Abstract
Current antiviral strategies target viral gene products. Although initially successful, their severe toxicity and susceptibility to circumvention by the generation of drug-resistant variants limit their usefulness. By contrast, the central role of the host cell serine endoprotease furin in the proteolytic activation of numerous pathogens points to the endoprotease as a strategic target for therapeutics. Herein, we show that the production of infectious human cytomegalovirus is dramatically reduced by exogenous addition of a bioengineered serpin, α1-PDX. This protein is a potent and selective furin inhibitor (Ki = 0.6 nM) and is 10-fold more effective than currently used antiherpetic agents in cell-culture models. The requirement of furin for the processing of envelope glycoproteins from many pathogenic viruses and for the activation of several bacterial toxins suggests that selective inhibitors of furin have potential as broad-based anti-pathogens.
A common virulence-activating step shared by many pathogenic bacteria [e.g., Pseudomonas exotoxin A (exoA) and anthrax toxoid] and viruses (e.g., HIV and measles) is an absolute requirement for the proteolytic maturation of key proprotein substrates at consensus furin sites (-Arg-X-Lys/Arg-Arg↓-) by the host (1–3). For example, proteolytic maturation of many viral envelope glycoproteins (e.g., HIV-1 gp160 and measles F0) at consensus furin sites is absolutely required for the formation of infectious progeny (2, 4, 5). Key to the ability of furin to activate diverse pathogen molecules is the ability of this proprotein convertase (PC) to sort between three principal proprotein processing compartments within the trans-Golgi network (TGN)/endosomal system: the TGN (cleavage of viral envelope glycoproteins), the cell surface (activation of anthrax and proaerolysin toxins), and early endosomes (activation of exoA; ref. 3).
The requirement for furin in the proteolytic activation of virulence molecules from a wide variety of pathogens makes this endoprotease a compelling target for the development of a generation of broad-based antimicrobials. A highly potent and selective inhibitor of furin was engineered by construction of a variant of α1-antitrypsin, named α1-PDX, that contains the minimal furin consensus sequence (-Arg-X-X-Arg↓-; Ki = 0.6 nM; ref. 6). Intracellular expression of the α1-PDX cDNA showed that α1-PDX blocks the furin-dependent processing of HIV-1 gp160 and measles virus F0 and the production of infectious progeny (5, 7). However, use of this inhibitor as a therapeutic requires the recombinant protein, when applied externally to the cell, to block the generation of infectious virus.
Human cytomegalovirus (HCMV), the prototypical member of the β-herpesviridae, is a major cause of morbidity and mortality in organ transplant recipients and patients with AIDS (8). Similar to other viral envelope glycoproteins, gB, the UL55 gene product and the predominant HCMV envelope glycoprotein (9), is generated by cleavage of pro-gB at a consensus furin site to generate the disulfide-linked gp110/gp55 gB heterodimer present within the virion (ref. 10; Fig. 1A). Several studies indicate the importance of gB for the infectivity of many herpes viruses, including HCMV (11). For example, use of blocking agents showed that HCMV gB participates in virus adsorption to target cell membranes (12), and the correlate protein in herpes simplex virus is essential for fusion (13). Herein, we show that the production of infectious HCMV is dramatically reduced by exogenous addition of the furin inhibitor α1-PDX. Comparison to the broadly used antiherpetic foscarnet shows that α1-PDX is 10-fold more effective than currently used therapeutics in cell-culture models. α1-PDX specifically and reversibly depletes cellular furin, resulting in a complete block of the furin-dependent processing of HCMV gB and the mislocalization of this envelope proprotein. Together with a lack of toxicity, our results suggest that protein-based inhibitors of furin have potential as broad-based anti-pathogens.
Figure 1.
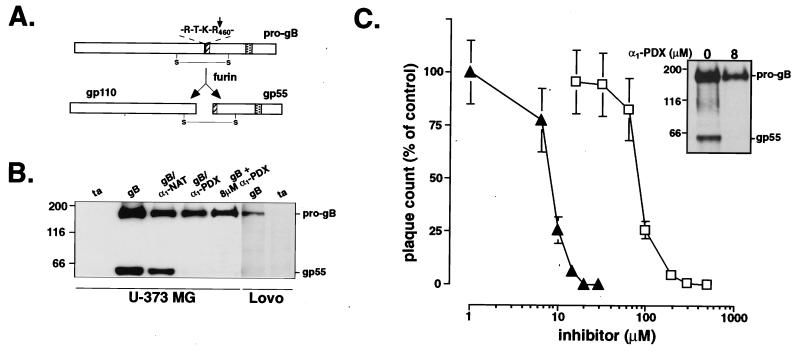
α1-PDX is a potent inhibitor of HCMV. (A) Schematic of HCMV gB. The 907-amino acid pro-gB is cleaved during transit through the TGN at the C-terminal side of the consensus furin site (black bar) to yield the disulfide-linked gp110/55 heterodimer. The inserted FLAG tag (hatched) and transmembrane domain (stippled) are also shown. (B) Inhibition of the furin-dependent processing of HCMV gB by α1-PDX. U-373 MG cells on replicate plates were coinfected with adenovirus recombinants expressing the tet transactivator [ta; multiplicity of infection (moi) = 5] and HCMV pro-gB (gB; total moi = 10) or triple-infected with the ta and gB recombinants together with adenovirus recombinants expressing either α1-antitrypsin (α1-NAT) or α1-PDX for 24 h (total moi = 15). Alternatively, U-373 MG cells were pretreated with 8 μM α1-PDX for 24 h and then coinfected with the ta and gB recombinants for an additional 24 h in the presence of 8 μM α1-PDX. As a control, furin-deficient LoVo cells were infected with either adenovirus ta alone (moi = 5) or together with adenovirus gB (total moi = 10) for 24 h. Cells were harvested in RIPA minus SDS and analyzed by Western blotting (SDS/8% PAGE) by using anti-gB mAb 27-156. The gp55 band could also be detected with the anti-FLAG mAb M1 (requires free N terminus), indicating correct cleavage of pro-gB (data not shown). (C) Dose response of inhibition of HCMV by α1-PDX and foscarnet. U373 cells on replicate plates were infected with the HCMV Towne (moi = 0.1) in the absence or presence of increasing concentrations of α1-PDX (triangles) or the pyrophosphate analog foscarnet (squares). At 5 days after infection, cells were harvested, and infectious cell-associated HCMV was quantified by plaque assay on human foreskin fibroblast cells (HCMV remains cell-associated in U373 MG cells; J.A.N., unpublished results). Data represent averages of three experiments. Error bars depict standard deviation. (Inset) U-373 MG cells on replicate plates were infected with HCMV Towne (moi = 0.2). At 24 h after infection, the medium was replaced with fresh medium containing either 0 or 8 μM α1-PDX. At 72 h after infection, cells were metabolically labeled with [35S]Met/Cys for 3 h, chased for 2 h in the absence of label, and harvested. gB proteins were immunoprecipitated with mAb 7-17. The minor protein band migrating below the 116-kDa marker likely represents coimmunoprecipitated gp110.
Methods
Cell Culture and α1-PDX Production.
Human foreskin fibroblasts and human astrocytoma U-373 MG cells were cultured in DMEM (high glucose) containing 10% (vol/vol) FCS (GIBCO) and 50 μg/ml gentamicin sulfate. LoVo cells were cultured in Ham's F-12 containing 20% (vol/vol) FCS supplemented with 2 mM l-glutamine and 50 μg/ml gentamicin sulfate. Bacterially expressed His- and FLAG-tagged α1-PDX was purified as described (6). Foscarnet was obtained from Sigma.
Viruses.
HCMV Towne stocks (provided by J.A.N.) were grown in human foreskin fibroblast cells as described (14). Plaque assays were performed as described (14). Construction and characterization of vaccinia recombinants expressing furin, FLAG-tagged furin, FLAG-tagged PC6B, and FLAG-tagged PC7 have been described (6). The adenovirus recombinant expressing FLAG-tagged HCMV gB was constructed by insertion of the FLAG epitope at the furin consensus cleavage site by PCR mutagenesis with an oligonucleotide primer encoding the mutation (5′-GCCAATGCATCCAGTCTGAATATCACTCATAGGACCAGAAGAGACTACAAGGATGACGAT- GACAAGAGTACGAGTGACAATAATACAAC-3′). Adenovirus recombinants expressing FLAG-tagged α1-PDX and α1-NAT were constructed by insertion of the FLAG-epitope at the α1-antitrypsin signal peptide cleavage site by PCR mutagenesis with an oligonucleotide primer encoding the mutation (5′-CTGGGGATCCTCCTTGTCATCGTCGTCCTTGTAG- TCAGCCAGGGAGACAGGGACCAGGCA-3′) and a second primer corresponding to the T7 promoter (5′-TAATACGACTCACTATAGGG-3′). The FLAG-tagged cDNAs were subcloned into the adenovirus shuttle vector pE1SP1Btet. The production of recombinant adenovirus vectors was performed as described (16).
Immunoprecipitations, Membrane Fractionation, and Western Blotting.
Cells were incubated in Met−/Cys− MEM (ICN) for 30 min, washed, and then labeled in the same medium containing 1% FCS and 500 μCi/ml [35S]Met/Cys (Express label, NEN) as described in the legend to Fig. 1. Cells were harvested in RIPA (50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/1% NP-40/1% NaDOC/0.1% SDS). In some experiments (where indicated), cells were harvested in RIPA minus SDS. Extracts were incubated with the anti-gB mAb 7-17 (1:100) followed by capture with protein A Sepharose. Immunoprecipitates were resolved by SDS/8% PAGE, and radiolabeled proteins were detected by using Kodak AR x-ray film. Membrane fractionation and Western blot analysis were performed as described (17).
Immunofluorescence Microscopy and Antibodies.
Immunofluorescence analyses were performed as described (17). Anti-FLAG mAbs M1 and M2 were from IBI. The human transferrin receptor mAb was from C. Enns (Oregon Health Sciences University). The anti-α1-human antitrypsin antiserum was from Bachem. The anti-furin antiserum PA1–062 was from Affinity Bioreagents (Neshanic Station, NJ). Fluorescent secondary antibodies were from Fisher.
Results
α1-PDX Blocks Processing of HCMV gB.
The processing of HCMV pro-gB at a consensus furin site raised the possibility that α1-PDX may represent an HCMV inhibitor. To begin testing this possibility, cell expression studies were conducted and showed that the processing of HCMV gB depends on furin and can be blocked by α1-PDX (Fig. 1). A specific requirement for furin in the processing of pro-gB was shown by the inability of LoVo cells, a furin-deficient colon carcinoma line, to process the 165-kDa pro-gB precursor. By contrast, expression of pro-gB in all furin-containing cell lines examined, including HCMV-permissive human astrocytoma U-373 MG cells (Fig. 1B), resulted in the efficient processing of pro-gB at the consensus furin site (-Arg-Thr-Lys-Arg460↓-; see Fig. 1A) to generate mature gp55. In agreement with these results, coexpression of pro-gB with the furin-directed inhibitor α1-PDX (reactive site sequence, -Arg355-Ile-Pro-Arg358-), but not the control serpin native α1-antitrypsin (α1-NAT, -Ala355-Ile-Pro-Met358-), blocked pro-gB processing in U-373 MG cells (Fig. 1B).
The effectiveness of intracellularly expressed α1-PDX in blocking the furin-dependent processing of pro-gB is consistent with similar gene transfer studies (5) and further supports the possibility of employing α1-PDX in gene therapy approaches to pathogen defense. However, use as a broad-based antimicrobial would require extracellular addition of recombinant α1-PDX to block also the processing of viral envelope glycoproteins. To test this possibility, U-373 MG cells expressing pro-gB were treated with 8 μM recombinant α1-PDX and analyzed by Western blotting. The extracellularly applied α1-PDX completely blocked the maturation of pro-gB (Fig. 1B).
Inhibition of HCMV by Extracellular α1-PDX.
The ability of α1-PDX, when applied as an extracellular compound, to inhibit the processing of pro-gB suggested an approach to block the production of infectious HCMV in a cell-culture model. To assess this approach, the relative effectiveness of α1-PDX against HCMV replication was compared with foscarnet, a clinically used pyrophosphate analog that inhibits viral DNA polymerase (ref. 18; Fig. 1C). U-373 MG cells on replicate plates were infected with HCMV Towne in the absence or presence of increasing concentrations of either α1-PDX or foscarnet. At 5 days after infection, cells were harvested, and the amount of cell-associated infectious viral progeny was determined by plaque assay. In both cases, a dose-dependent inhibition of HCMV production was observed; however, α1-PDX was much more potent. Treatment of U-373 MG cells with 8 μM α1-PDX reduced the amount of infectious virus by 50%, and treatment with 20 μM resulted in a 3-log reduction in the HCMV titer (Fig. 1C; ED50 = 8 μM). Comparison of the effectiveness of α1-PDX to that of foscarnet (ED50 = 80 μM) showed that the potency of the furin-directed serpin is approximately 10-fold greater than that of the currently used HCMV antivirals.
Consistent with the gB-processing analysis shown in Fig. 1, the α1-PDX-mediated reduction in the titer of infectious HCMV was coupled with a block in the processing of viral pro-gB. Parallel plates of HCMV-infected U-373 MG cells were treated with 8 μM α1-PDX. Immunoprecipitation analysis of [35S]Cys/Met-labeled proteins showed that in untreated cells, the 165-kDa pro-gB was cleaved to gp55. However, treatment with 8 μM α1-PDX blocked the processing of the HCMV envelope proprotein (Fig. 1C Inset). The lower expression of pro-gB in α1-PDX-treated cells was not due to toxicity of the furin inhibitor (see below) but likely resulted from a reduced spread of the virus in the infected cell cultures.
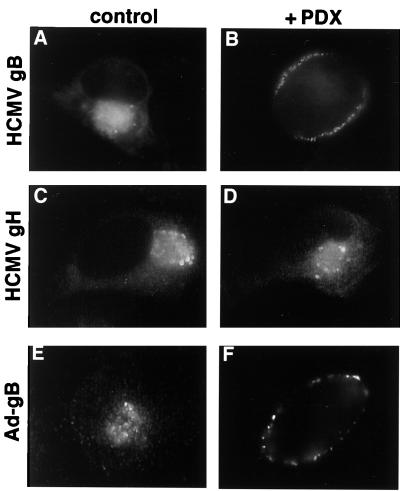
The α1-PDX-mediated inhibition of pro-gB processing was concomitant with a marked mislocalization of the uncleaved envelope glycoprotein precursor in the U-373 MG cells (Fig. 2). Although gB is localized to a paranuclear compartment in HCMV-infected cells, treatment with α1-PDX causes the unprocessed envelope precursor to redistribute to the cell periphery (Fig. 2 A and B). The mislocalization was specific for pro-gB, because the localization of the HCMV envelope glycoprotein gH (which is not processed by furin) was unaffected by α1-PDX treatment (Fig. 2 C and D). The effect of the recombinant serpin was also independent of HCMV infection, because the α1-PDX-dependent mislocalization of the envelope glycoprotein was observed when the adenovirus recombinant expressing pro-gB was used (Fig. 2 E and F).
Figure 2.
Inhibition of HCMV pro-gB processing results in mislocalization of the envelope precursor. (A–D). U-373 cells grown on coverslips were infected with HCMV Towne (moi = 0.2) and cultured in the absence (A and C) or presence (B and D) of 8 μM α1-PDX. At 5 days after infection, cells were fixed, permeabilized, and processed for immunofluorescence microscopy. HCMV gB molecules (A and B) were detected with mAb 27-156, and HCMV gH molecules (C and D) were detected with mAb 14-4B. (E and F) U-373 MG cells grown on coverslips were infected with a recombinant adenovirus expressing HCMV gB (moi = 10) and incubated for 48 h in the absence (E) or presence (F) of 8 μM α1-PDX. Cells were fixed and permeabilized, and epitope-tagged gB molecules were localized by immunofluorescence microscopy with an anti-FLAG mAb.
Reversible and Selective Depletion of Furin by Extracellular α1-PDX.
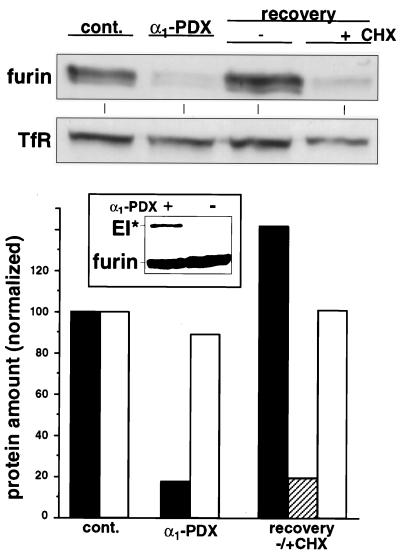
The ability of extracellularly applied α1-PDX to block the processing of pro-gB suggested that this serpin was capable of inhibiting the intracellular pool of furin in the TGN. Western blot analysis showed that a 6-h treatment with 8 μM α1-PDX reduced the intracellular furin pool by 80%, whereas the level of a control protein, the transferrin receptor, was relatively unaffected (Fig. 3). In agreement with kinetic analyses (6), the inhibition of intracellular furin by α1-PDX seemed to be mediated by a classic serpin-based mechanism, because extracellularly applied α1-PDX formed an SDS-stable complex with cellular furin within 30 min (Fig. 3 Inset). The α1-PDX-mediated depletion of intracellular furin is fully reversible, because removal of the furin inhibitor from the culture medium resulted in a protein synthesis-dependent restoration of the intracellular furin pool (Fig. 3).
Figure 3.
Depletion of cellular furin by exogenous α1-PDX. Replicate plates of U-373 MG cells were incubated in the absence or presence of 8 μM α1-PDX for 6 h. The cells were then either harvested directly (cont., control) or washed and incubated in fresh medium for an additional 10 h in the absence or presence of 50 μM cycloheximide (CHX) before harvesting. Crude membrane preparations from each sample were resolved by SDS/PAGE, and the levels of endogenous furin and transferrin receptor (TfR) were analyzed by Western blotting. The signals from the Western blot were quantified with National Institutes of Health image software. Black bars, furin; open bars, transferrin receptor; hatched bar, furin in the presence of cycloheximide. (Inset) U-373 MG cells were incubated with or without 8 μM α1-PDX for 30 min. Membrane preparations from washed cells were resolved by SDS/PAGE, and furin molecules were identified by Western blotting by using antiserum PA1-062. Formation of the kinetically trapped SDS-stable complex between furin and α1-PDX (EI*) causes furin to migrate at 160 kDa.
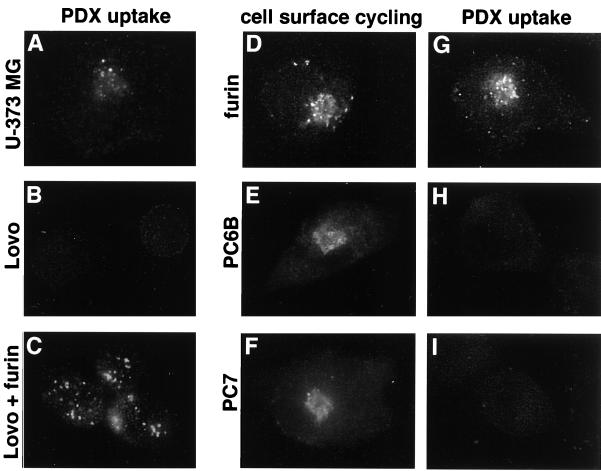
Three sets of experiments showed that extracellularly applied α1-PDX requires furin for cellular uptake and that it is a specific inhibitor of cellular furin (Fig. 4). First, intracellular accumulation of α1-PDX depends strictly on furin expression (Fig. 4 A–C). Parallel plates of furin-containing U-373 MG and furin-deficient LoVo cells were incubated with α1-PDX. Internalized α1-PDX molecules were detected in U-373 MG cells (Fig. 4A), whereas LoVo cells (Fig. 4B) failed to accumulate detectable levels of the furin inhibitor. Second, expression of furin in either LoVo or U-373 MG cells (Fig. 4 C and D) resulted in a marked increase in the amount of internalized α1-PDX. Third, the specificity of α1-PDX for furin was demonstrated by comparing the amount of internalized α1-PDX in furin-overexpressing cells to cells overexpressing the two other membrane-anchored PCs, PC6B and PC7 (19) (Fig. 4). Like furin, both PC6B and PC7 sort through TGN/endosomal compartments and cycle to the cell surface (15, 20). Studies in vitro show that PC6B (Ki = 2.3 nM) but not PC7 (Ki < 5,000 nM) is potently inhibited by α1-PDX (6). Replicate plates of U-373 MG cells expressing epitope (FLAG)-tagged furin, PC6B, or PC7 were incubated with α1-PDX for 1 h. Cells expressing furin internalized α1-PDX (Fig. 4G), whereas cells expressing either PC6B or PC7 failed to internalize the furin inhibitor (Fig. 4 H and I). The inability of PC7 and PC6B to internalize α1-PDX was not caused by a lack of cell-surface cycling of the PCs, because cells expressing each epitope (FLAG)-tagged enzyme efficiently internalized FLAG-directed antibody (Fig. 4 D–F).
Figure 4.
Uptake of α1-PDX requires cellular furin. (A–C) Parallel coverslips of U-373 MG (A) and LoVo (B and C) cells either noninfected (A and B) or infected with a vaccinia recombinant expressing nontagged furin (moi = 10) for 4 h (C) were incubated with 8 μM α1-PDX (FLAG-tagged) for 1 h before fixation and staining with an anti-FLAG mAb (mAb M2) to detect internalized α1-PDX. LoVo cells infected with wild-type vaccinia virus showed no uptake of α1-PDX (data not shown). (D–I) U-373 MG cells on replicate coverslips were infected with vaccinia recombinants expressing epitope (FLAG)-tagged furin (D and G), epitope (FLAG)-tagged PC6B (E and H), or epitope (FLAG)-tagged PC7 (F and I) for 4 h. The cultures were then incubated with an anti-FLAG mAb (mAb M1) for 1 h (D–F) to demonstrate cell-surface cycling of each FLAG-tagged convertase or with α1-PDX (G–I). The cells were fixed, permeabilized, and analyzed by immunofluorescence microscopy. Internalized mAb M1 was detected with a goat anti-mouse IgG2b-TxRD. Internalized α1-PDX was detected with an anti-α1-anti-trypsin antiserum followed by goat anti-rabbit-FITC. The lower immunofluorescent signal detected in H and I compared with A (i.e., PDX uptake by endogenous furin) was caused by lower sensitivity of the α1-anti-trypsin antiserum relative to mAb M2.
Analysis of α1-PDX Toxicity.
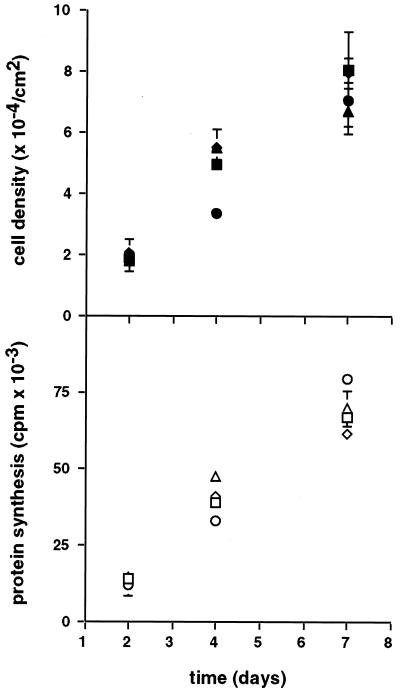
Because furin catalyzes the maturation of a large number of proprotein substrates, the toxicity of α1-PDX was tested in U-373 MG cells. Replicate plates of cells were seeded at low density and cultured in the absence or presence of either 8 μM (ED50 for HCMV) or 80 μM α1-PDX for 1 week. Treatment with either concentration of serpin had no effect on cell growth or viability as determined by cell proliferation and [35S]methionine incorporation (Fig. 5). The lack of detectable inhibitor toxicity shows that α1-PDX has a large selective index (IC50/ED50 > 10).
Figure 5.
Toxicity analysis of α1-PDX. At 6 h after seeding, replicate wells of U-373 MG cells were incubated in the absence (squares) or presence of either 8 μM (diamonds) or 80 μM (circles) α1-PDX or with 13% (vol/vol) PBS (buffer control, triangles). At 2, 4, and 7 days after seeding, duplicate cultures either were harvested for cell counting (filled symbols) or were incubated with [35S]Met/Cys for 30 min to measure protein synthesis (trichloroacetic acid precipitable counts, open symbols). Error bars depict standard deviation.
Discussion
Our studies clearly show that α1-PDX is a potent HCMV inhibitor that is effective at a concentration 10-fold lower than that of currently used therapeutics in cell-culture models. Together with the central role of furin in the activation of numerous viral and bacterial pathogens, our findings suggest the development of furin inhibitors as an approach to the generation of broad-based anti-pathogens.
Compared with other furin inhibitors (21–25), α1-PDX is highly selective for furin and shows no detectable toxicity, and the α1-PDX-mediated depletion of cellular furin is fully reversible. These findings point to a key advantage of using the α1-AT scaffold for the design of furin-selective inhibitors. In addition, the established pharmacokinetics of α1-AT further support the development of α1-PDX as a broad-based antipathogen (26, 27). Further, the highly selective and reversible depletion of secretory-pathway-localized furin by extracellular application of α1-PDX protein explains the molecular basis for the inhibition of pathogen activation. Although furin is localized to the TGN, it cycles to the cell surface and is retrieved to the TGN via endosomal compartments. The lack of detectable uptake of α1-PDX in cells lacking furin suggests that this serpin binds to furin molecules as they are sorted to the cell surface. The high affinity of α1-PDX for furin (Ki = 0.6 nM; ref. 6) then promotes tight binding of the two molecules and the formation of an SDS-stable complex that is subsequently degraded. Surprising, however, was the striking selectivity of α1-PDX for furin compared with a second PC, PC6B, that also cycles to the cell surface and is potently inhibited by α1-PDX in vitro (Ki = 2.3 nM; ref. 6). The difference, however, may be explained in part by the relative efficiency by which each PC forms an SDS-stable complex with α1-PDX. Once formed, the enzyme⋅serpin complex may partition by one of two pathways: formation of an SDS-stable complex or enzymatic cleavage of the serpin by the enzyme. The partitioning between these two pathways is measured as the stoichiometry of inhibition and is a gauge of the efficiency of the serpin. The efficiency of SDS-stable complex formation for furin and α1-PDX (stoichiometry of inhibition = 2, equal partitioning) is much greater than that for PC6B (stoichiometry of inhibition = 8; ref. 6). Together with the higher Ki, the differential stoichiometry of inhibition apparently contributes the specific targeting of furin by extracellular α1-PDX.
Although envelope glycoprotein processing is a required step for the generation of infectious progeny of a large number of pathogenic viruses, the molecular consequence for the processing block can vary greatly. For example, inhibition of the furin-dependent processing of measles virus F0 does not block incorporation of the uncleaved envelope precursor into viral progeny (5). However, such virions are completely noninfectious. Failure to cleave HIV-1 gp160 and avian influenza virus HA0 has different effects. Uncleaved HIV-1 gp160 envelope precursors transit to the cell surface but fail to incorporate into budding virions (4, 28). By contrast, failure to cleave the HA0 blocks transport of the envelope precursor through the secretory pathway (28). Similarly, in our studies, inhibition of furin blocked proteolytic maturation of HCMV gB and caused mislocalization of the proprotein (pro-gB). The extent to which inhibition of pro-gB cleavage and its mislocalization each contribute to the block in the generation of infectious progeny must be determined in future studies.
Antivirals that target viral gene products such as DNA polymerase and viral protease invariably lead to the generation of drug-resistant variants (18, 29). For example, treatment of HCMV infections with foscarnet, cidofovir, or ganciclovir leads to the generation of drug-resistant variants by mutation of either the viral DNA polymerase (cidofovir or foscarnet) or phosphotransferase (ganciclovir) genes (18). However, because the target of α1-PDX is a cellular protein, resistance by mutation of the virus is highly unlikely. Indeed, the virulence of numerous pathogenic viruses (e.g., HIV-1, Ebola, New Castle Disease, and avian influenza viruses) is directly coupled to the presence of a consensus furin site within their envelope glycoprotein sequences (2, 30–33). Therefore, strategic manipulation of furin levels, alone or in combination with other therapeutics, may provide a means to inhibit HCMV as well as a large number of acute bacterial (e.g., anthrax and Pseudomonas aeruginosa) and viral (infectious avian influenza virus and respiratory syncytial virus) pathogens. Future development of appropriate animal models will enable us to test these strategies.
Acknowledgments
We are grateful to D. Streblow, F. Ruchti, and H. L. Meyer for help in production of the adenovirus recombinants and D. Groves for technical assistance. We thank D. Johnson for helpful discussions and C. Enns for antibodies. F.J. was supported in part by the Medical Research Council (Canada). This work was supported by the National Institutes of Health and the Cystic Fibrosis Foundation (G.T.).
Abbreviations
- PC
proprotein convertase
- TGN
trans-Golgi network
- HCMV
human cytomegalovirus
- moi
multiplicity of infection
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050504297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050504297
References
- 1.Gordon V M, Leppla S H. Infect Immun. 1994;62:333–340. doi: 10.1128/iai.62.2.333-340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klenk H D, Garten W. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 3.Molloy S S, Anderson E D, Jean F, Thomas G. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 4.Guo H G, Veronese F M, Tschachler E, Pal R, Kalyanaraman V S, Gallo R C, Reitz M S., Jr Virology. 1990;174:217–224. doi: 10.1016/0042-6822(90)90070-8. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Hirano A, Stenglein S, Nelson J, Thomas G, Wong T C. J Virol. 1995;69:3206–3210. doi: 10.1128/jvi.69.5.3206-3210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason A J, Thomas G. Proc Natl Acad Sci USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson E D, Thomas L, Hayflick J S, Thomas G. J Biol Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- 8.Alford C A, Britt W J. In: The Human Herpesviruses. Lopez C, Whitley R, Roizman B, editors. New York: Raven; 1993. pp. 227–255. [Google Scholar]
- 9.Britt W J, Vugler L G. J Virol. 1992;66:6747–6754. doi: 10.1128/jvi.66.11.6747-6754.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk H D, Radsak K. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 11.Britt W J, Mach M. Intervirology. 1996;39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 12.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 13.Pereira L. Infect Agents Dis. 1994;3:9–28. [PubMed] [Google Scholar]
- 14.Wentworth B B, French L. Proc Soc Exp Biol Med. 1970;135:253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- 15.Xiang, Y., Molloy, S. S., Thomas, L. & Thomas, G. (2000) Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 16.Hitt M, Bett A J, Addison C L, Prevec L, Graham F L. In: Methods in Molecular Genetics. Celis J, editor. Vol. 7. New York: Academic; 1995. pp. 13–30. [Google Scholar]
- 17.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swierkosz E, Hodinka R. In: Manual for Clinical Microbiology. Murry P, editor. Washington, DC: Am. Soc. Microbiol.; 1999. pp. 1624–1639. [Google Scholar]
- 19.Steiner D F. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 20.van de Loo J W, Creemers J W, Bright N A, Young B D, Roebroek A J, Van de Ven W J. J Biol Chem. 1997;272:27116–27123. doi: 10.1074/jbc.272.43.27116. [DOI] [PubMed] [Google Scholar]
- 21.Dahlen J R, Jean F, Thomas G, Foster D C, Kisiel W. J Biol Chem. 1998;273:1851–1854. doi: 10.1074/jbc.273.4.1851. [DOI] [PubMed] [Google Scholar]
- 22.Lu W, Zhang W, Molloy S S, Thomas G, Ryan K, Chiang Y, Anderson S, Laskowski M., Jr J Biol Chem. 1993;268:14583–14585. [PubMed] [Google Scholar]
- 23.Van Rompaey L, Ayoubi T, Van De Ven W, Marynen P. Biochem J. 1997;326:507–514. doi: 10.1042/bj3260507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garten W, Hallenberger S, Ortmann D, Schafer W, Vey M, Angliker H, Shaw E, Klenk H D. Biochimie. 1994;76:217–225. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 25.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk H D, Garten W. Nature (London) 1992;360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 26.Barker A F, Iwata-Morgan I, Oveson L, Roussel R. Chest. 1997;112:607–613. doi: 10.1378/chest.112.3.607. [DOI] [PubMed] [Google Scholar]
- 27.Vogelmeier C, Kirlath I, Warrington S, Banik N, Ulbrich E, Du Bois R M. Am J Respir Crit Care Med. 1997;155:536–541. doi: 10.1164/ajrccm.155.2.9032191. [DOI] [PubMed] [Google Scholar]
- 28.Moulard M, Hallenberger S, Garten W, Klenk H D. Virus Res. 1999;60:55–65. doi: 10.1016/s0168-1702(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 29.Condra J H. Haemophilia. 1998;4:610–615. doi: 10.1046/j.1365-2516.1998.440610.x. [DOI] [PubMed] [Google Scholar]
- 30.Steinhauer D A. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 31.Nagai Y, Klenk H D, Rott R. Virology. 1976;72:494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- 32.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 33.Volchkov V E, Feldmann H, Volchkova V A, Klenk H D. Proc Natl Acad Sci USA. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]