Abstract
Objective
To determine the effects of interleukin-4 (IL-4) on IL-1 induction of collagenase (matrix metalloproteinase 1 [MMP-1]) and stromelysin-1 (MMP-3) in human synovial fibroblasts.
Methods
Northern blot analysis was performed to determine the effects of IL-4 on IL-1 induction of MMP messenger RNA (mRNA). MMP protein levels were determined by enzyme-linked immunosorbent assay, and prostaglandin E2 (PGE2) levels were measured by enzyme immunoassay. Run-on transcription assays and transient transfection experiments were performed to determine whether the effects of IL-4 occur at the level of transcription. Activator protein 1(AP-1) binding was assessed by electrophoretic mobility shift assay.
Results
Northern blot analysis revealed that coincubation of synovial fibroblasts with IL-1 and IL-4 resulted in a significant decrease in both collagenase and stromelysin mRNA levels compared with IL-1 alone, with a concomitant decrease in MMP protein levels. This inhibition is dose dependent, with an IC50 (50% inhibition concentration) for both MMPs of ~0.3 ng of IL-4 per ml, and is at least somewhat selective, since IL-1 induction of c-fos mRNA is not affected. Nuclear run-on experiments and transient transfection studies demonstrated that the suppression of IL-1–induced collagenase and stromelysin expression by IL-4 occurs at least in part at the transcriptional level, and that binding of transcription factor AP-1 is not affected. Although IL-1–induced levels of PGE2 are reduced by IL-4, exogenous addition of PGE2 does not abrogate the inhibitory effects of IL-4 on MMP expression.
Conclusion
IL-4 inhibits IL-1 induction of both collagenase and stromelysin, as well as PGE2 production, in human synovial fibroblasts. The inhibition occurs at least in part at the level of transcription, does not affect binding of transcription factor AP-1, and appears to involve a mechanism that is independent of the ability of IL-4 to inhibit production of PGE2.
Rheumatoid arthritis (RA) is a chronic inflammatory disorder in which a primary inflammatory event occurs in the synovium. The inflamed synovium is characterized by infiltrating T cells and macrophages, as well as activated fibroblasts. The macrophages produce inflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor (TNF), which stimulate production of chemotactic factors and matrix metalloproteinases (MMPs). These MMPs (e.g., collagenase [MMP-1] and stromelysin [MMP-3]) are believed to play a major role in the joint and tissue destruction associated with chronic inflammatory diseases such as RA (1) and periodontitis (2).
Production of MMPs by synovial fibroblasts is regulated by a variety of growth factors and cytokines, including IL-1. Although there are an increasing number of exceptions (3–7), the 2 genes are coordinately regulated under many conditions (8). The mechanism(s) by which IL-1 increases transcription of the MMP genes are not yet fully understood, but transcription factor activator protein 1 (AP-l), which has a binding site at a similar location in both promoters, is thought to play an important role (9,10).
Synovial fluid from RA patients and crevicular fluid from patients with periodontitis has been shown to contain high levels of proinflammatory cytokines IL-1 and TNF (11–13), while antiinflammatory cytokines IL-4 and IL-10 are virtually undetectable (14–16). IL-4 has been shown to inhibit collagenase expression in lipopolysaccharide (LPS)-stimulated monocytes (17,18) and in superantigen-induced synovial fibroblasts (19), as well as stromelysin expression in human skin fibroblasts stimulated with IL-1 (20). However, its effects on MMP expression in IL-1–stimulated synovial fibroblasts have not yet been examined. In addition, although the inhibitory effects of IL-4 have been associated with decreased production of prostaglandin E2 (PGE2) (18–22) and lack of protein kinase C activation (20), the mechanism of IL-4 inhibition of MMP expression has not been well established in any model system.
Herein we report that coincubation of human synovial fibroblast cultures with IL-1 and IL-4 results in a significant, dose-dependent reduction in the IL-1–stimulated production of both collagenase and stromelysin messenger RNA (mRNA) and protein, as well as PGE2. However, the mechanism of IL-4 inhibition of MMP expression appears to be independent of its inhibition of PGE2 production, since exogenous addition of PGE2 was unable to restore the MMP induction. Although this inhibition takes place at least in part at the transcriptional level, binding of transcription factor AP-1 is not affected. These findings suggest that the inhibitory effects of IL-4 may be mediated through other, less well-characterized, transcription factors involved in modulating the expression of these genes.
MATERIALS AND METHODS
Cell culture
Human synovial tissue was obtained from patients with osteoarthritis (OA) or RA who were undergoing reconstructive and restorative surgery. The tissue was processed by enzymatic dispersion to produce a primary culture, as described previously (23,24). Cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum and antibiotic/antimycotic (penicillin, streptomycin, amphotericin; Gibco BRL, Grand Island, NY). Cells between passages 5 and 8 were used for experiments.
Cells were serum deprived for 24 hours in serum-free EMEM supplemented with 10% ITS (insulin, transferrin, sodium selenite; Sigma, St. Louis, MO) prior to the addition of 100 ng of IL-1β/ml (a generous gift of R. Newton, Wilmington, DE) in the presence or absence of various doses of IL-4 (a generous gift from L. Marshall, King of Prussia, PA). Human foreskin fibroblasts (American Type Culture Collection [ATCC], Rockville, MD) were maintained and treated in the same manner described above, and used in passage 14.
RNA isolation and Northern blotting
Total RNA was isolated according to the acid-phenol method of Chomczynski and Sacchi (25) at various times after treatment, and run on 1% agarose–formaldehyde gels. Probes were made by random priming (Stratagene, La Jolla, CA) of complementary DNA fragments corresponding to human procollagenase (MMP-1; a generous gift of G. Coldberg, St. Louis, MO), stromelysin 1 (MMP-3; ATCC), c-fos (a generous gift of K. J. Soprano, Philadelphia, PA), and GAPDH (a generous gift of R. Newton). Northern blots were quantitated by densitometric scanning and normalized to GAPDH.
Nuclear extract isolation and electrophoretic mobility shift assay
Nuclear extracts were isolated according to the method of Schreiber et al (26) and quantitated in mini-Bradford assays (Pierce, Rockford, IL). Synthetic oligonucleotides corresponding to the stromelysin AP-1 binding site were annealed and kinased by T4 polynucleotide kinase in the presence of γ32P-ATP. Binding reactions contained 5 μg of protein, 20 mM HEPES-OH, pH 7,50 mM NaCl, 0.2M EDTA, 5% glycerol, 4 μg of deoxyinosine-deoxycytosine, and 10,000 counts per minute of probe.
Run-on transcription assay
Nuclei were isolated according to the method of Greenberg and Bender (27) from human synovial fibroblasts (HSF): untreated controls and cells treated for 6 hours with 100 ng of IL-1/ml in the presence or absence of 10 ng of IL-4/ml. Transcription reactions were carried out with equal numbers of nuclei in the presence of 32P-UTP for 30 minutes at 26°C with shaking. RNA was then isolated and hybridized to cDNA fragments corresponding to collagenase, stromelysin, and GAPDH at 32°C overnight. Results were quantitated by densitometry and normalized to GAPDH.
Quantitation of collagenase and stromelysin proteins and PGE2
Collagenase and stromelysin protein levels were quantitated in conditioned media of HSF that were untreated, treated with 100 ng of IL-1/ml alone, or with both 100 ng of IL-1/ml and 10 ng of IL-4/ml for 24 hours. Levels of PGE2 were measured in 6-hour conditioned media. The experiments were done in triplicate for 1 or 2 different cell strains by enzyme-linked immunosorbent assay (for MMP) and enzyme immunoassay (for PGE2) using the Biotrak system (Amersham, Arlington Heights, IL).
Transient transfections
A 2.3-kb fragment of the human stromelysin 5′-flanking region (a gift of D. Tewari, Philadelphia, PA) was subcloned to the pGL3Basic luciferase reporter vector (Promega, Madison, WI). This reporter construct was then cotransfected in triplicate in human foreskin fibroblasts, along with a β-galactosidase (β-gal) reporter under control of the SV40 promoter (SVβ-gal; Promega) using 20 μg of lipofectin (Gibco BRL) and 48 μg of transferrin (Sigma). Approximately 16 hours after transfection, the transfection medium was replaced with serum-free medium supplemented with 10% ITS along with the appropriate interleukin(s). Cells were harvested 24 hours later, and luciferase and β-gal activity were determined using reagents and protocols from Promega and ClonTech (Palo Alto, CA), respectively.
RESULTS
IL-4 inhibition of IL-1 induction of both collagenase and stromelysin 1
To determine the effect of IL-4 on IL-1 induction of collagenase and stronielysin 1 mRNA expression in HSF, total RNA was isolated at various times after the addition of either IL-1 alone or IL-1 plus IL-4. Northern blot analysis revealed a significant reduction (~65–80% at 6 hours) in IL-1–induced expression of both genes (Figure 1). This inhibition was evident as early as 3 hours after stimulation. Similar results were obtained with synovial fibroblasts isolated from a patient with RA (Figure 2).
Figure 1.
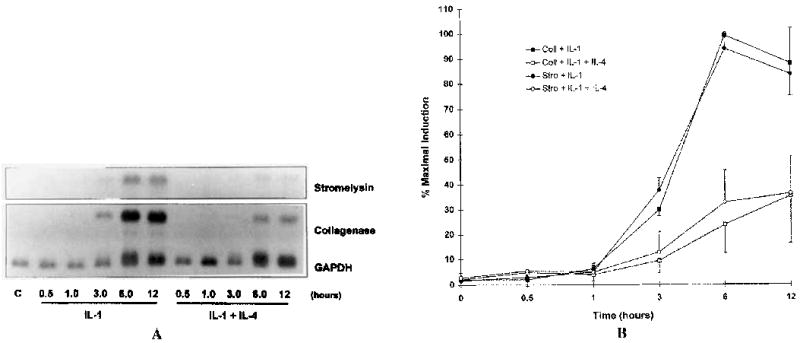
Interleukin-4 (IL-4) inhibits IL-1 induction of collagenase (Coll) and stromelysin (Stro) mRNA in human synovial fibroblasts (HSF). Total RNA was isolated at the indicated time points after addition of 100 ng of IL-1/ml alone or in combination with 10 ng of IL-4/ml to cultures of human synovial fibroblasts. A, Northern blots were hybridized to cDNA probes corresponding to collagenase (matrix metalloproteinase 1 [MMP-1]), stromelysin 1 (MMP-3), and GAPDH. B, Blots were quantitated by scanning densitometry and normalized to levels of GAPDH. Shown are data from 3 independent experiments with RNA isolated from HSF.
Figure 2.
Interleukin-4 (IL-4) inhibits IL-1 induction of collagenase and stromelysin mRNA in rheumatoid arthritis synovial fibroblasts. Total RNA was isolated 6 hours after addition of 100 ng of IL-1/ml alone or in combination with 10 ng of IL-4/ml to a single culture of rheumatoid arthritis synovial fibroblasts. A Northern blot was hybridized and quantitated as described in Figure 1.
Levels of MMP proteins were measured in conditioned media from control cell cultures and from HSF treated for 24 hours in the presence of 1L-1 alone or together with IL-4. Results of this analysis (Figure 3) showed a 70% inhibition of IL-1-induced collagenase levels and a 75–80% inhibition of stromelysin levels, similar to the degree of inhibition observed at the mRNA level.
Figure 3.
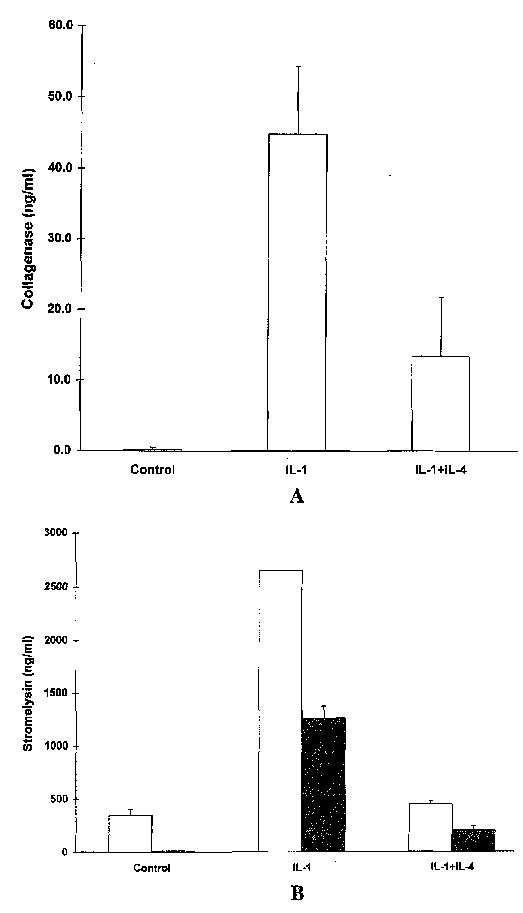
Interleukin-4 (IL-4) inhibits the IL-1 induction of collagenase and stromelysin proteins. Conditioned medium was harvested from human synovial fibroblast (HSF) cultures incubated for 24 hours with 100 ng of IL-1β/ml alone or in the presence of 10 ng of IL-4/ml. Levels of collagenase (A) and stromelysin (B) were measured in triplicate by enzyme-linked immunosorbent assay. For stromelysin, HSF cultures derived from 2 different individuals were used.
Selective IL-4 suppression of MMP
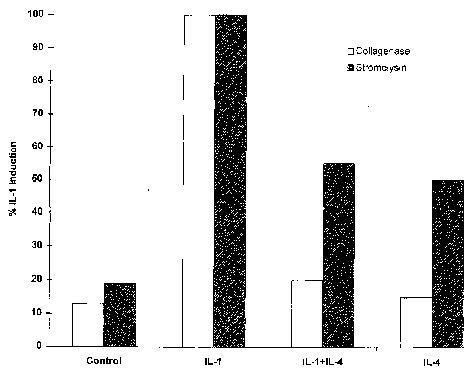
To determine whether the ability of IL-4 to interfere with IL-1 induction of collagenase and stromelysin is specific to these genes or represents a more global phenomenon, the same Northern blots described above were hybridized with a probe specific for c-fos. c-fos is an early response gene known to be induced by IL-1 (28), and as part of the transcription factor AP-1, the protein it encodes is involved in IL-1 induction of both genes (9,10). Figure 4 shows that IL-1 induction of c-fos mRNA was not appreciably affected by IL-4. These results demonstrate that the inhibitory activity of IL-4 is at least somewhat specific, and suggest that it is not simply interfering with IL-1 receptor activation or signal transduction. In addition, expression of another IL-1- induced gene, melanocyte growth stimulatory activity (29), was also unaffected by addition of IL-4 (results not shown).
Figure 4.
Interleukin-l (IL-I) induction of c-fos mRNA is not affected by IL-4. The same Northern blots described in Figure 1 were rehybridized with a cDNA probe corresponding to c-fos. Shown are data from 3 independent experiments.
Dose-dependent IL-4 inhibition
Dose curve analysis was done by adding incremental doses of IL-4 simultaneously with a constant dose of IL-1. Total RNA was isolated 6 hours after stimulation, and Northern blot analysis was performed to determine the effects of each dose on IL-1 induction of the MMP. Figure 5 shows that the suppressive action of IL-4 was dose dependent, with some inhibition seen with as little as 0.01 ng of IL-4/ml. Maximum inhibition was seen with 0.5 ng of IL-4/ml for collagenase and 10 ng of IL-4/ml for stromelysin. The IC50 (50% inhibition concentration), as determined by regression analysis of these data, was ~0.3 ng/ml.
Figure 5.
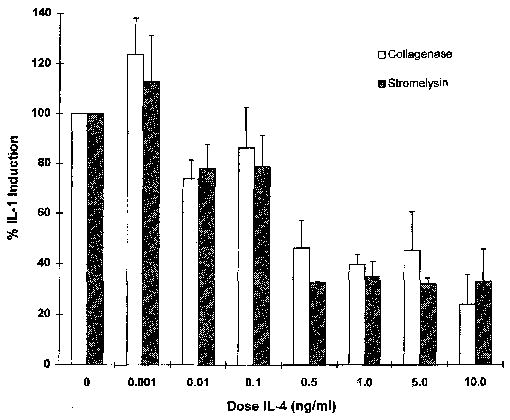
Interleukin-4 (IL4) inhibition of IL-1-induced matrix metalloproteinase mRNA is dose dependent. Total RNA was isolated from human synovial fibroblast cultures 6 hours after addition of 100 ng of IL-1β/ml alone or in the presence of the indicated doses of IL-4. Shown are data from 3 independent experiments. The IC50(50% inhibition concentration) calculated from these data is ~0.3 ng of IL-4/ml.
Occurrence of IL-4 inhibition of IL-1-induced MMP expression at the transcriptional level
To determine whether and to what extent the suppressive activity of IL-4 occurs at the level of transcription, run-on transcription assays were performed. Nuclei were isolated from HSF cultures 6 hours after stimulation with IL-1 alone or in the presence of IL-4. The results, shown in Figure 6A, demonstrated that IL-4 inhibited IL-1-induced transcription of both MMP genes.
Figure 6.
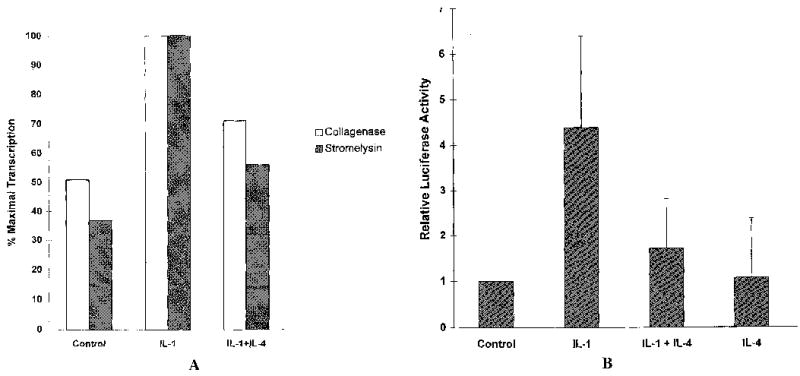
Interleukin-4 (IL-4) inhibition of IL-1-induccd matrix metalloproteinase expression occurs, at least in part, at the transcriptional level. A, Nuclei were isolated 6 hours after incubation of human synovial fibroblasts with 100 ng of IL-1β/ml alone or in the presence of 10 ng of IL4/ml. Nuclear run-on transcription assays were performed and the rcsulting RNA was hybridized to filters containing cDNA corresponding to collagenase, stromelysin, and GAPDH. Results were quantitated by densitometric scanning and normalized to GAPDH. The average of 2 independent experiments is shown. B, Human foreskin fibroblasts were transiently transfected with a luciferase reporter construct containing a 2-kb fragment of the human stromelysin promoter, along with SVβ-galactosidase as a control for transfection efficiency. Cytokines were added 16 hours after transfection, and cells were harvested 24 hours later. Shown are data from 2 independent experiments performed in triplicate, normalized to levels of β-galactosidase, and expressed relative to the control.
To further confirm this finding, transient transfection experiments were done in human foreskin fibroblasts, using a luciferase reporter construct containing 2 kb of the human stromelysin 5′-flanking region. Figure 6B shows that IL-1-induced transcription from the stromelysin promoter was reduced ~60% in the presence of IL-4. Similar results were obtained using a transformed synovial cell line (results not shown).
Association of IL-4 inhibition of IL-1-induced MMP production with decreased production of PGE2
Although IL-4 has been reported to inhibit the production of PGE2 in several model systems (18,19,21,22), the few results available in synovial fibroblasts have been conflicting. IL-4 was found to inhibit the production of PGE2 in synovial fibroblasts stimulated with superantigen (19), and Scitz et a1 (22) found that IL-4 inhibited production of PGE2 in RA and OA synoviocytes stimulated with IL-1β or TNF. However, Sugiyama et a1 (21) reported that the effects of IL-4 were cell-type specific in that IL-1α-induced PGE2 levels were inhibited in monocytes but not in rheumatoid synovial fibroblasts. In addition, there are conflicting reports on the role of PGE2 in IL-1-induced production of MMP (4,8,24,30–34).
To determine whether IL-4 inhibition of MMP production is associated with changes in PGE2 levels in HSF, levels of PGE2 were measured in 6-hour conditioned media from HSF cultures stimulated with IL,-1β in the presence or absence of IL-4. The results, shown in Figure 7, demonstrated that coincubation of HSF with IL-4 plus IL-1 resulted in decreased production of PGE2 compared with incubation with IL-1 alone.
Figure 7.
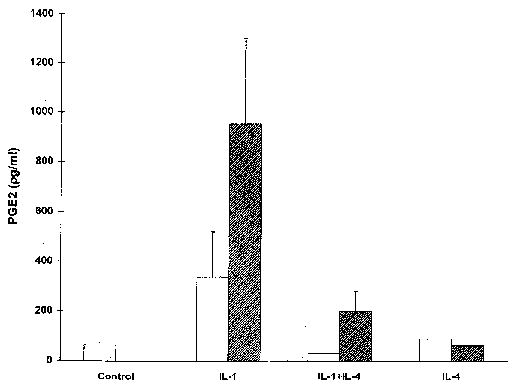
Interleukin-4 (IL-4) inhibits IL-1-induced production of prostaglandin E2 (PGE2) in human synovial fibroblasts (HSF). Levels of PGE2 were measured in triplicate in conditioned media from HSF cultures treated with the appropriate cytokine(s) for 6 hours. HSF cultures isolated from 2 different individuals were used.
Lack of abrogation of IL-4 inhibition of IL-1- induced MMP expression by the addition of exogenous PGE2.
To determine whether IL-4 inhibition of IL-1- induced PGE2 production plays a causal role in the inhibition of MMP expression, increasing doses of exogenous PGE2 were added in the presence of IL-1 and IL-4. Results of this experiment showed that addition of PGE2 had no effect on the ability of IL-4 to inhibit the IL-1 induction of MMP mRNA (Figure 8).
Figure 8.
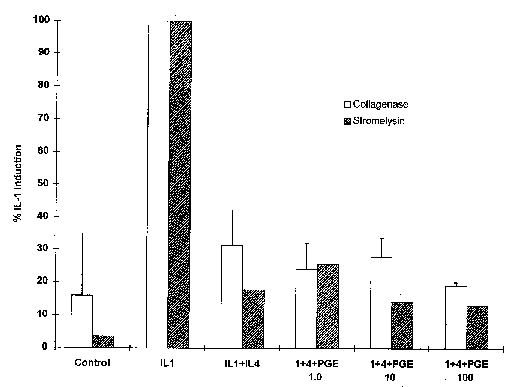
Interleukin-4 (IL-4) inhibition of IL-1-induced expression of matrix metalloproteinase is independent of prostaglandin E2 (PGE2). Indicated amounts (ng/ml) or PGE2 (PGE) were added to cultures of human synovial fibroblasts simultaneously with 100 ng of IL-1β/ml and 10 ng of IL-4/ml. Total RNA was isolated after 6 hours, and Northern blots were hybridized with cDNA probes corresponding to collagenase, stromelysin, and GAPDH. The blot was quantitated and normalized to GAPDH as described abovc. Shown are data from 3 independent experiments for collagenase and 2 for stromelysin.
Lack of effect of IL-4 on binding of transcription factor AP-1
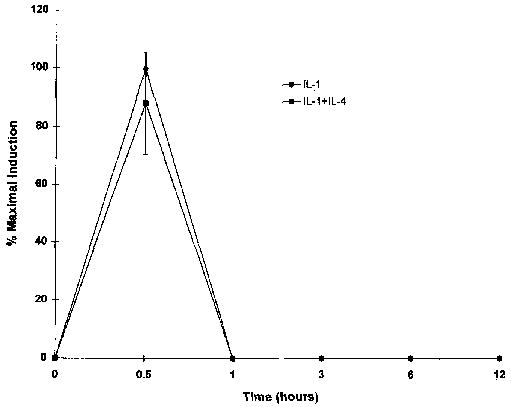
Transcription factor AP-1 is thought to be an important factor in the regulation of both collagenase and stromelysin genes (9,l0). Since the inhibition of IL-1-induced MMP expression is at least partly transcriptional, and since IL-4 has been shown to inhibit gene expression by interfering with LPS-induced binding of AP-1 in monocytes (35), the effects of IL-4 on AP-1 binding were investigated. Nuclear extracts were isolated from synovial fibroblast cultures 1 hour after addition of IL-1 alone, IL-4 alone, or IL-1 in the presence of IL-4.
As shown in Figure 9, IL-4 had no effect on IL-1-induced AP-1 binding activity. In addition, binding to another promoter element present in both genes, the polyoma virus enhancer 3 (PEA-3) site (36,37), was also unaffected by IL-4 (results not shown). These results are consistent with our earlier finding that induction of c-fos mRNA is unaffected, and with the conclusion that IL-4 does not interfere generally with IL-1 signal transduction events.
Figure 9.
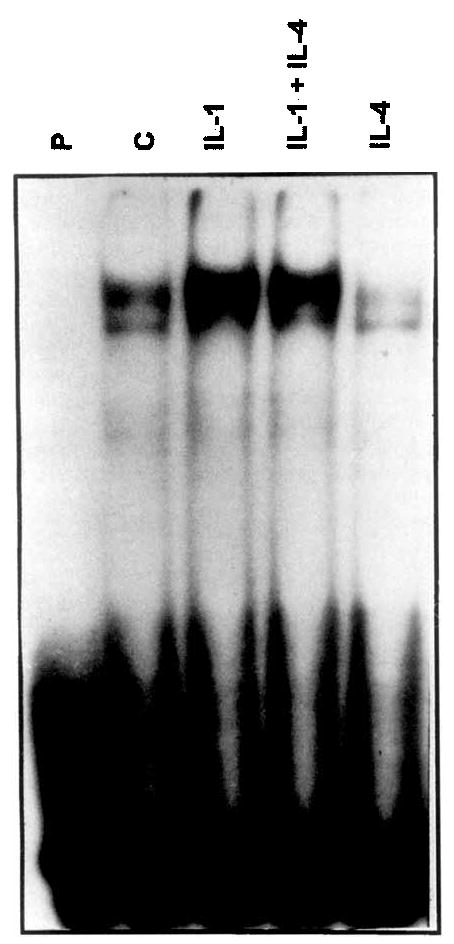
Binding of transcription factor activator protein 1(AP-1) is not affected by interleukin-4 (IL-4). Nuclear extracts were isolated from human synovial fibroblast cultures treated for 1hour with 100 ng of IL-1β/ml, 10 ng IL-4/m1, or both IL-1 and IL-4, as well as from control cultures. Binding of 5 μg of nuclear extract to a 32P-labeled oligo(dT) probe corresponding to the AP-1 site in the stromelysin promoter was determined by electrophoretic mobility shift assay. P = probe alone, without nuclear extract, C = control, without interleukin.
DISCUSSION
Joint and tissue destruction in chronic inflammatory conditions such as RA and periodontitis is thought to be largely a result of increased production of MMP by cytokine-stimulated fibroblasts (1,2). We have shown here that the antiinflammatory cytokine IL-4 has a dose-dependent inhibitory effect on IL-1 induction of both collagenase (MMP-1) and stromelysin 1 (MMP-3). Although similar results have been reported in LPS- stimulated monocytes (17,18) and superantigen-induced) synovial cells (19), as well as stromelysin expression in human skin fibroblasts stimulated with IL-1 (20), to our knowledge, this is the first report of such findings in IL-1-stimulated synovial fibroblasts.
Our data suggest that the inhibitory action of IL-4 takes place, at least in part, at the transcriptional level. The apparent discrepancy between the 30% (for collagenase) and 45% (for stromelysin) inhibition observed in the run-on transcription assay and the 60% seen in transfections may be due either to cell-specific variations or to the different experimental protocols. However, the level of suppression of stromelysin transcription observed in transfection experiments is very similar to that observed at both the mRNA and protein levels in synovial fibroblasts. Although the present data cannot exclude the possibility that IL-4 may also destabilize the MMP mRNA or inhibit protein production at the translational or posttranslational level, the effects of IL-4 on transcription appear to be significant.
Collagenase and stromelysin genes are, in most cases, coordinately regulated (8), and both genes contain AP-1 and PEA-3 sites at similar locations in their promoter regions that are thought to be important in their regulation (9,10,36,37). Based on these facts, along with results reported by Dokter et a1 (35) showing IL-4 inhibition of the induction of both c-jun and c-fos mRNA, as well as AP-1 binding activity, in LPS-stimulated monocytes, it was somewhat surprising to find that IL-1 induction of c-fos, as well as AP-1 and PEA-3 binding activity were not affected by the addition of IL-4 in synovial fibroblasts.
The fact that induction of c-fos mRNA and AP-1 binding are not affected suggests that IL-4 does not interfere with IL-1 receptor signaling or signal transduction in general, but rather, is at least somewhat specific. In addition, this result is consistent with evidence suggesting that although AP-1 is important for basal expression of these genes, other transcription factors are likely to be involved in determining the magnitude of induction. For example, the magnitude of the induction of both genes has been shown to decrease as promoter constructs are deleted from the 5′ end (9,10,38–40), and additional promoter elements have been identified for each of the 2 genes (7,41,42). Our results therefore suggest the possibility that the inhibitory effects of IL-4 on MMP expression may be mediated by other transcription factors that are not yet fully characterized. However, since the possibility remains that IL-4 may be affecting AP-1 transactivation potential in ways not reflected in DNA binding (e.g., phosphorylation or availability of coactivators), it cannot presently be definitively concluded that IL-4 inhibition of IL-1 induction of collagenase and stromelysin is independent of AP-1.
Previous reports showing IL-4 inhibition of gene expression have focused on inhibition of prostaglandin synthesis, presumably leading to decreased production of cAMP (18,19,21,22). However, Sugiyama et a1 (21) found that IL-4 inhibition of IL-1α–induced cyclooxygenase 2 mRNA and PGE2 production was cell-type specific, occurring in phorbol myristate acetate–differentiated U937 cells and freshly prepared adherent synoviocytes, but not in rheumatoid synovial fibroblasts. In addition, there are several conflicting reports concerning the role of prostaglandins in the IL-1 induction of collagenase and stromelysin genes. Inhibition of prostaglandin synthesis by indomethacin has been shown to both augment (32) as well as inhibit (33) MMP expression, as has exogenous addition of PGE2 (4,8,30–32,34) and alterations in levels of cAMP (8,31,32,34).
Our results, showing an IL-4 inhibition of IL-1-induced PGE2 production by synovial fibroblasts, are in conflict with the results reported by Sugiyama et al (21). However, these data are consistent with earlier reports that IL-4 inhibits PGE2 production in other systems (18,19,22). Although it has been suggested by some investigators that the induction of collagenase is dependent on PGE2 (18) and that the inhibitory effects of IL-4 are mediated by inhibition of PGE2 production (18,19,22), our finding that addition of exogenous PGE2 was unable to restore the IL-1 induction of the MMP suggests that IL-4 inhibition of MMP expression is through some other mechanism. This conclusion is consistent with that of Mauviel et al (4), who found that although addition of exogenous PGE2 induced collagenase expression in human dermal fibroblasts, inhibition of PGE2 production had no effect on the IL-1 induction. They concluded that while PGE2 and IL-1 induce collagenase expression by a common mechanism, the IL-1 induction is not dependent on the production of PGE2. In addition, Prontera et al (20) have suggested that the IL-4 inhibition of IL-1–induced stromelysin expression in human skin fibroblasts is independent of protein kinase A or cAMP levels. Taken together, these results suggest that although IL-4 inhibits both IL-1-induced PGE2 production and MMP expression, the two events do not appear to be causally linked in synovial fibroblasts.
In summary, we have presented evidence that coincubation of HSF with IL-4 and IL-1 results in a dose-dependent reduction in the IL-1-induced production of both collagenase and stromelysin. This inhibition takes place, at least in part, at the level of transcription and is accompanied by decreased production of PGE2. However, binding of transcription factor AP-1 is not affected, raising the possibility that the effects of IL-4 may be mediated by other, less well-characterized, transcription factors.
Lastly, it has been suggested that the chronic inflammation and ensuing joint and tissue destruction associated with diseases such as RA and periodontitis may be perpetuated by an imbalance between proinflammatory and antiinflammatory cytokines (22,43). In addition to inhibiting the production of MMP by synovial fibroblasts as demonstrated here, IL-4 has also been shown to: 1) inhibit the production of IL-1β while increasing the production of IL-1 receptor antagonist in rheumatoid synovial explants (44); 2) decrease the number of TNF receptors on rheumatoid synovial fibroblasts (45); 3 ) inhibit mitogen-induced proliferation of rheumatoid synoviocytes (46); and 4) increase production of tissue inhibitor of metalloproteinases in chondrocytes while inhibiting IL-1β-mediated cartilage matrix degradation (47). Taken together, these results suggest that restoring the cytokine balance might elicit a variety of beneficial effects.
Acknowledgments
The authors wish to thank Elizabeth Pease and Ruth Thornton for helpful discussions, and Yinkang Qian, Christian Lopez, Tshela Lewis-Smith, Bill Laidlaw, and Billie Johnson for technical assistance.
Footnotes
Dr. Carter Borghaei’s work was supported in part by NIH/NIDR grant RO3-DE11577-01.
References
- 1.Harris ED., Jr . Pathogenesis of rheumatoid arthritis. In: Kelley WN, Harris ED Jr, Ruddy S, Sledge CB, editors. Textbook of rheumatolog. Philadelphia: WB Saunders; 1981. pp. 896–927. [Google Scholar]
- 2.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1993;28:500–10. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 3.MacNaul KL, Chartrain N, Lark M, Tocci MJ, Hutchinson NL. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases genes by prostaglandin E2. J Bid Chem. 1990;265:17238–45. [PubMed] [Google Scholar]
- 4.Mauviel A, Halcin C, Vasiloudes P, Parks WC, Kurkinen M, Uitto J. Uncoordinate regulation of collagenase, stromelysin, and tissue inhibitor of metalloproteinases genes by prostaglandin E2: selective enhancement of collagenase gene expression in human dermal fibroblasts in culture. J Cell Biochem. 1994;54:465–72. doi: 10.1002/jcb.240540413. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Kurkinen M. Different mechanisms of regulation of the human stromelysin and collagenase genes: analysis by reverse-transcriptase-coupled PCR assay. Eur J Biochem. 1994;222:651–8. doi: 10.1111/j.1432-1033.1994.tb18909.x. [DOI] [PubMed] [Google Scholar]
- 6.Ni X, Borghaei H, Borghaei RC, Thornton R, Pease E, Mochan E. DGLA discoordinately suppresses IL- 1 induced metalloproteinase mRNA levels in human synovial fibroblasts. Inflamm Res. 1997;46:S124–6. doi: 10.1007/s000110050137. [DOI] [PubMed] [Google Scholar]
- 7.Borghaei H, Borghaei RC, Ni X, Pease E, Thornton R, Mochan E. Evidence that suppression of IL-1 induced collagenase mRNA expression by dihomo-γ-linolenic acid involves inhibition of NFκB binding . lnflamm Res. 1997;46:S177–8. doi: 10.1007/s000110050168. [DOI] [PubMed] [Google Scholar]
- 8.DiBattista JA, Pelletier JP, Zafarullah M, Fujimoto N, Obata K, Martel-Pelletier J. Coordinate regulation of matrix metalloproteinases and tissue inhibitor of metalloproteinase expression in human synovial fibroblasts. J Rheumatol Suppl. 1995;43:123–8. [PubMed] [Google Scholar]
- 9.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′ flanking region . Mol Cell Biol. 1987;7:2256–66. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinones S, Saus J, Otani Y, Harris ED, Jr, Kurkinen M. Transcriptional regulation of human stromelysin. J Biol Chem. 1989;264:8339–44. [PubMed] [Google Scholar]
- 11.Nouri AME, Panayi GS, Goodman SM. Cytokines and chronic inflammation of rheumatic disease. I. The presence of IL-1 in synovial fluids . Clin Exp lmmunol. 1984;55:295–302. [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CQ, Field M, Feldmann M, Maini RN. Localization of tumor necrosis factor α in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–32. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- 13.Tokoro Y, Yamamoto T, Hara K. IL-1β mRNA as the predominant inflammatory cytokine transcript: correlation with inflammatory cell infiltration into human gingiva . J Oral Pathol Med. 1996;25:225–31. doi: 10.1111/j.1600-0714.1996.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 14.Miossec P, Naviliat M, dupuy D’Angeac A, Sany J, Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor β in rheumatoid synovitis. Arthritis Rheum. 1990;33:1180–7. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- 15.Dolhain RJEM, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AMM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 16.Kabashima H, Nagata K, Hashiguchi I, Toriya Y, Iijima T, Maki K, et al. Interleukin 1 receptor antagonist and interleukin 4 in gingival crevicular fluid of patients with inflammatory periodontal disease. J Oral Pathol Med. 1996;25:449–55. doi: 10.1111/j.1600-0714.1996.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 17.Lacraz S, Nicod L, Galve-de Rochemonteix B, Bauberger C, Dayer JM, Welgus HG. Suppression of metalloproteinase biosynthesis in human alveolar macrophages by interleukin-4. J Clin Invest. 1992;90:382–8. doi: 10.1172/JCI115872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcoran ML, Stetler-Stevenson WG, Brown PD, Wahl LM. Interleukin 4 inhibition of prostaglandin E2 synthesis blocks interstitial collagenase and 92-kDa type IV collagenase/gelatinase production by human monocytes. J Biol Chem. 1992;267:515–9. [PubMed] [Google Scholar]
- 19.Mehindate K, Al-Daccak R, Aoudjit F, Damdoumi F, Fortier M, Borgeat P, et al. Interleukin-4, transforming growth factor β1, and dexamethasone inhibit superantigen-induced prostaglandin E2- dependent collagenase gene expression through their action on cyclooxygenase-2 and cytosolic phospholipase A2. Lab Invest. 1996;75:529–38. [PubMed] [Google Scholar]
- 20.Prontera C, Crescenzi G, Rotilio D. Inhibition by interleukin-4 of stromelysin expression in human skin fibroblasts: role of PKC. Exp Cell Res. 1996;224:183–8. doi: 10.1006/excr.1996.0126. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama E, Taki H, Kuroda A, Mino T, Yamashita N, Kobayashi M. Interleukin-4 inhibits prostaglandin E2 production via freshly prepared adherent rheumatoid synovial cells via inhibition of biosynthesis and gene expression of cyclo-oxygenase II but not of cyclo-oxygenase I. Ann Rheum Dis. 1996;55:375–82. doi: 10.1136/ard.55.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seitz M, Loetscher P, Dewald B, Towbin H, Ceska M, Boggioloini M. Production of interleukin-1 receptor antagonist, inflammatory chemotactic proteins, and prostaglandin E by rheumatoid and osteoarthritic synoviocytes-regulation by IFN-γ and IL-4. J Immunol. 1994;152:2060–5. [PubMed] [Google Scholar]
- 23.Mochan E, Uhl J, Newton R. IL-1 stimulation of synovial plasminogen activator production. J Rheumatol. 1986;13:15–9. [PubMed] [Google Scholar]
- 24.Mochan E, Uhl J, Newton R. Evidence that interleukin 1 induction of synovial cell plasminogen activator is mediated via prostaglandin E2 and cAMP. Arthritis Rheum. 1986;29:1078–84. doi: 10.1002/art.1780290904. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid-guanidinium-thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber E, Matthais P, Muller MM, Schaffner W. Rapid detection of octamer binding protein with “mini-extracts’ prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg ME, Bender JP. Current protocols in molecular biology. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al., editors. New York: John Wiley & Sons; 1997. pp. 4.10.1–4.10.11. [Google Scholar]
- 28.Lin JX, Vilcek J. Tumor necrosis factor and interleukin-1 cause a rapid and transient stimulation of c-fos and c-myc mRNA levels in human fibroblasts. J Biol Chem. 1987;262:11908–11. [PubMed] [Google Scholar]
- 29.Bedard PA, Golds EE. Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol. 1993;154:433–41. doi: 10.1002/jcp.1041540227. [DOI] [PubMed] [Google Scholar]
- 30.Wahl LM, Lampel LL. Regulation of human peripheral blood monocyte collagenase by prostaglandins and anti-inflammatory drugs. Cell Immunol. 1987;105:411–22. doi: 10.1016/0008-8749(87)90088-8. [DOI] [PubMed] [Google Scholar]
- 31.Salvatori R, Guidon PT, Jr, Rapauno BE, Bockman RS. Prostaglandin E1 inhibits collagenase gene expression in rabbit synoviocytes and human fibroblasts. Endocrinology. 1992;131:21–8. doi: 10.1210/endo.131.1.1377121. [DOI] [PubMed] [Google Scholar]
- 32.Case JP, Lafyatis R, Kumkumian GK, Remmers EF, Wilder RL. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990;145:3755–61. [PubMed] [Google Scholar]
- 33.Vignon E, Mathieu P, Louisot P; Richard M. In vitro effect of nonsteroidal antiinflammatory drugs on proteoglycanase and collagenase activity in human osteoarthritic cartilage. Arthritis Rheum. 1991;34:1332–5. doi: 10.1002/art.1780341021. [DOI] [PubMed] [Google Scholar]
- 34.DiBattista JA, Martel-Pelletier J, Fujimoto N, Obata K, Zafarullah M, Pelletier JP. Prostaglandins E2 and E1 inhibit cytokine-induced metalloproteinase expression in human synovial fibroblasts: mediation by cyclic-AMP signaling pathway. Lab Invest. 1994;71:270–8. [PubMed] [Google Scholar]
- 35.Dokter WHA, Koopmans SB, Vellenga E. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL-6, and NF-κB) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–16. [PubMed] [Google Scholar]
- 36.Gutman A, Wasylyk B. The collagenase promoter contains a TPA and oncogene responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–6. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buttice G, Kurkinen M. A polyoma virus enhancer A binding protein 3 site and ets-2 protein have a major role in the 12-O-tetradecanoylphorbol-13-acetate response of the human stromelysin gene. J Biol Chem. 1993;268:7196–204. [PubMed] [Google Scholar]
- 38.Buttice G, Quinones S, Kurkinen M. The AP-I site is required for basal expression but is not necessary for TPA response of the human stromelysin gene. Nucleic Acids Res. 1991;19:3723–31. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auble DT, Brinckerhoff CE. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991;30:4629–35. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- 40.Quinones S, Buttice G, Kurkinen M. Promoter elements in the transcriptional activation of the human stromelysin-1 gene by the inflammatory cytokine, interleukin 1. Biochem J. 1994;302:471–7. doi: 10.1042/bj3020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sam L, Moscat J, Diaz-Meco MT. Molecular characterization of a novel transcription factor that controls stromelysin expression. Mol Cell Biol. 1995;15:3164–70. doi: 10.1128/mcb.15.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyle GAR, Pierce RA, Parks WC. Transcriptional induction of collagenase-1 in differentiated monocyte-like (U937) cells is regulated by AP-1 and an upstream CEBP-β site. J Biol Chem. 1997;272:11840–9. doi: 10.1074/jbc.272.18.11840. [DOI] [PubMed] [Google Scholar]
- 43.Shapira L, van Dyke TE, Hart TC. A localized absence of interleukin 4 triggers periodontal disease activity: a novel hypothesis. Med Hypotheses. 1992;39:319–22. doi: 10.1016/0306-9877(92)90056-i. [DOI] [PubMed] [Google Scholar]
- 44.Chomarat P, Vannier E, Dechanet J, Rissoan MC, Banchereau J, Dinarello CA, et al. Balance of IL-1 receptor antagonist/IL-1β in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432–9. [PubMed] [Google Scholar]
- 45.Taylor DJ. Interleukin 4 (IL-4) induces down-modulation and shedding of the p55 tumour necrosis factor receptor and inhibits TNFα’s effect on rheumatoid synovial fibroblasts. Rheumatol Int. 1994;14:21–5. doi: 10.1007/BF00302667. [DOI] [PubMed] [Google Scholar]
- 46.Dechanet J, Briolay J, Rissoan MC, Chomarat P, Galizzi JP, Banchereau J, et al. IL-4 inhibits growth factor-stimulated rheumatoid synoviocyte proliferation by blocking the early phases of the cell cycle. J Immunol. 1993;151:4908–17. [PubMed] [Google Scholar]
- 47.Shingu M, Miyauchi S, Nagai Y, Yasutakes C, Horie K. The role of IL-4 and IL-6 in IL-1 dependent cartilage matrix degradation. Br J Rheumatol. 1995;34:101–6. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]


