Abstract
The RAG4 gene encodes for the sole transmembrane glucose sensor of Kluyveromyces lactis. A rag4 mutation leads to a fermentation-deficient phenotype (Rag− phenotype) and to a severe defect in the expression of the major glucose transporter gene RAG1. A recessive extragenic suppressor of the rag4 mutation has been identified. It encodes a protein (KlRgt1) 31% identical to the Saccharomyces cerevisiae Rgt1 regulator of the HXT genes (ScRgt1). The Klrgt1 null mutant displays abnormally high levels of RAG1 expression in the absence of glucose but still presents an induction of RAG1 expression in the presence of glucose. KlRgt1 is therefore only a repressor of RAG1. As described for ScRgt1, the KlRgt1 repressor function is controlled by phosphorylation in response to high glucose concentration and this phosphorylation is dependent on the sensor Rag4 and the casein kinase Rag8. However, contrary to that observed with ScRgt1, KlRgt1 is always bound to the RAG1 promoter. This article reveals that the key components of the glucose-signaling pathway are conserved between S. cerevisiae and K. lactis, but points out major differences in Rgt1 regulation and function that might reflect different carbon metabolism of these yeasts.
IN contrast to Saccharomyces cerevisiae, Kluyveromyces lactis is a predominantly aerobic yeast that exhibits a distinct regulation pattern for glucose uptake. In the cells of S. cerevisae, the low-affinity glucose uptake appears to be constitutively expressed, whereas the high-affinity system is glucose repressed (Bisson and Fraenkel 1984). This situation results from the superimposition of multiple regulatory mechanisms of many different glucose transporter genes (Ozcan and Johnston 1999). In K. lactis, the low-affinity glucose uptake is glucose inducible and the high-affinity glucose uptake is constitutive (Wesolowski-Louvel et al. 1992a). The two systems are encoded by two single genes: RAG1 encoding the low-affinity permease (Wesolowski-Louvel et al. 1992a) and HGT1 encoding the high-affinity permease (Billard et al. 1996). While HGT1 is constitutively expressed, RAG1 expression is activated in the presence of high concentrations of glucose (Chen et al. 1992; Wesolowski-Louvel et al. 1992a). The Rag1 permease supports fermentative growth, which requires a high substrate flow. In the absence of Rag1, growth of the cell on high-glucose media depends on respiration. Consequently, rag1 cells and mutants that do not express the Rag1 glucose transporter cannot grow on 5% glucose in the presence of antimycin A, which blocks respiration (the Rag− phenotype) (Goffrini et al. 1989; Wesolowski-Louvel et al. 1992b).
Several Rag− mutants have been isolated and at least four carry a mutation in genes that positively regulate the transcription of RAG1: RAG4, which encodes a glucose sensor (Betina et al. 2001); RAG5, which encodes a hexokinase (Prior et al. 1993); RAG8, which encodes an essential casein kinase 1 (Blaisonneau et al. 1997); and RAG17, which codes for enolase (Lemaire and Wesolowski-Louvel 2004). These genes are single-copy genes in K. lactis. These regulatory elements appear to define two different pathways: (i) a pathway involving the glucose sensor Rag4 that responds to extracellular glucose availability (pathway 1), and (ii) a pathway responding to an intracellular signal generated by glycolysis (pathway 2) in which Rag5 hexokinase and Rag17 enolase participate (Lemaire and Wesolowski-Louvel 2004). The participation of Rag8 casein kinase 1 in either of these two pathways has not yet been clearly established.
We looked for genetic suppressors of the rag4 mutation to help in finding new elements of the Rag4-dependent glucose-sensing pathway. We found that when grown on glucose, a rag4 null mutant spontaneously reverted to the Rag+ phenotype at a very high rate. This allowed us to identify recessive mutations in the KlRGT1 (SRA1) gene that bypass the need for Rag4 for growth on high-glucose media in the presence of antimycin A. We show that KlRGT1 encodes a repressor protein very similar to Rgt1 of S. cerevisiae (ScRgt1) that regulates HXT gene expression in this yeast (Ozcan et al. 1996). Our results show major differences between the two yeast systems: (i) KlRgt1 binds to the RAG1 promoter in a glucose-independent manner, whereas ScRgt1 is removed from HXT promoters in response to glucose; and (ii) KlRgt1 is only a repressor of RAG1 expression in K. lactis, whereas ScRgt1 is thought to both repress and activate HXT1 expression in S. cerevisiae (Ozcan et al. 1996; Polish et al. 2005). Despite these differences, KlRgt1, like ScRgt1, seems to be regulated post-translationally through a glucose-dependent pathway involving the glucose sensor.
MATERIALS AND METHODS
Yeast strains, growth conditions, and yeast transformation:
Yeast strains used are described in Table 1. Yeast cells were grown at 28° in a complete YP medium containing 1% Bacto yeast extract, 1% Bactopeptone (Difco, Detroit), supplemented with either 2% glucose (YPG) or a specified carbon source. Minimal medium contained 0.7% yeast nitrogen base without amino acids (Difco) and 2% glucose with auxotrophic supplements as required. The Rag phenotype was tested on GAA medium (YP medium containing 5% glucose and 5 μm antimycin A). For the G418 medium, YPG plates were supplemented with Geneticin (200 μg/ml; Life Technologies).
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| K. lactis | ||
| MW270-7B | MATauraA1-1 leu2 metA1-1 | Billard et al. (1996) |
| MW159-1C | MATauraA1-1adeT-600lysA1-1rag8-2 | Blaisonneau et al. (1997) |
| PM6-7A/VV30 | MATauraA1-1adeT-600rag8-1 | Blaisonneau et al. (1997) |
| MW109-8C/FA49 | MATαlysA1-1 rag4-5 | Wesolowski-Louvel et al. (1992b) |
| MWK8/SRA | Isogenic to MWK8 except sra1-6 | This study |
| MW360-5C | MATα uraA1-1leu2metA1-1rag4Δ2∷LEU2sra1-6 | This study |
| JLS4-11A | MATauraA1-1 lysA1-1 rag4-5 sra1-6 | This study |
| MWK6 | Isogenic to MW270-7B except rag4Δ2∷LEU2 | This study |
| MWK7 | Isogenic to MW270-7B except rgt1Δ1∷URA3 | This study |
| MWK7/F | Isogenic to MW270-7B except rgt1Δ1∷ura3 | This study |
| MWK8a | Isogenic to MW270-7B except rag4Δ1∷URA3 | Betina et al. (2001) |
| MWK9 | Isogenic to MW270-7B except rgt1Δ1∷URA3 rag4Δ2∷LEU2 | This study |
| MWK9/F | Isogenic to MW270-7B except rgt1Δ1∷ura3rag4Δ2∷LEU2 | This study |
| MWK21 | Isogenic to MW159-1C except rgt1Δ1∷URA3 | This study |
| MWK22b | MATαura3 trp1 ade1-600 adeT-600 rag4Δ1∷URA3 | Betina et al. (2001) |
| S. cerevisiae | ||
| THY.AP4 | MATaura3leu2lexA∷lacZ∷trp1lexA∷HIS3lexA∷ADE2 | Obrdlik et al. (2004) |
| THY.AP5 | MATαURA3 leu2 trp1 his3 loxP∷ade2 | Obrdlik et al. (2004) |
| BY4741 | MATahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 met15Δ0 | EUROSCARF |
Previously MW270-7B/Δrag4.
Previously JA6/Δrag4.
Genetics methods have been described previously (Wesolowski et al. 1982).
To monitor the behavior of KlRgt1 under repressing and inducing conditions, cells expressing KlRgt1-3HA inserted into the pCXJ22 vector (Chen 1996) bearing the URA3 gene for complementation were pregrown to an OD600 of 2–3 in uracil-less minimal medium containing 2% glycerol and 2% galactose. The harvested cells were washed with water, divided into two fractions, and further incubated for 6 hr in uracil-less minimal medium containing 2% glycerol or 5% glucose. Yeast transformation was carried out as previously reported (Lemaire and Wesolowski-Louvel 2004).
Construction of deletion strains and plasmids:
We constructed Klrgt1 strains by inserting the 3.3-kb XhoI–BamHI fragment containing the promoter region and two-thirds of the KlRGT1 ORF into the XhoI and BamHI sites of pBluescript KS phagemid (Stratagene, La Jolla, CA). The resulting plasmid was digested with BglII, which removed a 1.7-kb fragment. This internal fragment was replaced by a 0.9-kb BamHI fragment containing the URA3 marker from the pAF101 vector (Thierry et al. 1990). A 2.5-kb XhoI–BamHI fragment containing the disrupted KlRGT1 cassette was used to transform various host strains to disrupt the KlRGT1 copy.
A 7.3-kb SacI–EcoRV fragment containing the entire KlRGT1 gene was inserted into the Klori-ScARS-CEN-based shuttle vector pCXJ22 (Chen 1996) using SacI and SmaI sites. The resulting plasmid, pCXJ22-KlRGT1, was a source of KlRGT1 gene used for further constructions and yeast transformations.
The Klrgt1-6 allele was amplified by PCR using MWK8/SRA genomic DNA as a template and the primers P5′ RGT1/SacI (5′-ATCCGAGCTCCTTGAAACTGCCGTACAAACCA-3′) and P3′ RGT1/SmaI (5′-ATCCCCCGGGTTCCAACAGAAGGTGATGGCTA-3′). The PCR product was digested with SmaI and SacI and the fragment was inserted into the pCXJ22 vector, yielding pML203 plasmid.
We introduced the Klrgt1-6 mutation in the wild-type KlRGT1 gene by exchanging the 3′ region of the two alleles. A SpeI/SalI digestion of pCXJ22-KlRGT1 removed a 2.8-kb fragment, which was replaced by a 0.54-kb SpeI–SalI-digested fragment from pML203.
KlRgt1 was epitope tagged by in vivo recombination in S. cerevisiae using the BY4741 strain transformed with the pCXJ22-KlRGT1 plasmid. The resulting transformant was transformed with a 1898-bp DNA fragment made of the triple HA tag coding sequence followed by the ADH1 gene terminator and the kanMX6 module as a selectable marker. This DNA fragment (flanked by 40-bp sequences of the 3′ region of the KlRGT1 locus to target recombination) was obtained by PCR with the pFA6a-3HA-kanMX6 plasmid as a template (Longtine et al. 1998) and the primers F2-3HA-RGT1 (5′-TGGTGCATTGCAGACACTGAAGAACTAGGATGGTTTAATCAGCGGATCCCCGGGTTAATAA-3′) and R1-3HA-RGT1 (5′-GCTTGGTGACCTTTCCTTATTTAACTTTGTTTTATTCAGTTAGAATTCGAGCTCGTTTAAAC-3′). Plasmids contained in G418R transformants were rescued in Escherichia coli. After molecular analysis, K. lactis strains were transformed with pCXJ22-KlRGT1-3HA plasmid.
Preparation of yeast RNA and probes:
Total RNA was extracted from cells grown to an OD600 of ∼2 by the acid phenol method. Northern blot analysis was carried out as described by Prior et al. (1993). Probes were obtained by PCR amplification using either K. lactis genomic DNA or the cloned genes as templates. The oligonucleotides used for RAG1, RAG5, RAG17, and 18S have been previously described (Lemaire and Wesolowski-Louvel 2004). The KlACT probe was amplified by PCR using the following primers: Actin-up (5′-GAGGTATCTTGACTCTACGTTACC-3′) and Actin-down (5′-GACATGACGATGTTACCGTAC-3′).
Yeast cell extracts and immunoblotting:
Crude cell extracts were prepared as previously described (Kushnirov 2000). For Western blotting analysis, equal quantities of proteins were loaded onto SDS–PAGE. Gels were transferred to a PVDF membrane (Qbiogene) and probed with 12CA5 anti-HA monoclonal antibodies (1:5000; Roche Diagnostics). Primary antibodies were detected with horseradish peroxidase-conjugated anti-mouse secondary antibody (Upstate Biotechnology) and an enhanced chemiluminescence system (SuperSignal Western Femto; Pierce).
Chromatin immunoprecipitation :
Chromatin immunoprecipitation (ChIP) experiments were carried out as previously reported (Kim et al. 2003) using 1010 cells cross-linked with formaldehyde (final concentration of 1%) by incubation for 15 min at room temperature. The reaction was quenched with glycine (final concentration of 125 mm). The cells were washed in TBS buffer and disrupted by vortexing six times for 1 min in 1 ml of lysis buffer (50 mm Tris–HCl pH 7.5, 100 mm NaCl, 1 mm EDTA) in the presence of protease inhibitor cocktail, PMSF, and 650 μl of glass beads. The lysate was supplemented with Triton X-100 (final concentration, 1%) and sodium deoxycholate (final concentration, 0.1%), sonicated, and then centrifuged at 15,000 × g at 4° for 10 min. The genomic DNA fragments, averaging 500–1500 bp and cross-linked to KlRgt1-3HA, were immunoprecipitated by incubation for 3 hr at 4° with 2 μl of 12CA5 anti-HA monoclonal antibodies. The immunocomplexes were recovered by further incubation for 1 hr with 50 μl of Protein A-conjugated agarose bead suspension (Sigma). The beads were then washed twice by 1 min incubation at room temperature in 1 ml of IP buffer (lysis buffer supplemented with Triton X-100 and Na-deoxycholate), twice with 1 ml of ChIP high salt lysis buffer (IP buffer containing 500 mm NaCl), twice with 1 ml of wash buffer (10 mm Tris–HCl pH 8, 250 mm LiCl, 1 mm EDTA, 0.5% NP-40 and 0.5% Na-deoxycholate), and then once with 1 ml of TBS buffer. Immunocomplexes were then eluted from the beads by incubation at 65° for 10 min in 150 μl of elution buffer (50 mm Tris–HCl pH 8, 1% SDS, 10 mm EDTA). The tubes were briefly vortexed during the incubation to help the elution. The cross-linking was reversed by incubating the elution fraction at 65° for 15 hr. The immunoprecipitated and input DNA were purified using a PCR purification kit (QIAGEN) and were used as templates in a 25-cycle PCR. PCR amplifications were carried out on 1/25 of the immunoprecipitation (IP) and 1/5000 of the chromatin before immunoprecipitation (input, IN). The reactions, in a 50-μl reaction volume, comprised 6 min at 95° followed by 25 cycles of 30 sec at 95°, 30 sec at 47°, 30 sec at 72°, and then 7 min at 72°. The primer sequences were as follows: for pRAG1 (H51: 5′-GGAGAAACTTGTCCCTCTTCC-3′ and H52: 5′-TTTAGAGGGTCAAAGGCACCG-3′), for pACT (H1: 5′-TTTTTCTAGAGATCCGCCTTTGAAGCTG-3′ and H15: 5′-AAAGAATTCAAGTCAGTAACCTGCGCATGG-3′), for pRAG5 (RAG5CHIP5′: 5′-CATCTTCATTTTCGCAGCG-3′ and RAG5CHIP3′: 5′-CTGTGGTTGTATCTTATTC-3′), for pENO (ENOCHIP5′: 5′-CACTCGTTCATCAGGTTTG-3′ and ENOCHIP3′: 5′-ATGTCTCATAATGTCTCGTC-3′).
Immobilized DNA binding assay:
Immobilized DNA binding assay (IDBA) experiments were carried out as described by Kim (2004). Briefly, KlRgt1-3HA was immunoprecipitated from crude yeast cell extracts (3 × 109 cells) with anti-HA antibodies as reported above. The beads coated with KlRgt1-3HA were washed twice for 10 min at 4° in 500 μl of IP buffer and once in 500 μl of TGZD buffer (20 mm Tris–HCl pH 8.0, 75 mm KCl, 10 μM ZnCl2, 5% glycerol). The beads were separated into two fractions and incubated under agitation at 25° for 30 min in 500 μl of TGZD buffer containing 1 μg of poly-dI-dC (Sigma-Aldrich) and 2 × 104 counts per minute of 32P-labeled DNA fragments corresponding to either pRAG1 or pACT. (32P-labeled pRAG1 or pACT DNA were prepared by PCR amplification using the primer sets indicated in ChIP experimental section, followed by end-labeling with [32P]ATP and T4 polynucleotide kinase.) The beads were then washed at 25° for 5 min with 500 μl of TGZD buffer containing 250 mm NaCl. The bound DNA was eluted from the beads by incubation with 30 μl of Tris–EDTA buffer containing 1 m NaCl at room temperature for 5 min. KlRgt1-3HA was recovered by boiling the beads for 5 min in SDS–PAGE sample buffer. The eluted DNA and KlRgt1-3HA were analyzed by electrophoresis in polyacrylamide gels and visualized by autoradiography and Western blotting, respectively.
Phosphatase treatment of protein extracts:
Immunoprecipitated KlRgt1 was treated with phosphatase. After washing with lysis buffer and phosphatase buffer, beads decorated with KlRgt1-3HA were incubated with 10 units of calf intestinal alkaline phosphatase (CIP) (Fermentas) at 37° for 30 min. After elution, the proteins were analyzed by Western blotting.
Split-ubiquitin interaction assays:
The RAG4 ORF was inserted in vivo into the pMetYCgate vector (LEU2-CEN/ARS-kanMX) (gift from P. Obrdlik) so that Cub-PLV was fused to the C-terminus of Rag4. The RAG4 ORF was amplified by using B1-RAG4 (5′-acaagtttgtacaaaaaagcaggctctccaaccaccATGACTACTGATTCTGTTCCA-3′) and B2-RAG4 (5′-tccgccaccaccaaccactttgtacaagaaagctgggtaAGTGTTACTATTAATATCGGT-3′) primers (lowercase letters are the linkers B1 and B2 present in the pSUgate vectors) (Obrdlik et al. 2004) and MW270-7B strain genomic DNA (Table 1) as a template. The THY.AP4 strain was cotransformed with the 2.4-kb PCR product and the pMetYCgate vector digested with PstI and HindIII (Table 1). Transformants were selected as previously reported (Obrdlik et al. 2004). The RAG8 ORF was inserted in vivo into the pNXgate vector (TRP1-2-kanMX) (gift from P. Obrdlik) so that NubG was fused to the N-terminus of Rag8. The RAG8 ORF was amplified by using B1-RAG8 (5′-acaagtttgtacaaaaaagcaggctctccaaccaccATGAGTATTACAGCGGGACCT-3′) and B2-RAG8 (5′-tccgccaccaccaaccactttgtacaagaaagctgggtaACAACAGCCAAGCTTGCTGAA-3′) primers and MW270-7B strain genomic DNA as a template. The THY.AP5 strain was cotransformed with the 1.7-kb PCR product and the pNXgate vector digested with EcoRI and SmaI (Table 1). Transformants were selected as previously described (Obrdlik et al. 2004).
For interaction assays, THY.AP4 and THY.AP5 transformants were mated on YPD plates by incubation for 6 hr at 28°. Diploids were selected by replica plating on tryptophan-less, uracil-less, leucine-less medium and then incubated at 28°. For growth assays (activation of HIS3 and ADE2 reporter genes), diploids were replicated on minimal media supplemented or not with 1 mm methionine. Growth was monitored for 3 days at 28°.
β-Galactosidase assays:
β-Galactosidase activity assays were carried out with permeabilized yeast cells. Results are given in Miller units [1000 OD420 (ONPG)/OD600 (cells) volume (ml) time (min)]. Transformants were pregrown to log phase (OD600 1–2) in minimal medium containing 2% glucose supplemented with adenine, histidine, and 1 mm methionine (repressing conditions). The harvested cells were washed with water, diluted 10-fold in minimal medium containing 2% glucose supplemented with adenine, histidine (inducing conditions), and further incubated to log phase (OD600 1–2).
RESULTS
sra mutations restore RAG1 expression in rag4 mutant:
We observed that rag4 null mutant cells (MWK8) reverted to the Rag+ phenotype at a high rate (Figure 1; Δrag4 srat1-6). We analyzed six of these revertants. When these strains were individually crossed with the rag4 MWK22 strain (Table 1), the Rag− phenotype of the diploids indicated that the suppressor mutations were recessive (sra for suppressor of rag4 mutation). The 2:2 segregation of the Rag phenotype in the tetrads from meiosis of these diploids showed that they were single gene mutations. Moreover, when the revertant strains were crossed with each other, the resulting diploids had a Rag+ phenotype, indicating that the six mutations affected a single locus, SRA1. We analyzed one revertant, sra1-6 (MWK8/SRA strain, Table 1), further.
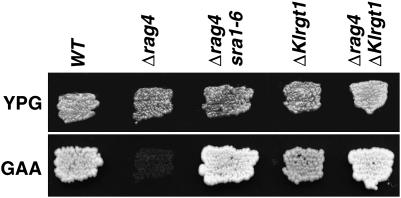
Figure 1.—
Phenotypes of the revertants and various mutants. Strains grown on YPG plates were replicated on GAA plates. The photographs were taken after 24 hr incubation at 28°. MW270-7B (WT); MWK8 (Δrag4); MWK8/SRA (Δrag4 sra1-6); MWK7 (ΔKlrgt1); MWK9 (Δrag4 ΔKlrgt1).
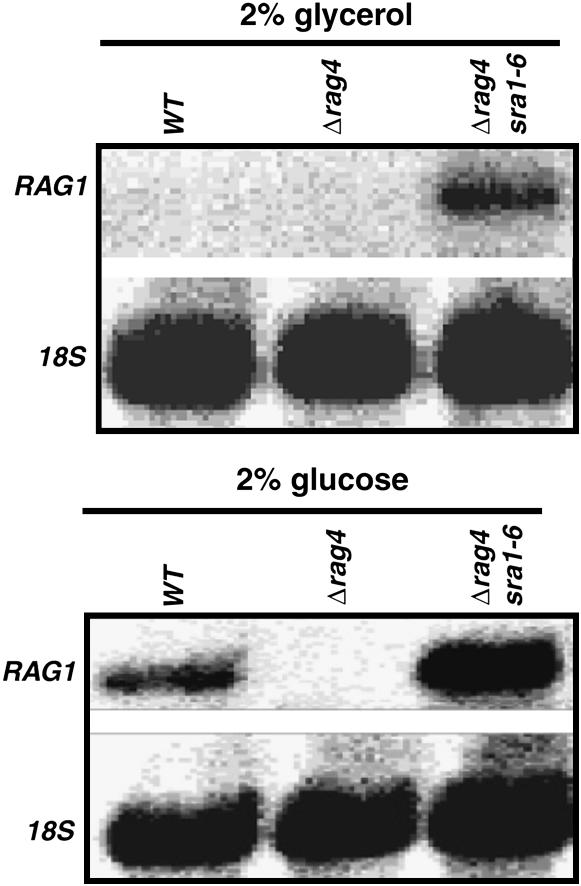
As the Rag− phenotype of the Δrag4 mutant comes from a severe reduction in the expression of the low-affinity glucose transporter gene (Chen et al. 1992; Betina et al. 2001), we studied RAG1 transcription in the revertant strain. Northern blot analysis showed that RAG1 expression returned to high levels when the Δrag4 sra1-6 cells were grown on 2% glucose and was derepressed in the absence of glucose (Figure 2). The complete suppression of the Δrag4 mutation phenotypes by mutations in SRA1 indicates that the SRA1 gene product plays a negative role in RAG1 expression and could act downstream from Rag4.
Figure 2.—
Northern blot analysis of RAG1 mRNA in the revertant cells. Total RNA was extracted from cells grown in the presence of either 2% glycerol or 2% glucose. Each slot was loaded with 5–10 μg of RNA and run on a 1.2% agarose-formaldehyde gel. The probes used are described in materials and methods. rRNA (18s) was used as a standard. MW270-7B (WT); MWK8 (Δrag4); MWK8/SRA (Δrag4 sra1-6).
SRA1 encodes a protein similar to the Rgt1 repressor of S.cerevisiae:
We cloned the SRA1 gene by in vivo complementation by transforming rag4 sra1-6 cells (Rag+) with a wild-type genomic library of K. lactis made in the KEp6 vector (Wesolowski-Louvel et al. 1988) and then screening Rag− transformants. One Rag− transformant contained an 8.7-kb complementing DNA. The nucleotide sequence of the extremities of this complementing DNA fragment showed that it spanned a region of chromosome VI (http://cbi.labri.fr/Genolevures/) containing two entire ORFs. The best candidate was KLLA0F25630g (EMBL accession no CR382126), which had some similarities with the ScRgt1 regulator of S. cerevisiae HXT genes (Ozcan and Johnston 1995; Ozcan et al. 1996). The DNA region containing KLLA0F25630g was inserted into the pCXJ22 vector (materials and methods) and was used to transform MWK8/SRA cells. Rag− phenotype of the obtained transformants (data not shown) indicated that the SRA1 locus did correspond to this gene encoding a protein similar to the ScRgt1 repressor.
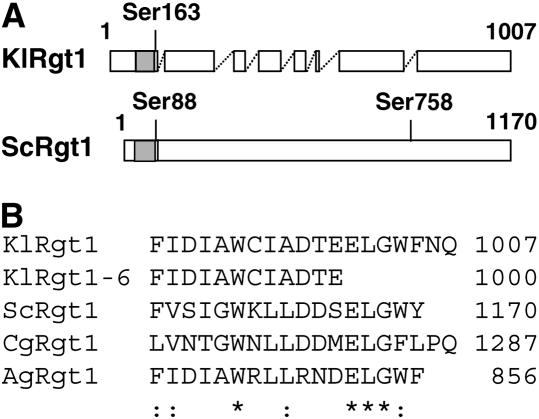
The predicted protein (1007 amino acids) encoded by SRA1 gene is 31% identical to ScRgt1 of S. cerevisiae (1170 amino acids), 32% identical to the AGL083wp ORF (856 amino acids) of Ashbya gossypii (Dietrich et al. 2004), and 29% identical to the CAGL0L01903g ORF (1287 amino acids) of Candida glabrata (Dujon et al. 2004). Alignment of these proteins showed that they have similar structural organizations, with a typical zinc-finger DNA binding motif consisting of six cysteine residues present in their N-terminal regions (Figure 3A shows the alignment of S. cerevisiae and K. lactis products). As the SRA1 gene product is similar to ScRgt1 of S. cerevisiae, we have called it KlRgt1 and renamed the sra1-6 mutation Klrgt1-6. By extension, we have called the A. gossypii and C. glabrata products AgRgt1 and CgRgt1, respectively.
Figure 3.—
Alignment of KlRgt1-orthologous sequences. (A) Alignment of ScRgt1 (S. cerevisiae) and KlRgt1 (K. lactis). Open and shaded boxes indicate aligned regions and the Zn2Cys6 binuclear DNA binding domain, respectively. Gaps >20 amino acids are indicated by dashed lines. Conserved serine residues that are phosphorylated in ScRgt1 (Polish et al. 2005) are also indicated. (B) Alignment of C-terminal region of KlRgt1, KlRgt1-6 (K. lactis), ScRgt1 (S. cerevisiae), CgRgt1 (Candida glabrata), and AgRgt1 (Ashbya gossypii). Asterisks and colons indicate identical or similar residue, respectively.
We then amplified and sequenced the mutant allele Klrgt1-6 (materials and methods) to identify the suppressor mutation. We found that the mutation was a nonsense mutation resulting in a truncated KlRgt1 protein (1000 amino acids). Three of the 7 missing amino acids are highly conserved in Rgt1 orthologs (Figure 3B), suggesting that the C-terminal region of the protein may be essential for KlRgt1 function.
KlRgt1 is a repressor of RAG1 expression:
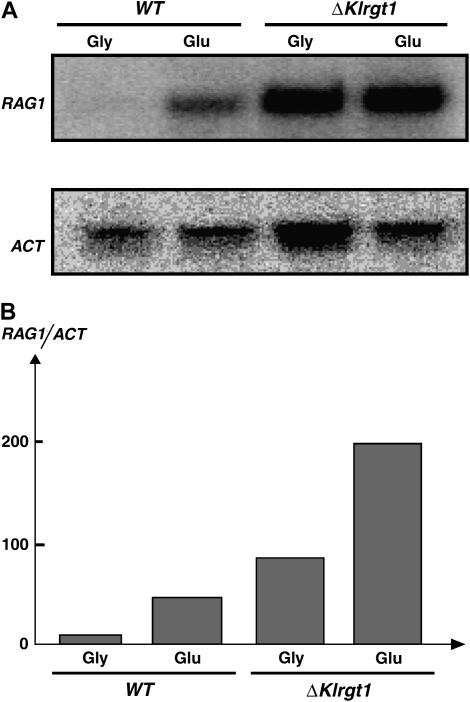
We constructed a ΔKlrgt1 null mutant to determine more precisely the role of KlRgt1 in RAG1 expression. This mutant displayed a Rag+ phenotype (Figure 1) as the isogenic wild-type strain, indicating that the loss of KlRgt1 does not lead to a fermentation defect. Northern blot analysis showed that RAG1 transcription was deregulated in ΔKlrgt1 null mutant (Figure 4), as we observed in the revertant strain (Figure 2). RAG1 was strongly transcribed in cells grown in glycerol-containing medium, suggesting that in the absence of glucose, KlRgt1 has a repressor function. In the presence of glucose, the level of RAG1 expression was still strong and even higher than in the wild-type cells. This indicates that the induction of RAG1 expression in glucose mainly involves getting rid of KlRgt1 repression. However, RAG1 expression could still be induced by glucose in the Klrgt1 mutant (Figure 4B). Such a finding suggests that whether KlRgt1 has any activator function in RAG1 expression, it is not the only one involved. Altogether, these data are consistent with KlRgt1 being a transcriptional repressor at the RAG1 promoter in the absence of glucose.
Figure 4.—
RAG1 transcription in Klrgt1 cells. (A) Northern blot analysis was carried out as in Figure 2. MW270-7B (WT); MWK7 (ΔKlrgt1). (B) Densitometric scanning. The hybridization signals were quantified by means of a Cyclone PhosphoImager (Packard). The ratio between RAG1 and ACT transcript is represented.
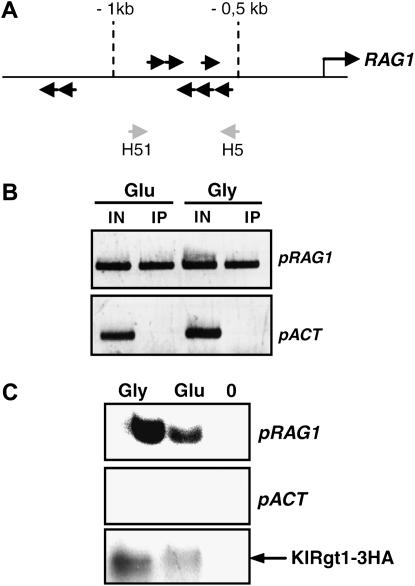
KlRgt1 binds to the RAG1 promoter in vivo and in vitro:
We analyzed the interaction of KlRgt1 with the RAG1 promoter in vivo by ChIP assay. We used a KlRgt1-3HA construct expressed from pCXJ22 vector (materials and methods) transformed in Klrgt1 mutant cells (MWK7/F strain, Table 1). The fusion protein was functional because RAG1 transcription was repressed in the absence of glucose in the transformants (not shown). After KlRgt1-3HA immunoprecipitation, we used the immunoprecipitated and total input DNA as templates for PCR analysis using primers designed for the RAG1 gene promoter. The amplified sequence contains six 5′-CGGANNA-3′ putative binding sites (Figure 5A), corresponding to ScRgt1 target sites (Kim 2004). The promoter region of the actin gene was used as a control. We found that KlRgt1-3HA was associated with the RAG1 promoter in cells grown both in glycerol-containing medium and in glucose-containing medium (Figure 5B). We also used an in vitro DNA-binding assay, IDBA, to further determine the KlRgt1 DNA-binding activity (Kim 2004). In this experiment, the immunoprecipitated KlRgt1-3HA was used as an affinity matrix to retain radiolabeled promoter sequences. We found that KlRgt1 can bind to sequences upstream from RAG1 in the absence of glucose and that this binding capacity remained in the presence of glucose (Figure 5C). The apparent weaker binding of KlRgt1 to the RAG1 promoter in the presence of glucose could be explained by a weaker level of immunoprecipitated KlRgt1 (Figure 5C). However, we cannot exclude the existence of a modification of the affinity of KlRgt1 for the RAG1 promoter in response to glucose. Nevertheless, in K. lactis, the derepression of RAG1 gene expression is not due to the complete removal of the repressor protein from its promoter.
Figure 5.—
Binding of KlRgt1 to the RAG1 promoter. (A) Diagram of RAG1 promoter (pRAG1). Location and orientation of putative KlRgt1 binding sites (5′-CGGANNA-3′) are indicated by solid arrows. The location of the H51 and H52 primers used to generate pRAG1 DNA used for the ChIP and IDBA experiments are indicated by shaded arrows. (B) The association of KlRgt1 with the RAG1 promoter was determined by ChIP experiments. Chromatin from cells expressing KlRgt1-3HA (MWK7/F) grown on media containing either 5% glucose (Glu) or 2% glycerol (Gly) was immunoprecipitated with anti-HA antibodies. Immunoprecipitated DNA (IP) and input DNA (IN) were amplified by PCR using the pRAG1 primer set and an actin promoter (pACT) primer set as a negative control. PCR products were then analyzed by electrophoresis in 1.5% agarose gel. (C) In vitro DNA-binding activity of KlRgt1 was analyzed by IDBA. KlRgt1-3HA was immunoprecipitated as in B and incubated with 32P-radiolabeled pRAG1 or pACT DNA (both promoters were amplified using the same primer sets as above). The bound DNA was resolved in 6% polyacrylamide gel and visualized by autoradiography. KlRgt1 was analyzed by Western blotting using anti-HA antibodies (KlRgt1-3HA). A control (0) experiment was also carried out with cells containing an untagged KlRgt1 protein.
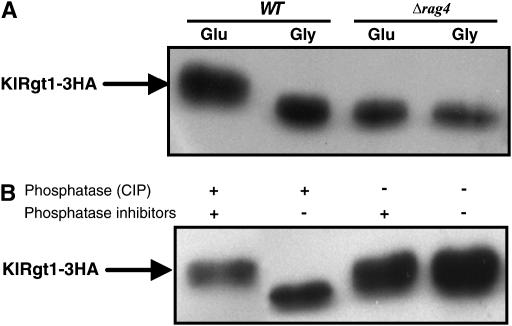
KlRgt1 is phosphorylated in response to glucose:
As KlRgt1 binds to the RAG1 promoter whatever the carbon source (glycerol or glucose) tested, we investigated whether it undergoes a post-translational modification in response to glucose. We found that KlRgt1-3HA from cells grown in the presence of glucose had a lower mobility in SDS–PAGE than KlRgt1-3HA from cells grown in the presence of glycerol (Figure 6A). This showed that KlRgt1 is modified in the presence of glucose. We tested whether phosphorylation caused the change in KlRgt1-3HA mobility in response to glucose by incubating with phosphatase (CIP) extracts from KlRgt1-3HA-containing cells grown in the presence of glucose. We found that phosphatase treatment increased the mobility of the protein (Figure 6B). This shows that glucose induces the phosphorylation of KlRgt1 and is consistent with the idea that glucose-induced phosphorylation inhibits the repressor function of KlRgt1. This glucose-induced phosphorylation of KlRgt1-3HA did not occur in Δrag4 mutant cells grown with high levels of glucose (Figure 6A). As rag4 mutants, affected in the glucose sensor, exhibit a constitutive repression of RAG1 gene expression, this suggests that the Rag4-dependent glucose pathway abolishes KlRgt1 repressor function through its phosphorylation.
Figure 6.—
KlRgt1 was phosphorylated in a Rag-4-dependent manner in response to glucose. (A) Cells transformed with the KlRgt1-3HA construct (MWK7/F, WT and MWK9, Δrag4) were pregrown on 2% glycerol and the transferred to 5% glucose or 2% glycerol for 6 hr. After cell lysis, the protein extracts were separated in 6% SDS–PAGE and analyzed by Western blotting. (B) Phosphatase treatment of cell extracts from WT cells grown in the presence of glucose. KlRgt1-3HA was immunoprecipitated as in Figure 6 and incubated with (+) or without (−) 10 units of CIP in the presence (+) or absence (−) of phosphatase inhibitors at 37° for 30 min and then analyzed by Western blotting.
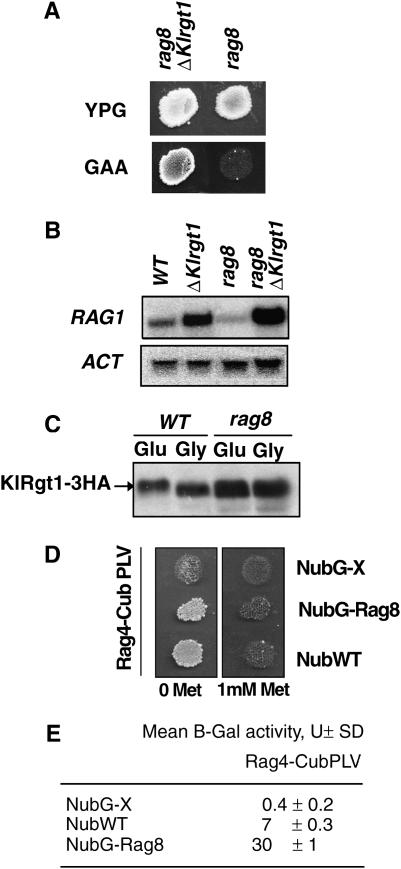
Involvement of Rag8 casein kinase 1 in the glucose-signaling pathway:
We have previously shown that the Rag8 casein kinase 1 positively regulates RAG1 gene transcription (Blaisonneau et al. 1997). Therefore, we investigated whether it may be a component of the glucose-signaling pathway regulating the KlRgt1 repressor. Therefore, we constructed a rag8 ΔKlrgt1 double mutant. We found that the loss of KlRgt1 in the rag8 mutant strain suppressed its Rag− phenotype (Figure 7A) and Northern blot analysis showed that RAG1 expression was restored when the rag8 ΔKlrgt1 mutant was grown on glucose (Figure 7B). We also observed no KlRgt1 phosphorylation in response to glucose in rag8 cells (Figure 7C). Thus, membrane-bound casein kinase Rag8 appears to be a component of the Rag4-dependent glucose-induction pathway acting upstream from KlRgt1.
Figure 7.—
Involvement of Rag8 casein kinase 1 in the glucose-signaling pathway. (A) The rag8 mutation was suppressed by the Klrgt1 mutation. The Rag phenotype of rag8 (MW159-1C) and rag8 Klrgt1 cells (MWK21) was determined as described in the legend of Figure 1. (B) RAG1 expression was restored in a rag8 Klrgt1 double mutant. Northern blot analysis of RAG1 mRNA in cells grown on 2% glucose medium was carried out as indicated in Figure 2; an actin gene transcript (ACT) was used as an internal control. WT (MW270-7B); Klrgt1 (MWK7); rag8 (MW159-1C); rag8 Klrgt1 cells (MWK21). (C) The rag8 mutant was defective for KlRgt1 phosphorylation in response to glucose. Western blot analysis of the KlRgt1 modification in WT (MW270-7B) and rag8 (MW159-1C) cells was carried out as described for Figure 6. (D) Interaction of Rag4-CubPLV with the NubG-Rag8 fusion protein. Replica plates showing diploid cells selected on minimal media with and without 1 mm methionine (Met) after 3 days incubation. NubWT and NubG are positive and negative controls, respectively. (E) Liquid β-galactosidase assay. Results are expressed in Miller units (U) and are the average of assays carried out in triplicate.
This was further supported by showing that the Rag8 casein kinase interacts with the Rag4 glucose sensor. We tested for such an interaction by using the mating-based split ubiquitin system (mb SUS) developed to test interactions between membrane-associated proteins (Obrdlik et al. 2004). In this system, the interaction between “prey” and “bait” can be detected through the reconstitution of a functional ubiquitin that leads to the release of an artificial transcription factor, PLV (A-LexA-VP16), that can activate the lexA-driven reporter genes HIS3, ADE2, and lacZ. In addition, one of the fusion protein-encoding genes is under the control of MET25-regulated promoter, which can be repressed by methionine. We constructed pMET25-RAG4-CubPLV and pADH1-NubG-RAG8 (materials and methods). Diploids harboring Rag4 and Rag8 fusion proteins grew on adenine/histidine-free medium in the absence of methionine (Figure 7D), indicating a physical interaction between Rag8 and Rag4 proteins. We confirmed this interaction by β-galactosidase assays, which indicate the level of activation of the lacZ reporter gene (Figure 7E). Therefore, Rag4 (the K. lactis glucose sensor) and Rag8 casein kinase 1 physically interact, as previously reported for the glucose sensor Rgt2 and the casein kinase 1 Yck1 of S. cerevisiae (Moriya and Johnston 2004).
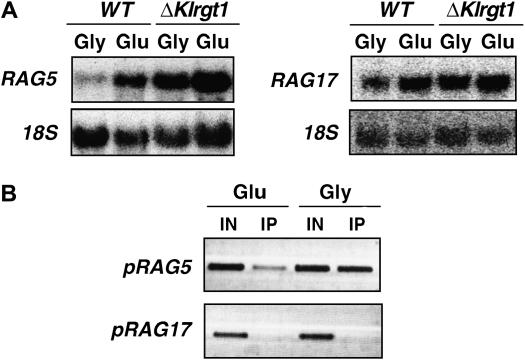
KlRgt1 controls hexokinase gene expression:
Rag8 casein kinase, which is involved in RAG1 expression regulation, is also required for the maximal expression of glycolytic genes (Lemaire et al. 2002) (our unpublished results). Therefore, the Rag− phenotype of rag8 mutants is due to defects in both glucose transport and glycolysis. As the Rag− phenotype of rag8 mutant is suppressed by Klrgt1 mutation, we tested whether KlRgt1 is also involved in glycolytic gene expression. We measured the transcription of hexokinase- and enolase-encoding genes (RAG5 and RAG17, respectively) in WT and Klrgt1 cells grown in the absence or presence of 2% glucose (Figure 8A). As previously reported, the expression of both genes is induced by glucose (Neil et al. 2004). The level of transcription of RAG5 was clearly increased in the absence of glucose in Klrgt1 cells, whereas that of RAG17 was affected to a lesser extent. Therefore, we tested whether KlRgt1 could interact with the promoter sequence of the two genes. The regions of the RAG5 gene promoter (−1052 to −640 bp) and the RAG17 gene promoter (−933 to −512 bp) used as templates for PCR amplification contain three and one putative KlRgt1 binding sites, respectively (not shown). We found that KlRgt1 interacts with the RAG5, whereas no ChIP amplification of RAG17 promoter sequence was detected (Figure 8B). These results clearly show that KlRgt1 represses hexokinase gene expression in the absence of glucose. Under the same conditions enolase gene appears indirectly affected by the repressor. However, we cannot exclude that the slight effect seen on RAG17 expression in the Klrgt1 mutant (Figure 8A) is due to the binding of KlRgt1 on its single site present in the RAG17 promoter (but not detected in our ChIP experiments). As seen for the RAG1 gene promoter, KlRgt1 binds to the RAG5 promoter in both the absence and the presence of glucose. In this case the binding of KlRgt1 to DNA in the presence of glucose appears to be different than in the presence of glycerol (Figure 8B). This indicates that KlRgt1 gene regulation could occur through a differential binding affinity, modulated by its phosphorylation status.
Figure 8.—
Effect of KlRgt1 on the expression of glycolytic genes. (A) Northern blot analysis of RAG5 (hexokinase encoding gene) and RAG17 (enolase encoding gene) transcription in a Klrgt1 mutant vs. wild-type cells. Yeast cell growth conditions and electrophoresis conditions were as indicated in Figure 2. WT (MW270-7B), Klrgt1 (MWK7). (B) The interaction of KlRgt1 with the pRAG5 and pRAG17 promoters was analyzed by ChIP assay as for Figure 6. pRAG5 and pRAG17 DNA were amplified using sets of primers described in materials and methods.
DISCUSSION
We show that the Rag− phenotype and the transcriptional defect, seen in cells grown on glucose, of the glucose permease gene RAG1 caused by a rag4 null mutation can be suppressed by recessive mutations in the KlRGT1 gene. We also show that RAG1 is expressed at a high level in the absence of glucose in both the revertant and the Klrgt1 mutant, demonstrating that KlRGT1 encodes a negative regulator of the RAG1 gene acting in the absence of glucose.
Amino acid sequence analysis of the KlRGT1 gene product revealed a good match with the S. cerevisiae glucose transporter gene regulator ScRgt1 (Ozcan et al. 1996). In addition to a well-conserved DNA-binding domain consisting of a Zn2-Cys6 cluster, there are several high-identity blocks in the two proteins. However, not all of these conserved regions correspond to functional domains identified in the ScRgt1 transcription factor of S. cerevisiae (Polish et al. 2005). The loss-of-function mutation Klrgt1-6 lies in a region (at the end of the C-terminal region of the protein) that escaped the functional analysis of ScRgt1 (Polish et al. 2005). Also, because the K. lactis protein is shorter, there are several amino acid regions present in ScRgt1 that are missing in KlRgt1. These differences may explain the functional discrepancies observed between them (see below).
In the absence of glucose, ScRgt1 from S. cerevisiae binds to the HXT gene promoters and represses their transcription (Ozcan et al. 1996; Flick et al. 2003; Kim et al. 2003; Mosley et al. 2003; Polish et al. 2005). Some reports also suggest that in the presence of glucose, ScRgt1 behaves as a transcriptional activator of the HXT genes (Ozcan and Johnston 1995; Ozcan et al. 1996; Polish et al. 2005). Our data suggest that in K. lactis, KlRgt1 represses RAG1 transcription only in the absence of glucose. This is supported, first, by the lack of Rag− phenotype of a Klrgt1 mutant. Indeed, the disruption of an activator of RAG1 gene is expected to generate the Rag− phenotype. For instance, even a twofold reduction of RAG1 transcription in sck1 mutant leads to a Rag− phenotype (Lemaire et al. 2002). Second, Northern blot analysis showed that RAG1 transcription in the Klrgt1 mutant grown on glucose was higher than that in wild-type cells. This last result also indicates that glucose induction of RAG1 gene expression results mostly from inactivation of KlRgt1. Nevertheless, RAG1 transcription was found to be still slightly glucose inducible. Therefore, additional activation mechanism of the RAG1 gene in response to glucose remains to be determined.
We tested the ability of KlRgt1 to bind to the RAG1 promoter in vivo and in vitro using ChIP and IDBA assays, respectively. We found that KlRgt1 binds continually to the RAG1 promoter, even when RAG1 is derepressed in the presence of glucose. This is unlike what is observed in S. cerevisiae, in which ScRgt1 dissociates from the HXT promoters in the presence of glucose. Under these conditions, glucose regulates the DNA-binding ability of ScRgt1 by inducing its phosphorylation (Flick et al. 2003; Kim et al. 2003; Mosley et al. 2003). The difference between the two yeasts may be due to the absence in KlRgt1 of the amino acids 750–760 of ScRgt1. When this region is deleted, ScRgt1 constitutively binds DNA (Polish et al. 2005). Consistent with this, we found that glucose regulates the repressor activity of KlRgt1 through post-translational modification: phosphorylated KlRgt1 does not repress RAG1 expression but can still bind to the RAG1 promoter. Furthermore, rag4 mutant cells, in which KlRgt1 always functions as a transcriptional repressor independent of the presence of glucose, show no glucose-induced phosphorylation of KlRgt1. This suggests that the Rag4-dependent-sensing pathway inhibits KlRgt1 transcriptional repression through the phosphorylation of KlRgt1. A similar situation has recently been reported for the yeast Mig1 repressor, which always binds to repressed promoters under both repression and activation conditions (Papamichos-Chronakis et al. 2004). However, as shown by the IDBA and ChIP assay performed with the promoter of RAG1 and RAG5, respectively, we cannot exclude that its binding affinity can be modified, even if KlRgt1 is present on the RAG1 promoter in the presence of glucose. In this case, KlRgt1 could either bind to its sites with a lower affinity or interact with different sites, thus allowing the binding of some activator protein to the promoter region.
Glycolytic genes, like the glucose transporter gene RAG1, are glucose inducible. Therefore, we tested whether their regulation involved the KlRgt1 repressor. Using hexokinase (RAG5)- and enolase (KlENO/RAG17)-encoding genes, we showed that only the hexokinase gene is repressed by the KlRgt1 repressor in the absence of glucose. This result indicates that yeast cells carefully regulate glucose use by tightly coregulating the first steps of glucose metabolism (its transport across the plasma membrane and its phosphorylation by hexokinase), glucose permease and hexokinase gene expression being repressed in the absence of glucose and induced in its presence. This finding could also be related to regulatory function of hexokinase, which is known to be involved in glucose induction of RAG1 expression (Chen et al. 1992; Prior et al. 1993; Lemaire and Wesolowski-Louvel 2004). We previously showed that hexokinase together with other glycolytic enzymes (enolase and phosphoglycerate kinase) participate in a pathway responding to an intracellular signal generated by glycolysis to regulate RAG1 expression (Lemaire and Wesolowski-Louvel 2004). As the first element of the intracellular glucose-sensing pathway, the hexokinase gene RAG5 is regulated by KlRgt1; this indicates that the extracellular glucose-sensing pathway would trigger off the intracellular pathway. Therefore, in response to glucose, KlRgt1 will have a double control on glucose uptake by directly regulating the RAG1 promoter and by controlling the intracellular signal mediated by glycolysis. Despite the fact that we found that the hexokinase gene RAG5 itself is a target of KlRgt1, we cannot exclude its direct participation in the extracellular glucose-sensing pathway to regulate RAG1. These two possibilities are not exclusive. It should be noted that in S. cerevisiae, ScRgt1 has been shown to control the hexokinase gene HXK2, although in this case a connection to HXT gene regulation has not been established (Palomino et al. 2005; Palomino et al. 2006).
The regulation of ScRgt1 in S. cerevisiae also involves the paralogous proteins Mth1 and Std1, known to be negative regulators of HXT gene expression (Flick et al. 2003; Lakshmanan et al. 2003; Mosley et al. 2003). Mth1/Std1, which have been shown to interact with the Snf3 and Rgt2 glucose sensors (Schmidt et al. 1999; Lafuente et al. 2000), Yck 1 casein kinase 1 (Moriya and Johnston 2004), and the repressor ScRgt1 (Tomas-Cobos and Sanz 2002; Lakshmanan et al. 2003) are good candidates for relaying the glucose signal from the plasma membrane to the nucleus. The K. lactis genome (http://cbi.labri.fr/Genolevures/) contains a single orthologous gene for the MTH1/STD1 paralogs.
Finally, the glucose-sensing and -signaling pathway appears to be well conserved between S. cerevisiae and K. lactis, although there are some important differences in the regulation of repressor function of KlRgt1 vs. ScRgt1. The most important difference is that KlRgt1 does not depart from the RAG1 and RAG5 promoters during growth in glucose to enable transcription. As a consequence the system would be less easily derepressed than in S. cerevisiae and is even not totally derepressed in the presence of high concentrations of glucose. This is especially shown by the higher level of RAG1 transcription in the ΔKlrgt1 mutant than in the wild type. This mechanism might provide a rapid reestablishment of repression under glucose limitation. In addition, RAG1 induction seems to be complicated by the involvement of an additional activator that is not KlRgt1 itself, as in the case of ScRgt1 in S. cerevisiae.
Therefore, RAG1 expression is tightly repressed in the absence or in the presence of low glucose. This regulation might reflect an adaptation of K. lactis to its natural environment, which is normally poor in glucose. Under these conditions, K. lactis metabolism would rely on respiration and the high-affinity glucose permease Hgt1. The presence of the regulated high-capacity glucose permease gene RAG1 would allow K. lactis to adapt and to compete in a high-glucose environment. In this case the cells would be able to utilize glucose by both fermentation and oxidation, since respiration is not glucose repressed.
Acknowledgments
We thank Petr Obrdlik for providing us with the mating-based split ubiquitin system (mbSUS). Martina Hnatova was a recipient of a Fellowship from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.
References
- Betina, S., P. Goffrini, I. Ferrero and M. Wesolowski-Louvel, 2001. RAG4 gene encodes a glucose sensor in Kluyveromyces lactis. Genetics 158: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard, P., S. Menart, J. Blaisonneau, M. Bolotin-Fukuhara, H. Fukuhara et al., 1996. Glucose uptake in Kluyveromyces lactis: role of the HGT1 gene in glucose transport. J. Bacteriol. 178: 5860–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson, L. F., and D. G. Fraenkel, 1984. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J. Bacteriol. 159: 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisonneau, J., H. Fukuhara and M. Wesolowski-Louvel, 1997. The Kluyveromyces lactis equivalent of casein kinase I is required for the transcription of the gene encoding the low-affinity glucose permease. Mol. Gen. Genet. 253: 469–477. [DOI] [PubMed] [Google Scholar]
- Chen, X. J., 1996. Low- and high-copy-number shuttle vectors for replication in the budding yeast Kluyveromyces lactis. Gene 172: 131–136. [DOI] [PubMed] [Google Scholar]
- Chen, X. J., M. Wesolowski-Louvel and H. Fukuhara, 1992. Glucose transport in the yeast Kluyveromyces lactis. II. Transcriptional regulation of the glucose transporter gene RAG1. Mol. Gen. Genet. 233: 97–105. [DOI] [PubMed] [Google Scholar]
- Dietrich, F. S., S. Voegeli, S. Brachat, A. Lerch, K. Gates et al., 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304: 304–307. [DOI] [PubMed] [Google Scholar]
- Dujon, B., D. Sherman, G. Fischer, P. Durrens, S. Casaregola et al., 2004. Genome evolution in yeasts. Nature 430: 35–44. [DOI] [PubMed] [Google Scholar]
- Flick, K. M., N. Spielewoy, T. Kalashnikova, M. Guaderrama, Q. Zhu et al., 2003. Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol. Biol. Cell 14: 3230–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffrini, P., A. A. Algeri, C. Donnini, M. Wesolowski-Louvel and I. Ferrero, 1989. RAG1 and RAG2: nuclear genes involved in the dependence/independence on mitochondrial respiratory function for growth on sugars. Yeast 5: 99–106. [DOI] [PubMed] [Google Scholar]
- Kim, J., 2004. Immobilized DNA-binding assay, an approach for in vitro DNA-binding assay. Anal. Biochem. 334: 401–402. [DOI] [PubMed] [Google Scholar]
- Kim, J. H., J. Polish and M. Johnston, 2003. Specificity and regulation of DNA binding by the yeast glucose transporter gene repressor Rgt1. Mol. Cell. Biol. 23: 5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov, V., 2000. Rapid and reliable protein extraction from yeast. Yeast 16: 857–860. [DOI] [PubMed] [Google Scholar]
- Lafuente, M. J., C. Gancedo, J. C. Jauniaux and J. M. Gancedo, 2000. Mth1 receives the signal given by the glucose sensors Snf3 and Rgt2 in Saccharomyces cerevisiae. Mol. Microbiol. 35: 161–172. [DOI] [PubMed] [Google Scholar]
- Lakshmanan, J., A. L. Mosley and S. Ozcan, 2003. Repression of transcription by Rgt1 in the absence of glucose requires Std1 and Mth1. Curr. Genet. 44: 19–25. [DOI] [PubMed] [Google Scholar]
- Lemaire, M., and M. Wesolowski-Louvel, 2004. Enolase and glycolytic flux play a role in the regulation of the glucose permease gene RAG1 of Kluyveromyces lactis. Genetics 168: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire, M., A. Guyon, S. Betina and M. Wesolowski-Louvel, 2002. Regulation of glycolysis by casein kinase I (Rag8p) in Kluyveromyces lactis involves a DNA-binding protein, Sck1p, a homologue of Sgc1p of Saccharomyces cerevisiae. Curr. Genet. 40: 355–364. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Moriya, H., and M. Johnston, 2004. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101: 1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley, A. L., J. Lakshmanan, B. K. Aryal and S. Ozcan, 2003. Glucose-mediated phosphorylation converts the transcription factor Rgt1 from a repressor to an activator. J. Biol. Chem. 278: 10322–10327. [DOI] [PubMed] [Google Scholar]
- Neil, H., M. Lemaire and M. Wesolowski-Louvel, 2004. Regulation of glycolysis in Kluyveromyces lactis: role of KlGCR1 and KlGCR2 in glucose uptake and catabolism. Curr. Genet. 45: 129–139. [DOI] [PubMed] [Google Scholar]
- Obrdlik, P., M. El-Bakkoury, T. Hamacher, C. Cappellaro, C. Vilarino et al., 2004. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101: 12242–12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan, S., and M. Johnston, 1995. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell. Biol. 15: 1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan, S., and M. Johnston, 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63: 554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan, S., T. Leong and M. Johnston, 1996. Rgt1p of Saccharomyces cerevisiae, a key regulator of glucose-induced genes, is both an activator and a repressor of transcription. Mol. Cell. Biol. 16: 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino, A., P. Herrero and F. Moreno, 2005. Rgt1, a glucose sensing transcription factor, is required for transcriptional repression of the HXK2 gene in Saccharomyces cerevisiae. Biochem. J. 388: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino, A., P. Herrero and F. Moreno, 2006. Tpk3 and Snf1 protein kinases regulate Rgt1 association with Saccharomyces cerevisiae HXK2 promoter. Nucleic Acids Res. 34: 1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis, M., T. Gligoris and D. Tzamarias, 2004. The Snf1 kinase controls glucose repression in yeast by modulating interactions between the Mig1 repressor and the Cyc8-Tup1 co-repressor. EMBO Rep. 5: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polish, J. A., J. H. Kim and M. Johnston, 2005. How the Rgt1 transcription factor of Saccharomyces cerevisiae is regulated by glucose. Genetics 169: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior, C., P. Mamessier, H. Fukuhara, X. J. Chen and M. Wesolowski-Louvel, 1993. The hexokinase gene is required for transcriptional regulation of the glucose transporter gene RAG1 in Kluyveromyces lactis. Mol. Cell. Biol. 13: 3882–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M. C., R. R. McCartney, X. Zhang, T. S. Tillman, H. Solimeo et al., 1999. Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 4561–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry, A., C. Fairhead and B. Dujon, 1990. The complete sequence of the 8.2 kb segment left of MAT on chromosome III reveals five ORFs, including a gene for a yeast ribokinase. Yeast 6: 521–534. [DOI] [PubMed] [Google Scholar]
- Tomas-Cobos, L., and P. Sanz, 2002. Active Snf1 protein kinase inhibits expression of the Saccharomyces cerevisiae HXT1 glucose transporter gene. Biochem. J. 368: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski, M., A. A. Algeri, P. Goffrini and H. Fukuhara, 1982. Killer DNA plasmids of the yeast Kluyveromyces lactis. I. Mutations affecting the killer phenotype. Curr. Genet. 150: 137–140. [DOI] [PubMed] [Google Scholar]
- Wesolowski-Louvel, M., P. Goffrini, I. Ferrero and H. Fukuhara, 1992. a Glucose transport in the yeast Kluyveromyces lactis. I. Properties of an inducible low-affinity glucose transporter gene. Mol. Gen. Genet. 233: 89–96. [DOI] [PubMed] [Google Scholar]
- Wesolowski-Louvel, M., C. Prior, D. Bornecque and H. Fukuhara, 1992. b Rag- mutations involved in glucose metabolism in yeast: isolation and characterization. Yeast 8: 711–719. [Google Scholar]
- Wesolowski-Louvel, M., C. Tanguy-Rougeau and H. Fukuhara, 1988. A nuclear gene required for the expression of the linear DNA-associated killer system in the yeast Kluyveromyces lactis. Yeast 4: 71–81. [DOI] [PubMed] [Google Scholar]