite manipulation of insect reproduction: who benefits? Parasitology. 1998;116(Suppl.):S13–S21. doi: 10.1017/s0031182000084900. [DOI] [PubMed] [Google Scholar]
- Hurd H. Host fecundity reduction: a strategy for damage limitation? Trends Parasitol. 2001;17:363–368. doi: 10.1016/s1471-4922(01)01927-4. [DOI] [PubMed] [Google Scholar]
- Hurd H, Warr E, Polwart A. A parasite that increases host lifespan. Proc R Soc London B Biol Sci. 2001;268:1749–1753. doi: 10.1098/rspb.2001.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Leal I, White EE, Sahota TS, Manville JF. Differential expression of the vitellogenin gene in the spruce terminal weevil feeding on resistant versus susceptible host trees. Insect Biochem Mol Biol. 1997;27:569–575. doi: 10.1016/s0965-1748(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Lee JM, Hatakeyama M, Oishi K. A simple and rapid method for cloning insect vitellogenin cDNAs. Insect Biochem Mol Biol. 2000;30:189–194. doi: 10.1016/s0965-1748(99)00127-7. [DOI] [PubMed] [Google Scholar]
- Michalik J, Chojnicka B, Cymborowski B. Vitellogenesis in virgin and mated females of the mealworm beetle, Tenebrio molitor. Acta Biochim Pol. 1996;43:623–631. [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshitzky P, Applebaum SW. The role of adipokinetic hormone in the control of vitellogenesis in locusts. Insect Biochem. 1990;20:319–323. [Google Scholar]
- Piulachs MD, Guidugli KR, Barchuk AR, Cruz J, Simoes ZL, Belles X. The vitellogenin of the honey bee, Apis mellifera: structural analysis of the cDNA and expression studies. Insect Biochem Mol Biol. 2003;33:459–465. doi: 10.1016/s0965-1748(03)00021-3. [DOI] [PubMed] [Google Scholar]
- Poulin R, Combes C. The concept of virulence: interpretations and implications. Parasitol Today. 1999;15:474–475. doi: 10.1016/s0169-4758(99)01554-9. [DOI] [PubMed] [Google Scholar]
- Romans P, Zhijian T, Ke Z, Hagedorn HH. Analysis of a vitellogenin gene of the mosquito, Aedes aegypti and comparisons to vitellogenins from other organisms. Insect Biochem Mol Biol. 1995;25:939–958. doi: 10.1016/0965-1748(95)00037-v. [DOI] [PubMed] [Google Scholar]
- Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol. 1998;28:277–300. doi: 10.1016/s0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Scott MP, Panaitof SC, Carleton KL. Quantification of vitellogenin-mRNA during maturation and breeding of a burying beetle. J Insect Physiol. 2005;51:323–331. doi: 10.1016/j.jinsphys.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Trewitt PM, Heilmann LJ, Degrugillier SS, Kumaran AK. The boll weevil vitellogenin gene: nucleotide sequence, structure, and evolutionary relationship to nematode and vertebrate vitellogenin genes. J Mol Evol. 1992;34:478–492. doi: 10.1007/BF00160462. [DOI] [PubMed] [Google Scholar]
- Tufail M, Takeda M. Vitellogenin of the cockroach, Leucophaea maderae: nucleotide sequence, structure and analysis of processing in the fat body and oocytes. Insect Biochem Mol Biol. 2002;32:1469–1476. doi: 10.1016/s0965-1748(02)00067-x. [DOI] [PubMed] [Google Scholar]
- Tufail M, Lee JM, Hatakeyama M, Oishi K, Takeda M. Cloning of vitellogenin cDNA of the American cockroach, Periplaneta americana (Dictyoptera), and its structural and expression analyses. Arch Insect Biochem Physiol. 2000;45:37–46. doi: 10.1002/1520-6327(200009)45:1<37::AID-ARCH4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ullmann SL. Oogenesis in Tenebrio molitor: histological and autoradiological observations on pupal and adult ovaries. J Embryol Exp Morphol. 1973;30:179–217. [PubMed] [Google Scholar]
- Warr E, Eggleston P, Hurd H. Apoptosis in the fat body tissue of the beetle Tenebrio molitor parasitised by Hymenolepis diminuta. J Insect Physiol. 2004;50:1037–1043. doi: 10.1016/j.jinsphys.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Webb TJ, Hurd H. The use of a monoclonal antibody to detect parasite-induced reduction in vitellin content of the ovaries of Tenebrio molitor. J Insect Physiol. 1995;41(9):745–751. [Google Scholar]
- Webb TJ, Hurd H. Hymenolepis diminuta:metacestode-induced reduction in the synthesis of the yolk protein, vitellogenin, in the fat body of Tenebrio molitor. Parasitology. 1996;112:429–436. doi: 10.1017/s0031182000066658. [DOI] [PubMed] [Google Scholar]
- Webb TJ, Hurd H. Direct manipulation of insect reproduction by agents of parasite origin. Proc R Soc London B Biol Sci. 1999;266:1537–1541. [Google Scholar]
- Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- Wyatt GR, Braun RP, Glinka AV, Zhang J. Hormonal control of gene expression in locust fat body. In: Suzuki A, Kataoka H, Matsumoto S, editors. Molecular Mechanisms of Insect Metamorphosis and Diapause. Industrial Publishing and Consulting, Inc.; Tokyo: 1995. pp. 271–281. [Google Scholar]