Abstract
While evolution of coding sequences has been intensively studied, diversification of noncoding regulatory regions remains poorly understood. In this study, we investigated the molecular evolution of an enhancer region located 5 kb upstream of the transcription start site of the maize pericarp color1 (p1) gene. The p1 gene encodes an R2R3 Myb-like transcription factor that regulates the flavonoid biosynthetic pathway in maize floral organs. Distinct p1 alleles exhibit organ-specific expression patterns on kernel pericarp and cob glumes. A cob glume-specific regulatory region has been identified in the distal enhancer. Further characterization of 6 single-copy p1 alleles, including P1-rr (red pericarp/red cob) and P1-rw (red pericarp and white cob), reveals 3 distinct enhancer types. Sequence variations in the enhancer are correlated with the p1 gene expression patterns in cob glume. Structural comparisons and phylogenetic analyses suggest that evolution of the enhancer region is likely driven by gene conversion between long direct noncoding repeats (∼6 kb in length). Given that tandem and segmental duplications are common in both animal and plant genomes, our studies suggest that recombination between noncoding duplicated sequences could play an important role in creating genetic and phenotypic variations.
EVOLUTION of cis-regulatory elements has been indicated as a major contributor to phenotypic variation, because changes in regulatory regions can induce temporal and spatial expression pattern changes (Doebley and Lukens 1998; Ludwig 2002; Wray et al. 2003; Carroll et al. 2004). Despite the importance of cis-elements in regulation of gene expression, the molecular basis of the cis-regulatory region evolution remains poorly understood. In recent studies, comparisons of cis-regulatory regions between natural variants have been applied to investigate their molecular evolution (Hanson et al. 1996; Wang et al. 1999; Ludwig et al. 2000; Purugganan 2000). In this study, we used natural variations of the gene specifying flavonoid pigment patterns in maize to gain insight into the evolutionary dynamic of cis-regulatory sequences. Several genetic loci, including the c1 (colorless1), p1 (pericarp color1), r1 (red1), b1 (booster1), and pl1 (purple plant1) genes, that regulate flavonoid biosynthetic pathways of maize have been well characterized at the molecular level. All of these regulatory genes display diverse patterns of gene expression in both floral and vegetative organs of maize (Ludwig and Wessler 1990; Cone et al. 1993; Procissi et al. 1997; Selinger et al. 1998). Allelic variation has been investigated at the c1, b1, and r1 loci in detail. Variations in regulatory regions have been linked to distinct allelic expression patterns and phenotypic diversity (Selinger et al. 1998; Li et al. 2001). Moreover, studies on the c1 gene have suggested that the cis-regulatory region could be subject to selection, resulting in increased frequency of one c1 haplotype and giving rise to the pigmented kernel aleurone phenotype (Hanson et al. 1996).
Recent analyses of the maize p1 gene provide further evidence that variations in cis-regulatory regions are involved in phenotypic diversification. The p1 gene encodes an R2R3 Myb-like transcription factor (Jiang et al. 2004). Expression of the p1 gene is observed predominantly in the floral organs, most notably the kernel pericarp and cob glumes. According to the pigmentation patterns in these two organs, p1 alleles are commonly classified into four major types: P1-rr (red pericarp/red cob), P1-wr (white pericarp/red cob), P1-rw (red pericarp/white cob) and p1-ww (white pericarp/white cob). Three distinct p1 alleles, P1-rr4B2, P1-rw1077, and P1-wr[w22], have been characterized and compared at the molecular level. All of these p1 alleles share highly similar coding regions, but differ in the flanking regulatory sequences (Chopra et al. 1998; Sidorenko et al. 2000). Our recent data have demonstrated that changes in the enhancer region, located 5 kb 5′ of the transcription start site, result in phenotypic variations between P1-rr4B2 and P1-rw1077 (Zhang and Peterson 2005).
More than 100 natural variants of p1 have been reported, each exhibiting distinctive patterns and intensity of pigmentation in kernel pericarp and cob glumes (Brink and Styles 1966). This rich collection of naturally occurring alleles provides an excellent system to study the evolution of cis-regulatory regions at the p1 locus. In this study, 6 distinct single-copy p1 alleles were characterized and compared. The goals of the study were: (i) to investigate DNA variations in noncoding regions of the p1 locus, particularly the distal enhancer regions; (ii) to examine correlation between variations in cis-regulatory regions and phenotypic changes; and (iii) to identify the forces affecting evolution of the regulatory regions and the generation of genetic and phenotypic diversity at the p1 locus.
MATERIALS AND METHODS
Maize germplasm:
The p1 alleles used in this study are homozygous in the 4Co63 inbred genetic background. As shown in Table 1, p1 alleles with an assigned CFS number were from Brink's p1 collection (Brink and Styles 1966). The P1-rr4B2 and P1-rw1077 alleles are the same as used in previous studies (Lechelt et al. 1989; Zhang and Peterson 2005).
TABLE 1.
Collections of single-copy p1 alleles
| Phenotype
|
||||
|---|---|---|---|---|
| Allelic name | Allelic type | Pericarp | Cob glume | Source |
| P1-rr4B2 | P1-rr4B2 | Red | Red | Derivative from P1-vva |
| P1-rrCFS181 | P1-rr4B2 | Red | Red | Brink's collectionb |
| P1-rr13:255 A10 | P1-rr4B2 | Red | Red | From P1-vv |
| P1-rr1088 | P1-rr1088 | Red | Red | To be determined |
| P1-rrCFS36 | P1-rrCFS36 | Red | Red | Brink's collection |
| P1-rrCFS33 | P1-rrCFS36 | Red | Red | Brink's collection |
| P1-rrCFS305 | P1-rrCFS36 | Red | Red | Brink's collection |
| P1-rrCFS548 | P1-rrCFS36 | Red | Red | Brink's collection |
| P1-rrCFS272 | P1-rrCFS36 | Red | Red | Brink's collection |
| P1-rw1077 | P1-rw1077 | Red | White | Maize Genetic Coop |
| P1-rwCFS325 | P1-rw1077 | Red | White | Brink's collection |
| P1-rwCFS302 | P1-rwCFS302 | Red | White | Brink's collection |
| P1-rwCFS332 | P1-rwCFS302 | Red | White | Brink's collection |
| P1-rwCFS342 | P1-rwCFS342 | Red | White | Brink's collection |
| P1-rwCFS334 | P1-rwCFS342 | Red | White | Brink's collection |
DNA gel blot analysis:
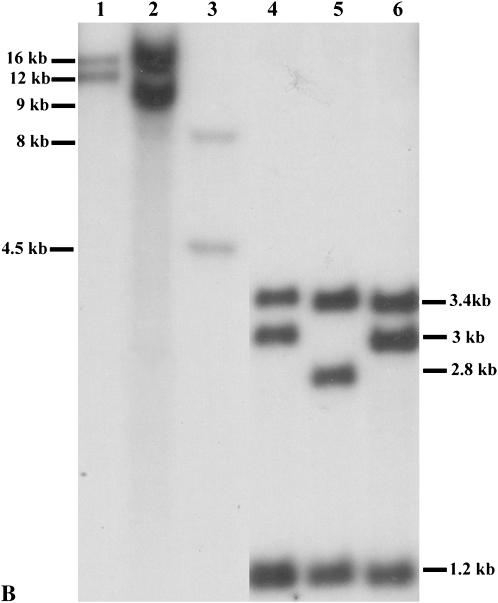
Genomic DNA was extracted from maize seedling leaves by the CTAB method (Saghai-Maroof et al. 1984) and digested with restriction enzymes according to manufacturer's instructions. Gel electrophoresis and hybridization procedures were conducted as described in previous studies (Sidorenko et al. 2000). The blot was probed with the p1-specific probe, genomic fragment 15 (Figures 1B and 2). p1 alleles that exhibited identical hybridization patterns following digestion with SalI, SacI, EcoRI, and XbaI and probing with fragment 15 were grouped as the same allelic type.
Figure 1.—
(A) Phenotypes of the natural p1 alleles. Mature ear pigmentation patterns specified by the p1 alleles: P1-rw1077, P1-rwCFS302, and P1-rwCFS342 (top row from left to right) and P1-rr1088, P1-rrCFS36, and P1-rr4B2 (bottom row from left to right). All alleles are homozygous. (B) DNA gel blot analyses on the p1 simplex alleles: lane 1, P1-rw1077; lane 2, P1-rwCFS302; lane 3, P1-rwCFS342; lane 4, P1-rr4B2; lane 5, P1-rr1088; and lane 6, P1-rrCFS36. Genomic DNA was digested with SalI and hybridized with the probe, p1 genomic fragment 15. The 1.2-kb band in P1-rr4B2 is a doublet, while P1-rr1088 and P1-rrCFS36 have only one copy of the 1.2-kb fragment.
Figure 2.—
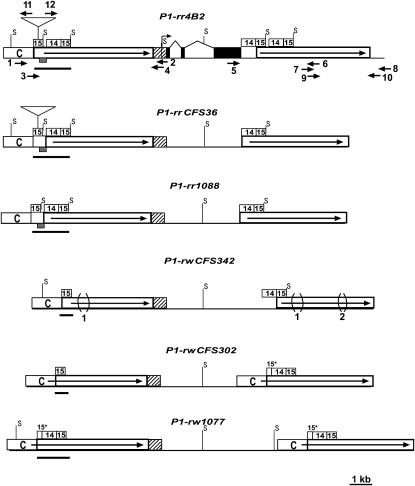
Structural comparisons of the simplex p1 alleles. The solid boxes connected by thin lines represent exon regions. The open boxes with solid arrows indicate long direct repeats in 5′ and 3′ noncoding regions. The bent arrow represents a transcription start site (Grotewold et al. 1991). The hatched boxes represent 5′-UTR and 90-bp promoter regions conserved among the p1 alleles. The open boxes labeled with C indicate a 1.1-kb region, termed fragment C, present at the 5′ direct repeats of all p1 alleles, but absent from the 3′ direct repeats in several p1 alleles. The numbered open boxes represent the sequences identified in the text as fragment 15 and fragment 14. The enhancer-containing regions in each p1 allele are highlighted by solid bars, which range from 1.6 kb (in the P1-rr alleles) to 602 bp (in the P1-rwCFS302 and P1-rwCFS342 alleles). The cob glume-specific regulatory region is indicated as shaded boxes in the enhancer-containing regions of the P1-rr alleles (which include the 189-bp sequence from fragment 15 and the 197-bp immediate downstream sequence). The regions marked 15* indicate partial fragment 15 sequences, which contain only the first 200 bp fragment 15 sequences. The triangles located in the first copy of fragment 15 in the 5′ noncoding repeats of P1-rr4B2 and P1-rrCFS36 represent insertions of a 1.6-kb transposon-like sequence. The bracketed regions marked 1 and 2 indicate 549- and 148-bp deletions, respectively, in the noncoding repeats of P1-rwCFS342. The solid arrows represent primers used to amplify 5′ and 3′ noncoding regions of p1: 1, P1rr-18; 2, PA-B4; 3, P1rr-14; 4, PA-B6; 5, EP5-16; 6, P1rr-16; 7, P1rr-13r; 8, P1rr-30; 9, P1rr-16r; 10, P1rr-29; 11, P1rr-32; and 12, P1rr-25. Positions of the restriction site, SalI, are shown on each p1 allele (only unmethylated SalI sites that flank fragment 15 are indicated on the map).
Amplification and sequencing of the p1 noncoding regions:
Nested genomic PCR was performed to amplify ∼6 kb from the 5′ noncoding regions. The primer pair, P1rr-18 and PA-B4, was used in the first-round reaction. A 1-μl aliquot of the first-round PCR product was subjected to a second-round PCR with the primer pair, P1rr-14 and PA-B6. For the p1 allele, P1-rrCFS36, two additional primers, P1rr-32 and P1rr-25, were used due to the presence of a 1.6-kb transposon-like sequence inserted in the 5′ copy of fragment 15 (Figure 2). The 3′ noncoding regions were amplified in two overlapping pieces separately by using the primers EP5-16 and P1rr-16, as well as nested primers, P1rr-13r and P1rr-30 and P1rr-16r and P1rr-29. The 723-bp sequences in the second intron were amplified with primers 723-5 and 723-3. Locations and sequences of all primers are shown in Figures 2 and 4 and Table 2. The PCR reactions were performed using enhanced DNA polymerase, Elongase (Invitrogen, Carlsbad, CA) with ∼1 min of extension time for every 1-kb fragment size. Optimal PCR parameters were followed as suggested by the manufacturer. To minimize the problems caused by PCR artifacts due to annealing of partial extension products to homologous regions in the template DNA pool, the PCR products from three independent reactions were mixed, purified using a gel extraction kit (QIAGEN, Valencia, CA), and sequenced directly from both directions. Sequencing reactions were provided by the Iowa State University DNA Sequencing Facility. The final sequences were inspected and assembled using the software package, Vector NTI Advance 9.0 (InforMax, Frederick, MD). 5′ and 3′ noncoding repeats of the newly sequenced p1 alleles, P1-rr1088, P1-rrCFS36, P1-rwCFS302, and P1-rwCFS342, have GenBank accession nos. DQ160218–DQ160223.
Figure 4.—
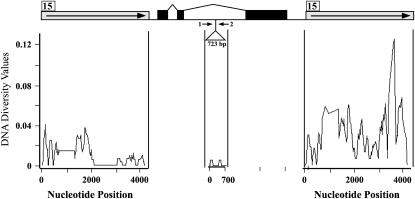
Nucleotide diversity analysis of noncoding regions at the p1 locus. Open boxes with solid arrows represent the 5′ and 3′ noncoding direct repeats. The position of fragment 15 is indicated with the numbered box. The solid boxes connected with thin lines represent the exon and intron structure of the p1 locus. The triangle in the second intron indicates the 723-bp sequence that is also subject to nucleotide diversity analysis. The solid arrows represent the primers for amplification of the 723-bp region: 1, 723-5; 2, 723-3.
TABLE 2.
Primers used to amplify maize p1 sequences
| Primer names | Primer sequences (5′–3′) |
|---|---|
| P1rr-18 | TGAGTCCTGACCGACAGTCT |
| PA-B4 | tgccttccatacttgcactgc |
| P1rr-14 | GAAGGCAGACGATGAGGAGA |
| PA-B6 | CACAACCTTTCACATACAGAG |
| EP5-16 | cgagacttggctcctgt |
| P1rr-16 | TCTCAGAGTATAGCAACAC |
| P1rr-13r | CTCATCAACGTGCTGTTCC |
| P1rr-30 | CGTCGTCAAGAACTCAAGAT |
| P1rr-16r | GTGTTGCTATACTCTGAGA |
| P1rr-29 | GGCTTGGTGCGTTGCTGA |
| 723-5 | TCTAGGCACTTTCTCGTG |
| 723-3 | GTAGAAATAAAGTCTGAGCA |
| P1rr-32 | TGTAAACCGTGCTCACTG |
| P1rr-25 | TGTAAACCGTGCCAGTGA |
Sequence analysis:
Sequences from P1-rr1088, P1-rrCFS36, P1-rwCFS302, and P1-rwCFS342 were aligned with those from P1-rr4B2 and P1-rw1077 using the software package, Vector NTI Advance 9.0 (InforMax). Phylogenetic analysis was carried out using a maximum-likelihood method in PAUP version 4.01 (Swofford 1998). Heuristic searching was conducted using a general time-reversible evolutionary model with estimated base frequencies and rate variation across sites modeled by gamma distribution. Support values for nodes on the maximum-likelihood tree were estimated with 500 bootstrap replicates (Felsenstein 1985). A 50% majority-rule consensus tree was generated using the sequence from teosinte as an outgroup. DNA diversity estimation and sliding-window analysis on the p1 alleles were conducted using the program package, DnaSP 4.0 (Rozas and Rozas 1999). The number of substitutions per nucleotide site between the distinct p1 alleles was estimated under the Kimura two-parameter (gamma) model as implemented in MEGA v. 2.1.
RESULTS
Characterization of p1 alleles:
More than 100 natural p1 variations have been collected and classified according to their pigmentation patterns in kernel pericarp and cob glumes (Brink and Styles 1966). Previous studies on three distinct p1 alleles indicated that the p1 gene could contain either a single coding sequence, as in P1-rr4B2 and P1-rw1077 (Lechelt et al. 1989; Zhang and Peterson 2005), or a multiple-copy complex, as in P1-wr[w22] (Chopra et al. 1998). A DNA gel blot survey with p1-specific probes has been conducted on 24 P1-rr, 6 P1-rw, and 9 P1-wr alleles (Cocciolone et al. 2001; M. D. McMullen, personal communication). The results showed that all examined P1-wr alleles contain multiple copies of p1 sequences, while all P1-rw alleles are single-copy genes; the P1-rr alleles could be either single copy or multiple copy. Because of the complicated nature of multiple-copy p1 alleles (Chopra et al. 1998), we focused on the characterization of single-copy p1 alleles in this study. According to the DNA gel blot patterns, the single-copy p1 alleles, including 6 P1-rw and 9 P1-rr alleles, can be classified into six allelic types: three P1-rr types and three P1-rw types (Table 1; Figure 1, A and B). The P1-rr4B2 and P1-rw1077 allelic types have been characterized and compared previously (Lechelt et al. 1989; Sidorenko et al. 2000; Zhang and Peterson 2005). Representative alleles from the other four allelic types (P1-rrCFS36, P1-rr1088, P1-rwCFS302, and P1-rwCFS342, phenotypes are shown in Figure 1A) were chosen for further analysis.
Structural comparisons of p1 alleles:
Previous studies have shown that the coding regions of different p1 alleles share high sequence similarity; and DNA polymorphisms in noncoding regulatory regions other than in coding regions could be responsible for phenotypic variations of the p1 alleles (Lechelt et al. 1989; Sidorenko et al. 2000; Zhang and Peterson 2005). To examine sequence polymorphisms in noncoding regions of the single-copy p1 alleles studied here, we used genomic PCR with p1-specific primers to amplify both 5′ and 3′ noncoding regions of the P1-rr and P1-rw alleles. In each allele, ∼6 kb PCR products from both 5′ and 3′ regions were sequenced and assembled, and the sequence assemblies match DNA gel blot patterns (see materials and methods). The structures of the noncoding regions of all six single-copy p1 alleles were compared as shown in Figure 2. Like those in P1-rr4B2 and P1-rw1077, the 5′ and 3′ noncoding regions in the four newly sequenced p1 alleles are also present as long direct repeats flanking the p1 coding sequences. The repeat unit could extend as long as 6.3 kb (as in P1-rw1077) from a 1.1-kb region, termed fragment C, to a 15-bp small repeat near the transcription start site (5′-GCGGGAGTGCGGCCT-3′)2.
Structural and sequence comparisons between the simplex p1 alleles revealed a number of DNA polymorphisms in both 5′ and 3′ noncoding direct repeats. In the 5′ repeats, the most notable polymorphic regions overlap with the previously identified enhancer regions (Figure 2). The distal enhancer region is located 5 kb upstream of the p1 transcription start site and includes the 405-bp fragment 15 and a 666-bp sequence termed fragment 14 (Sidorenko et al. 2000; Zhang and Peterson 2005; Figure 2). Based on the copy number of fragment 15 and fragment 14 sequences in the enhancer-containing regions, three distinct structural types can be defined in six simplex p1 alleles: (i) 1 copy of fragment 15 and 0 copy of fragment 14, i.e., the enhancer-containing region contains only fragment 15, as shown in P1-rwCFS302 and P1-rwCFS342; (ii) 1.5 copies of fragment 15 and 1 copy of fragment 14, i.e., the enhancer-contaning region contains a partial copy of fragment 15 followed by fragment 14 and a full copy of fragment 15, as seen in P1-rw1077; (iii) 2 copies of fragment 15 and 1 copy of fragment 14, i.e., the enhancer-containing region contains a full copy of fragment 15 followed by fragment 14 and an additional full copy of fragment 15, as shown in P1-rr1088, P1-rrCFS36, and P1-rr4B2. The type iii sequences can be further classified as two subtypes: (a) P1-rrCFS36 and P1-rr4B2, in which a 1.6-kb transposon-like sequence (Sidorenko et al. 2000) is present in the midpoint of the upstream fragment 15; (b) P1-rr1088, in which the 1.6-kb sequence is absent (Figure 2). The 1.6-kb transposon is flanked by an 8-bp direct repeat (CCAGTGAG), which may represent a target site duplication (TSD) generated upon insertion of the 1.6-kb transposon-like sequence. The P1-rr1088 allele contains only a single copy of the 8-bp sequence and no evidence of a sequence alteration (footprint), which commonly accompanies transposon excision. We conclude that the polymorphism between the two subtypes reflects a sequence insertion, rather than a deletion.
In the 3′ noncoding regions, the sequences composing fragment 15 and fragment 14 are also highly variable. Similar to the 5′ noncoding sequences, the 3′ regions of the simplex p1 alleles can also be classified as three distinct types: (i) 1 copy of fragment 14 and 1 copy of fragment 15 as shown in P1-rrCFS36, P1-rr1088, and P1-rwCFS342; (ii) 1.5 copies of fragment 15 and 1 copy of fragment 14 as seen in P1-rw1077; and (iii) 2 copies of fragment 14 and 2 copies of fragment 15 as shown in P1-rr4B2 (equivalent to two copies of type i sequences arranged in direct orientation).
Sequence alignment also revealed two large deletions in the 5′ and 3′ noncoding regions of the P1-rwCFS342 alleles: a 549-bp sequence (738 bp downstream of fragment 15) that is absent from both 5′ and 3′ noncoding direct repeats of P1-rwCFS342 (Figure 2) and a 148-bp sequence (∼3500 bp downstream of fragment 15 in the 3′ repeat, Figure 2) that is absent from the 3′ noncoding regions of P1-rwCFS342. Sequence analysis of a p1 homologous gene from teosinte (Zea mays subsp. parviglumis) (Zhang et al. 2000), the immediate progenitor of modern maize, indicated that both 549- and 148-bp sequences are present in teosinte (data not shown). Possibly, these two polymorphic regions resulted from deletions in P1-rwCFS342 rather than insertions in other p1 alleles.
Variations in the distal enhancers correlate with changes of pigmentation patterns in cob glumes:
Comparisons of the simplex p1 alleles reveal three distinct structural types in the 5′ enhancer-containing region. In all the simplex P1-rr alleles, this region carries duplicated fragment 15 sequences as well as a fragment 14 sequence between them. A 386-bp cob glume-specific regulatory region has been identified from the P1-rr4B2 allele, which overlaps with the upstream copy of fragment 15 plus the downstream 197-bp sequence (Figure 2). Although this particular sequence is present in both 5′ and 3′ direct noncoding repeats, the observation that the P1-rw1077 allele lacks this region in that specific location at the enhancer-containing region suggests that this cob glume enhancer region functions in a position-specific manner (Zhang and Peterson 2005). Absence of the cob glume-specific enhancer in that specific location is also observed in the newly sequenced P1-rw alleles, P1-rwCFS302 and P1-rwCFS342, which carry only one copy of fragment 15 (without duplication and fragment 14 sequence) in the enhancer-containing region (Figure 2). Therefore, in the single-copy p1 alleles examined in this study, the presence/absence of the cob glume-specific regulatory region in a particular position is correlated with gain/loss of pigmentation in cob glumes. Moreover, structural comparisons between the P1-rr and P1-rw alleles indicated that the cob glume-specific region is formed by complete duplication of fragment 15 and insertion of fragment 14 sequences. Our further analysis suggested that gene conversion between 5′ and 3′ noncoding repeats could account for the duplication and insertion events observed in the enhancer-containing regions of the simplex p1 alleles, which brought the cob glume-specific regulatory elements into the right position (see below).
Evidence for gene conversion between noncoding regions in the p1 alleles:
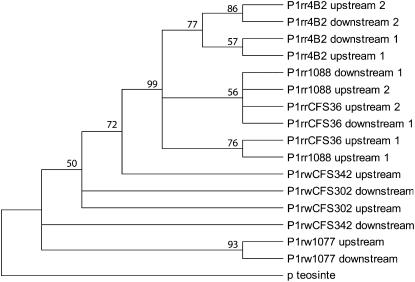
The 5′ and 3′ noncoding regions of the p1 locus are arranged as direct repeats, separated by the ∼6-kb transcribed region. The observation that a 549-bp deletion is located at identical positions in both 5′ and 3′ noncoding regions (P1-rwCFS342; Figure 2) suggests that gene conversion events occurred between the noncoding direct repeats in the p1 locus. Gene conversion has been indicated as a candidate mechanism for both homogenizing and diversifying tandem repeats (Teshima and Innan 2004). As mentioned above, the enhancer-containing regions in the 5′ noncoding repeats have diverse structures in the distinct p1 simplex alleles. The primary aim of this study is to understand how the 5′ enhancer-containing region was diversified and how the cob glume-specific sequence came to occupy its specific position in the P1-rr alleles. To assess the potential role of gene conversion in the creation of diversity in that region, phylogenetic analysis was performed on a 602-bp sequence, which includes fragment 15 and a 3′ adjacent 197-bp sequence. This 602-bp sequence is present in both 5′ and 3′ noncoding regions of all single-copy p1 alleles and can be viewed as a small repeat unit. Thus, it will be more informative to use the entire 602-bp region in the phylogenetic analysis than to use only the 386-bp cob glume enhancer-containing region. In the P1-rw alleles, only one copy of the 602-bp sequence is present in the 5′ noncoding regions, while two P1-rr alleles (P1-rr1088 and P1-rrCSF36) have duplicated 602-bp sequences in their 5′ repeats; and P1-rr4B2 has the 602-bp sequence duplicated in both 5′ and 3′ repeats (Figure 2). A total of 17 sequences were used to generate a maximum-likelihood consensus tree (Figure 3): 16 sequences from 5′ and 3′ repeats of the simplex p1 alleles and one sequence from the 3′ noncoding region of the teosinte p gene (p2t) used as the outgroup (Zhang et al. 2000). The 1.6-kb transposon-like sequence and a flanking 8-bp TSD were removed from the 5′ 602-bp sequence of P1-rr4B2 and P1-rr1088 for this analysis. If the 5′ repeats evolved independently from the 3′ repeats, then the 5′ sequences and the 3′ sequences should form distinct groups in the phylogenetic tree. Gene conversion events between the 5′ and 3′ repeats, however, could change the phylogenetic patterns by clustering the 5′ sequence with the 3′ sequence from the same p1 allele. On the basis of this logic, from the resulting phylogenetic tree, potential gene conversion events were detected between the 5′ and 3′ 602-bp sequences from four p1 alleles, i.e., P1-rw1077, P1-rr4B2, P1-rrCFS36, and P1-rr1088. Interestingly, the second copies (3′ copies) of the duplicated 602-bp sequences in the 5′ repeats of all P1-rr alleles are more closely related to the 602-bp sequences in the 3′ repeats than to their adjacent 5′ copies. This observation suggests that gene conversion events are involved in generating P1-rr-specific enhancer structures (see discussion). In contrast to the sequences from the P1-rr alleles, which form a monophyletic group with a high bootstrapping value (99%), those sequences from the P1-rw alleles are closely related to that from the p homologous gene in teosintes. This could suggest early divergence of the P1-rw alleles from the p1 ancestral gene during evolution (see discussion).
Figure 3.—
Phylogenetic analysis of the p1 distal enhancer regions. A rooted 50% majority-rule consensus maximum-likelihood tree was generated by using a 602-bp sequence, which includes fragment 15 plus a 197-bp downstream sequence. The sequence from the maize wild relative, teosinte parviglumis, was used as the outgroup. The numbers at nodes represent bootstrap values derived from 500 replicates. Sequences from the 5′ and 3′ noncoding repeats are identified as upstream or downstream, respectively. Sequences from the same repeat are indicated in numeric order from 5′ to 3′.
Nucleotide diversity in p1 noncoding regions:
Nucleotide diversity among the simplex p1 alleles was first estimated in both the 5′ and the 3′ noncoding repeats. As indicated in Figure 4, the regions that were sampled in this analysis are shared in all simplex p1 alleles. In the 5′ noncoding regions, a total of 19 indels and 83 polymorphic sites were detected, while, in the 3′ noncoding regions, a total of 42 indels and 197 polymorphic sites were detected. The estimated values (π and θ) for DNA diversity also indicated that the 3′ regions are more diverse than the 5′ regions (e.g., π-value 0.02929 vs. 0.00906; Table 3). This suggests that the 5′ noncoding repeats, but not the 3′ repeats, contain the critical regulatory regions for p1 expression and are therefore under functional constraints (Sidorenko et al. 2000).
TABLE 3.
Nucleotide diversity in the simplex p1 alleles
| Regions | n | No. of silent sites | No. of polymorphic sites | Θ | π | Tajima's D |
|---|---|---|---|---|---|---|
| Upstream repeat | 6 | 3526 | 83 | 0.01031 | 0.00906 | −0.7840, NS |
| Downstream repeat | 6 | 3278 | 197 | 0.02483 | 0.02929 | −1.1599, NS |
| 723-bp sequence | 5 | 723 | 2 | 0.00133 | 0.00111 | −0.97256, NS |
Nucleotide diversity was estimated on the simplex p1 haplotypes. NS, not significant.
To investigate the distribution of polymorphic sites, sliding-window analysis was performed on both 5′ and 3′ noncoding regions. This analysis revealed that the polymorphic sites are distributed throughout the 3′ noncoding regions, while, in the 5′ noncoding regions, polymorphic sites were frequently observed in the first 2-kbp region but are much less frequent in the remaining regions (Figure 4). Unequal functional constraints on the two regions could be one possible explanation for heterogeneity of nucleotide diversity in the 5′ noncoding regions. By Ac transposon mutagenesis assay, however, no functional elements were identified in the low-diversity region, while the distal enhancer was identified in the first 2-kb region (Moreno et al. 1992; Sidorenko et al. 2000). Alternatively, the uneven distribution of polymorphic sites in the 5′ noncoding regions could result from variation in recombination frequency across this region (see discussion).
Phylogenetic analysis has suggested that DNA diversity of 5′ and 3′ repeats at the simplex p1 alleles could have been affected by gene conversion. To further test this idea, we compared nucleotide diversity between duplicated and nonduplicated p1 sequences. An ideal single-copy p1 sequence for this analysis is the 723-bp sequence located in the second intron of p1 (Figure 4), because (i) this sequence is present only in the p1 locus but not in the p2 gene, the tightly linked paralogous gene of p1, which has potential to undergo gene conversion with p1 (Zhang et al. 2000; Zhang and Peterson 2005), and (ii) this sequence seems to be subject to no functional constraints as it is dispensable for expression of P1-rw1077 (Zhang and Peterson 2005). Interestingly, the values for DNA diversity of the 723-bp sequence (π-value is 0.00133 and θ-value is 0.00111) are 8–26 times lower than those of the 5′ and 3′ noncoding regions (Table 3). This result is consistent with the idea that homologous recombination, e.g., gene conversion, between duplicated sequences increased the diversity in the p1 noncoding repeats.
Estimated divergence time of the p1 alleles:
As stated above, the 723-bp sequence in the p1 second intron likely evolved neutrally; and nucleotide diversity of this region could not have been affected by local gene conversion events. Thus, this sequence can be used to estimate the divergence time of the p1 alleles. The pairwise nucleotide substitution rates were estimated between the simplex p1 alleles (Table 4). Because P1-rw1077 lacks the 723-bp sequence, the substitution rates between P1-rw1077 and other p1 alleles cannot be determined. On the basis of the published estimates of substitution rates in plant nuclear genes (ranging from 2.6 × 10−9 to 1.5 × 10−8 substitutions per synonymous site per year; Gaut 1998; Senchina et al. 2003), the divergence time of two P1-rw alleles, P1-rwCFS342 and P1-rwCFS302, is estimated as 93,000–540,000 years ago, while the time between P1-rw (either P1-rwCFS342 or P1-rwCFS302) and P1-rr is ∼47,000–270,000 years ago (Figure 5). The time of divergence between the P1-rr alleles cannot be estimated, because no substitutions were detected between these alleles (using the formula T = K/2r, where K represents divergence amount and r equals the rate of mutation).
TABLE 4.
Estimated number of substitutions per nucleotide site between the p1 alleles
| P1-rw CFS302 | P1-rw CFS342 | P1-rw 1077 | P1-rr CFS36 | P1-rr 1088 | P1-rr 4B2 | |
|---|---|---|---|---|---|---|
| P1-rw CFS302 | — | |||||
| P1-rw CFS342 | 0.0028 (2) | — | ||||
| P1-rw 1077 | NA | NA | — | |||
| P1-rr CFS36 | 0.0014 (1) | 0.0014 (1) | NA | — | ||
| P1-rr 1088 | 0.0014 (1) | 0.0014 (1) | NA | 0 (0) | — | |
| P1-rr 4B2 | 0.0014 (1) | 0.0014 (1) | NA | 0 (0) | 0 (0) | — |
Numbers in parentheses are the numbers of nucleotide substitutions over 723 sites.
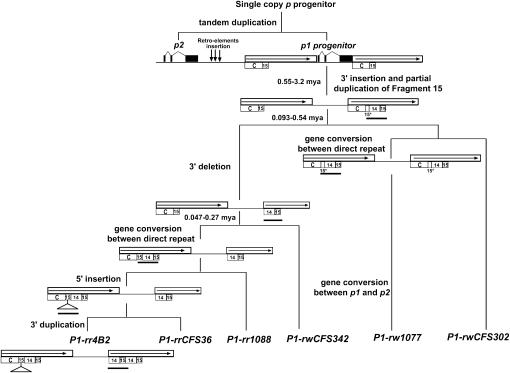
Figure 5.—
Sequential model for p1 evolution. The stepwise progression from p progenitor gene to the distinct p1 alleles is shown. The open boxes with solid arrows indicated the noncoding direct repeats flanking the p1 coding sequence. The numbered open boxes are the same as those shown in Figure 2. The solid boxes indicate exons of the p1 and p2 genes. The vertical arrows between the p1 and p2 regions represent retroelement insertions that separated the p1 gene from p2 (Zhang et al. 2000). In each step, the rearranged regions are highlighted by solid bars. The divergence time between the p1 alleles is indicated at the branch points.
DISCUSSION
Origin and diversification of the p1 gene:
Alleles of the p1 gene exhibit a great degree of genetic and phenotypic diversity (Cocciolone et al. 2001; Zhang and Peterson 2005). In a previous study, Zhang et al. (2000) suggested that the p1 gene was generated by a recent tandem duplication event. How was the observed high degree of diversity created at the p1 locus in this short period of evolutionary time? In this study, investigation of six distinct p1 alleles suggested that the noncoding repeats flanking the p1 coding sequence may have played an important role in diversification of the p1 locus during evolution.
Sequence comparisons of six distinct single-copy p1 alleles have revealed that they all contain the long direct noncoding repeats that flank the p1 coding sequence. The gene structure of the common ancestor of all p1 alleles has been deduced on the basis of analysis of a p-homologous gene in teosinte (Zhang et al. 2000). As indicated in Figure 5, in the p1 progenitor gene, the long direct repeat is ∼6 kb and extends from a 5′ sequence termed fragment C to a small 15-bp repeat. Subsequent sequence rearrangements in both 5′ and 3′ repeats diversified the p1 alleles (Table 3).
Structural comparisons of the distinct p1 alleles as well as evidence from phylogenetic analysis and nucleotide diversity analysis allowed us to propose a stepwise evolutionary model to most parsimoniously account for generation of the distinct single-copy p1 alleles. In this model, as indicated in Figure 5, the DNA rearrangements started at the 3′ noncoding repeats with insertion of fragment 14 and partial duplication of fragment 15, i.e., the structure shown in P1-rwCFS302. Such rearrangements could be transferred to the 5′ noncoding region by gene conversion as shown in P1-rw1077; or they can be further modified by deletion, giving rise to the structures of the 3′ noncoding regions observed in the P1-rwCFS342 and P1-rr alleles (Figures 2 and 5).
Because of low nucleotide polymorphisms in the enhancer-containing region, the relationship between the simplex p1 alleles is not well resolved in the maximum-likelihood tree. Although our proposed evolution model is not in agreement with the phylogenetic tree in every detail, both scenarios state that the P1-rw alleles were present early in the evolutionary pathway, while the P1-rr alleles likely originated more recently. The divergence time of the p1 alleles, estimated on the basis of the 723-bp sequence from the p1 second intron (Figure 4), seems to postdate the estimated birth date of the p1 progenitor gene (2.75 MYA; Zhang et al. 2000). This is consistent with the hypothesis that all p1 alleles studied to date are derived from tandem duplication of a single p1 ancestral gene (Zhang et al. 2000). On the other hand, the diversification of the p1 gene appears to predate domestication of maize from teosinte ∼7500 years ago (Iltis 1983). Most likely, introgression of various p1 alleles from teosinte into the domesticated maize gene pool was facilitated by positive human selection for the obvious pigmentation phenotypes. However, our estimation does not preclude the possibility that divergence of the p1 alleles occurred during or after maize domestication, as the DNA diversity of the p1 alleles is very low. Further investigation of p1-homologous genes in representative teosinte stocks is needed to more precisely estimate the divergence time.
Gene conversion could promote diversification of the p1 noncoding repeats:
In our model, the most interesting step in the evolution of the simplex p1 locus is the change from the P1-rwCFS342-like structure, which contains only one copy of fragment 15 in the enhancer-containing region, to a P1-rr-like structure that has two copies of fragment 15 and one copy of fragment 14 in that region (Figures 2 and 5). The phylogenetic analysis based on the sequences containing the distal enhancer indicated that the second copy of the duplicated fragment 15 sequences at the 5′ noncoding region is more closely related to fragment 15 in the 3′ noncoding region rather than to the 5′ duplicated copy. These results suggest that the 5′ P1-rr-type structure in the enhancer-containing region could be generated by gene conversion between the 5′ and 3′ repeats. More specifically, we propose that, in an allele with a structure similar to that of P1-rwCFS342, fragment 14 and 15 sequences were converted from the 3′ repeat into the 5′ repeat, with the original fragment 15 sequence adjoined to the newly transferred sequence. After gene conversion, the enhancer-containing region of the resultant p1 allele acquired an extra fragment 15 sequence as well as a fragment 14 sequence downstream of the original fragment 15. It has been suggested that gene conversion can create a new mosaic sequence by transferring a segment of differential sequence from a homologous donor region (Martinsohn et al. 1999). In this study, the conversion-generated mosaic sequences in the P1-rr alleles contain duplicated fragment 15 sequences with insertion of a fragment 14 sequence. As the result, a novel cob glume-specific regulatory region was created in the distal enhancer of P1-rr.
Diversifying gene conversion events between duplicated sequences could also explain the increased DNA diversity in the p1 noncoding repeats compared to the single-copy sequence within the second intron. Recent studies have identified gene conversion as a major force to generate nucleotide diversity between homologous sequences (Ohta 1995; Angers et al. 2002; Nielsen et al. 2003; Teshima and Innan 2004; Backstrom et al. 2005). It has been suggested that gene conversion could be an error-prone process due to biased tendency toward GC in repair systems as well as a higher rate of misincorporation relative to replicative DNA synthesis (Martinsohn et al. 1999; Marais 2003). In addition, polarity gradients of gene conversion rates have been reported for conversion tracts in yeast (Martinsohn et al. 1999). Unequal gene conversion rates may explain the uneven distribution of polymorphisms in the 5′ p1 noncoding repeats. For example, gene conversion rates may be higher in the first 2-kbp region than in the further downstream regions. As a result, DNA diversity in the first 2-kbp region in the 5′ noncoding region could have increased by converting nucleotide variations present in the 3′ noncoding regions, which probably accumulated more freely there due to fewer functional constraints. Uneven distribution of recombination events has also been reported in a1, b1, and r1 loci in maize (Eggleston et al. 1995; Patterson et al. 1995; Yao et al. 2002). Because of the small sample size (six p1 alleles) used in this study, however, investigation of additional p1 variations is necessary to test these hypotheses.
Evolution of the distal enhancer and phenotypic variations at the p1 locus:
The stepwise evolution model proposed that the p1 distal enhancer evolved from a simple to complex structures, i.e., from a single copy of fragment 15 to duplicated fragment 15 sequences with insertion of fragment 14. In a recent study (Zhang and Peterson 2005), a cob glume-specific regulatory region was identified within the complex enhancer types (Figure 2). This particular structure is associated with all P1-rr alleles, but is absent from the P1-rw alleles. Thus, the evolution of the distal enhancer region appears to accompany the evolution of phenotypic variations observed at the p1 gene. We propose that the early p1 alleles conferred the red kernel pericarp and white cob glumes (RW) phenotype and that a later event may have converted the distal enhancer into a type that contains the cob glume-specific regulatory region. Such change resulted in acquisition of p1 expression in cob glumes and thereby gave rise to the red kernel pericarp and red cob glumes (RR) phenotype.
Taken together, these results suggest that gene conversion between noncoding repeat sequences could be a major driving force for generation of genetic and phenotypic diversity at the p1 locus. Given that tandem and segmental duplications are common in both animal and plant genomes (Blanc et al. 2000; McLysaght et al. 2002; Yu et al. 2005), recombination between noncoding duplicated sequences could have a major impact on molecular evolution and diversity in gene expression.
Acknowledgments
We thank Johnathan Wendel, Xun Gu, Erik Vollbrecht, Diane Bassham, Steve Whitham, and Dan Voytas for advice and comments on this manuscript. This material is based upon work supported by the National Science Foundation under grant no. 9601285. This journal article of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, was supported by Hatch Act and State of Iowa funds.
References
- Angers, B., K. Gharbi and A. Estoup, 2002. Evidence of gene conversion events between paralogous sequences produced by tetraploidization in Salmoninae fish. J. Mol. Evol. 54: 501–510. [DOI] [PubMed] [Google Scholar]
- Backstrom, N., H. Ceplitis, S. Berlin and H. Ellegren, 2005. Gene conversion drives the evolution of HINTW, an ampliconic gene on the female-specific avian W chromosome. Mol. Biol. Evol. 22: 1992–1999. [DOI] [PubMed] [Google Scholar]
- Blanc, G., A. Barakat, R. Guyot, R. Cooke and M. Delseny, 2000. Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink, R., and D. Styles, 1966. A collection of pericarp factors. Maize Genet. Coop. Newsl. 40: 149–160. [Google Scholar]
- Carroll, S. B., J. K. Grenier and S. D. Weatherbee, 2004. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Blackwell Science, Malden, MA.
- Chopra, S., P. Athma, X. G. Li and T. Peterson, 1998. A maize Myb homolog is encoded by a multicopy gene complex. Mol. Gen. Genet. 260: 372–380. [DOI] [PubMed] [Google Scholar]
- Cocciolone, S. M., S. Chopra, S. A. Flint-Garcia, M. D. McMullen and T. Peterson, 2001. Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant J. 27: 467–478. [DOI] [PubMed] [Google Scholar]
- Cone, K. C., S. M. Cocciolone, F. A. Burr and B. Burr, 1993. Maize anthocyanin regulatory gene pl is a duplicate of c1 that functions in the plant. Plant Cell 5: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley, J., and L. Lukens, 1998. Transcriptional regulators and the evolution of plant form. Plant Cell 10: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston, W. B., M. Alleman and J. L. Kermicle, 1995. Molecular organization and germinal instability of R-stippled maize. Genetics 141: 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gaut, B. S., 1998. Molecular clocks and nucleotide substitution rates in higher plants, pp. 93–120 in Evolutionary Biology, edited by M. K. Hecht, W. C. Steere and B. Wallace. Plenum, New York.
- Grotewold, E., P. Athma and T. Peterson, 1991. Alternatively spliced products of the maize P gene encode proteins with homology to the DNA binding domain of Myb-like transcription factors. Proc. Natl. Acad. Sci. USA 88: 4587–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, M. A., B. S. Gaut, A. O. Stec, S. I. Fuerstenberg, M. M. Goodman et al., 1996. Evolution of anthocyanin biosynthesis in maize kernels: the role of regulatory and enzymatic loci. Genetics 143: 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iltis, H. H., 1983. From teosinte to maize: the catastrophic sexual transmutation. Science 222: 886–894. [DOI] [PubMed] [Google Scholar]
- Jiang, C., J. Gu, X. Gu, S. Chopra and T. Peterson, 2004. Ordered origin of the typical two- and three-repeat Myb genes. Gene 326: 13–22. [DOI] [PubMed] [Google Scholar]
- Lechelt, C., T. Peterson, A. Laird, J. Chen, S. L. Dellaporta et al., 1989. Isolation and molecular analysis of the maize P locus. Mol. Gen. Genet. 219: 225–234. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. P. Bernot, C. Illingworth, W. Lison, K. M. Bernot et al., 2001. Gene conversion within regulatory sequences generates maize r alleles with altered gene expression. Genetics 159: 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, M. Z., 2002. Functional evolution of noncoding DNA. Curr. Opin. Genet. Dev. 12: 634–639. [DOI] [PubMed] [Google Scholar]
- Ludwig, M. Z., C. Bergman, N. H. Patel and M. Kreitman, 2000. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature 403: 564–567. [DOI] [PubMed] [Google Scholar]
- Ludwig, S. R., and S. R. Wessler, 1990. Maize R gene family: tissue-specific helix-loop-helix proteins. Cell 62: 849–851. [DOI] [PubMed] [Google Scholar]
- Marais, G., 2003. Biased gene conversion: implications for genome and sex evolution. Trends Genet. 19: 330–338. [DOI] [PubMed] [Google Scholar]
- Martinsohn, J. T., A. B. Sousa, L. A. Guethlein and J. C. Howard, 1999. The gene conversion hypothesis of MHC evolution: a review. Immunogenetics 50: 168–200. [DOI] [PubMed] [Google Scholar]
- McLysaght, A., K. Hokamp and K. H. Wolfe, 2002. Extensive genomic duplication during early chordate evolution. Nat. Genet. 31: 200–204. [DOI] [PubMed] [Google Scholar]
- Moreno, M. A., J. Chen, I. Greenblatt and S. L. Dellaporta, 1992. Reconstitutional mutagenesis of the maize P gene by short-range Ac transpositions. Genetics 131: 939–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, K. M., J. Kasper, M. Choi, T. Bedford, K. Kristiansen et al., 2003. Gene conversion as a source of nucleotide diversity in Plasmodium falciparum. Mol. Biol. Evol. 20: 726–734. [DOI] [PubMed] [Google Scholar]
- Ohta, T., 1995. Gene conversion vs point mutation in generating variability at the antigen recognition site of major histocompatibility complex loci. J. Mol. Evol. 41: 115–119. [DOI] [PubMed] [Google Scholar]
- Patterson, G. I., K. M. Kubo, T. Shroyer and V. L. Chandler, 1995. Sequences required for paramutation of the maize b gene map to a region containing the promoter and upstream sequences. Genetics 140: 1389–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procissi, A., S. Dolfini, A. Ronchi and C. Tonelli, 1997. Light-dependent spatial and temporal expression of pigment regulatory genes in developing maize seeds. Plant Cell 9: 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan, M. D., 2000. The molecular population genetics of regulatory genes. Mol. Ecol. 9: 1451–1461. [DOI] [PubMed] [Google Scholar]
- Rozas, J., and R. Rozas, 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15: 174–175. [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof, M. A., K. M. Soliman, R. A. Jorgensen and R. W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81: 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger, D. A., D. Lisch and V. L. Chandler, 1998. The maize regulatory gene B-Peru contains a DNA rearrangement that specifies tissue-specific expression through both positive and negative promoter elements. Genetics 149: 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchina, D. S., I. Alvarez, R. C. Cronn, B. Liu, J. Rong et al., 2003. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol. Biol. Evol. 20: 633–643. [DOI] [PubMed] [Google Scholar]
- Sidorenko, L. V., X. Li, S. M. Cocciolone, S. Chopra, L. Tagliani et al., 2000. Complex structure of a maize Myb gene promoter: functional analysis in transgenic plants. Plant J. 22: 471–482. [DOI] [PubMed] [Google Scholar]
- Swofford, D. L., 1998. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods). Sinauer Associates, Sunderland, MA.
- Teshima, K. M., and H. Innan, 2004. The effect of gene conversion on the divergence between duplicated genes. Genetics 166: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. L., A. Stec, J. Hey, L. Lukens and J. Doebley, 1999. The limits of selection during maize domestication. Nature 398: 236–239. [DOI] [PubMed] [Google Scholar]
- Wray, G. A., M. W. Hahn, E. Abouheif, J. P. Balhoff, M. Pizer et al., 2003. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 20: 1377–1419. [DOI] [PubMed] [Google Scholar]
- Yao, H., Q. Zhou, J. Li, H. Smith, M. Yandeau et al., 2002. From the cover: molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc. Natl. Acad. Sci. USA 99: 6157–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J., J. Wang, W. Lin, S. Li, H. Li et al., 2005. The genomes of Oryza sativa: a history of duplications. PLoS Biol. 3: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F., and T. Peterson, 2005. Comparisons of maize pericarp color1 alleles reveal paralogous gene recombination and an organ-specific enhancer region. Plant Cell 17: 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., S. Chopra and T. Peterson, 2000. A segmental gene duplication generated differentially expressed myb-homologous genes in maize. Plant Cell 12: 2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]