Abstract
Objective:
To analyze the short-term surgical outcome of hepatobiliary resections for perihilar cholangiocarcinoma in the last 5 years.
Summary Background Data:
Hepatobiliary resection for perihilar cholangiocarcinoma remains a technically demanding procedure, calling for a high level of expertise in biliary and hepatic surgery, and is still associated with significant morbidity or mortality.
Methods:
Between 2000 and 2004, we surgically treated 102 consecutive patients with perihilar cholangiocarcinoma with a management strategy consisting of preoperative biliary drainage, portal vein embolization (for right-sided and extended left-sided resections), and major hepatobiliary resection. The data on all of the patients were analyzed retrospectively to identify the factors that might significantly affect the postoperative mortality and morbidity.
Results:
There were no cases of in-hospital mortality or postoperative liver failure. Major complications were encountered in 7 patients (6.9%), and the overall morbidity rate was 50%. Reoperation was required in 2 patients (2%). The overall median length of postoperative hospital stay was 26 days (range, 13–119 days). Univariate analysis in relation to the postoperative morbidity showed significant differences in the preoperative occurrence of segmental cholangitis or cholecystitis (P = 0.015), the severity of postoperative hyperbilirubinemia (P < 0.001), and the total amount of fresh frozen plasma administered (P = 0.002). Multivariate analysis revealed a single independent significant predictive factor for postoperative morbidity, namely, preoperative cholangitis or cholecystitis (odds ratio, 9.08; 95% confidence interval, 1.05–78.56, P = 0.045).
Conclusions:
Our experience indicates that hepatobiliary resections for perihilar cholangiocarcinoma can be conducted safely, without a single case of postoperative liver failure or mortality. Occurrence of preoperative cholangitis or cholecystitis is a significant indicator for morbidity of major hepatobiliary resection.
A single-institute retrospective review of 102 consecutive patients with perihilar cholangiocarcinoma with a management strategy consisting of preoperative biliary drainage, portal vein embolization, and major hepatobiliary resection in the last 5 years was performed. Our experience indicates that hepatobiliary resections for perihilar cholangiocarcinoma can be conducted safely, with zero mortality.
Hepatobiliary resection for perihilar cholangiocarcinoma remains a technically demanding procedure, calling for a high level of expertise in biliary and hepatic surgery. Performance of major hepatectomy with caudate lobectomy1 has increased the resection rate of hilar cholangiocarcinoma. However, hepatobiliary resection for perihilar cholangiocarcinoma is a complex procedure involving lymphadenectomy, vascular resection and reconstruction, and pancreaticoduodenectomy (HPD)2 in selected situations. In addition, the majority of patients with perihilar cholangiocarcinoma have cholestatic liver injury. Consequently, hepatobiliary resection carries with a considerable risk of mortality and serious postoperative morbidity, such as liver failure.
Large single-center experience of more than 100 hepatobiliary resections have been reported rarely,3–5 and postoperative mortality rates of less than 5% are rare even in high-volume centers;6–9 this is very different from hepatectomy for hepatocellular carcinoma or metastatic liver tumors.10–13 Curative hepatobiliary resection with a low postoperative mortality offers the only chance for long-term survival in patients with perihilar cholangiocarcinoma. Factors affecting the morbidity or mortality associated with hepatobiliary resection still remain unclear; therefore, audit-elicited data must be culled from medical records to elucidate such factors for further refinement of hepatobiliary resection.
We implemented a management strategy, consisting of preoperative biliary drainage, portal vein embolization (PVE),14 and major hepatobiliary resection in patients with perihilar cholangiocarcinoma. The aims of this study were to review the short-term outcome of our strategy for hepatobiliary resection conducted over the last 5 years to treat perihilar cholangiocarcinoma and to analyze the risk factors effecting the surgical morbidity and mortality.
MATERIALS AND METHODS
Patients
Between January 2000 and December 2004, 135 patients were admitted to our department with a tentative diagnosis of perihilar cholangiocarcinoma. Fifteen patients were diagnosed as having highly advanced disease or poor hepatic functional reserve during the preoperative workup, and were excluded. Laparotomy was conducted in the remaining 120 patients. Eventually, 111 patients underwent hepatobiliary resection (resection rate, 82.2%), and 9 did not undergo any resectional surgery, including 1 who underwent palliative bile duct resection. Of these 111 patients, 6 with gallbladder neck or cystic duct carcinoma and 3 with benign biliary stricture pathologically proven postoperatively were also excluded. The remaining 102 patients, consisting of 63 cases (61.8%) of hilar bile duct cancer (HBC), and 39 cases (38.2%) of intrahepatic cholangiocarcinoma involving the hepatic hilus (ICC), were enrolled in this study. There were 31 women and 71 men, with a median age of 66 years (range, 34–78 years).
Preoperative Evaluation and the Strategies for Management
Initially, the location and extent of the disease were evaluated by ultrasonography, multiphasic helical computed tomography (CT), or magnetic resonance imaging (Fig. 1). The strategy summarized in Figure 1 was applied during this study period. Principally, we select percutaneous transhepatic biliary drainage (PTBD) under ultrasound guidance for patient without biliary drainage, if necessary. Both proximal and distal extension of the disease along the bile duct was evaluated by cholangiography conducted through the PTBD catheter, endoscopic retrograde cholangiography (ERCP), or magnetic resonance cholangiopancreatography (MRCP).
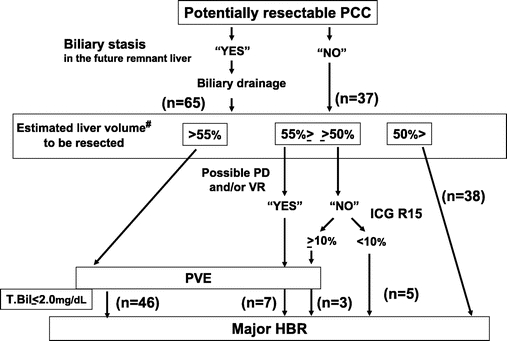
FIGURE 1. Decision criterion of strategy for preoperative management in perihilar cholangiocarcinoma. PCC, perihilar cholangiocarcinoma; PTBD, percutaneous transhepatic biliary drainage; PD, combined pancreaticoduodenectomy; VR, combined vascular resection and reconstruction; ICG R15, indocyanine green retention value at 15 minutes; PVE, portal vein embolization; HBR, hepatobiliary resection; T.Bil, serum total bilirubin level. #Three patients who did not undergo PVE due to the hepatic lobar atrophy to be resected or the portal vein stricture to be embolized were excluded.
Our indication criteria for biliary drainage included: 1) a relief of obstructive jaundice (total bilirubin value greater than 3 mg/dL), 2) a decompression of the biliary tree in the future remnant liver (maximum diameter of the biliary tree > 7 mm), and 3) a treatment of segmental cholangitis (clinically proven). Fifty-seven patients with obstructive jaundice (serum total bilirubin concentration of 11.5 mg/dL; range, 3.1–40.9 mg/dL) underwent preoperative biliary drainage. Six other patients were not jaundiced but received biliary drainage because their intrahepatic bile ducts in the future remnant liver was dilated of 9.9 mm in the maximum diameter (range, 7.6–12.8 mm). Two other patients also underwent biliary drainage for treating cholangitis (positive culture of the drainaged bile was confirmed). Thus, a total of 65 patients underwent biliary drainage before surgery, including PTBD in 58 patients and only endoscopic biliary drainage (ERBD) in 7. The remaining 37 patients underwent neither ERBD nor PTBD.
A single catheter was inserted in 39 patients and multiple catheters in 19 patients with PTBD. Thirty-seven patients (36%) underwent ERCP, and 14 of the 37 patients underwent ERBD at another institutes prior to referral to our hospital. Seven of the 14 patients with ERBD also eventually required PTBD owing to catheter obstruction and/or segmental cholangitis, and 1 patient required ERBD catheter exchange because of segmental cholangitis.
Standard contrast angiographic examination was performed to assess the extent of vascular involvement and to delineate the vascular anatomy in each case.
The volume of the entire liver and the part of the hepatic segment to be resected were calculated using CT volumetry.15 PVE for the liver segment to be resected has been advocated as a useful option to induce compensatory hypertrophy of the future remnant liver,14 and was indicated if the estimated resection volume exceeded 50% of the whole liver taking into consideration of the hepatic functional reserve or invasiveness of the procedure potentially concomitant vascular resection and/or HPD, and was carried out in 56 patients (55%). PVE was considered unnecessary in the following circumstances: documented atrophy of the hepatic lobe to be resected in 1 patient, and obvious portal venous stricture to be embolized due to tumor involvement in 2 patients. After PVE, 2 patients underwent additional transcatheter hepatic arterial partial embolization aiming at further atrophy of the liver to be resected and compensatory hypertrophy of the future remnant liver.
The estimated resection liver volume prior to the resectional surgery was 46.0% (range, 9.0%–71.9%). The mean indocyanine green retention rate at 15 minutes after relief of jaundice was 7.0% (range, 0.2%–63.2%). Definitive surgery was planned 2 to 4 weeks after PVE and was usually carried out when the serum total bilirubin level decreased to below 2 mg/dL. Pathologic staging of the disease was conducted according to the criteria of the International Union Against Cancer (6th Edition).
Surgery
At first, systematic lymphadenectomy for the nodes at the hepatoduodenal ligament and upper part of the retropancreatic, and celiac nodes was performed, followed by skeletonization of the hepatic hilus. After complete mobilization of the hemiliver to be resected, the caudate lobe was completely detached form the inferior vena cava (IVC). Liver transection was performed using the forceps clamp crushing method during both hepatic artery and portal vein clamping for 15 minutes with 5 minutes intervals (Pringle's maneuver). The surgical procedures are summarized in Table 1. All the patients underwent hemihepatectomy or more extensive resection with en bloc resection of the caudate lobe and extrahepatic bile duct, and biliary tract reconstruction was conducted by bilio-enterostomy using a Roux-en-Y jejunal limb, and external biliary stents were placed across the bilio-enteric anastomosis.
TABLE 1. Surgical Procedures
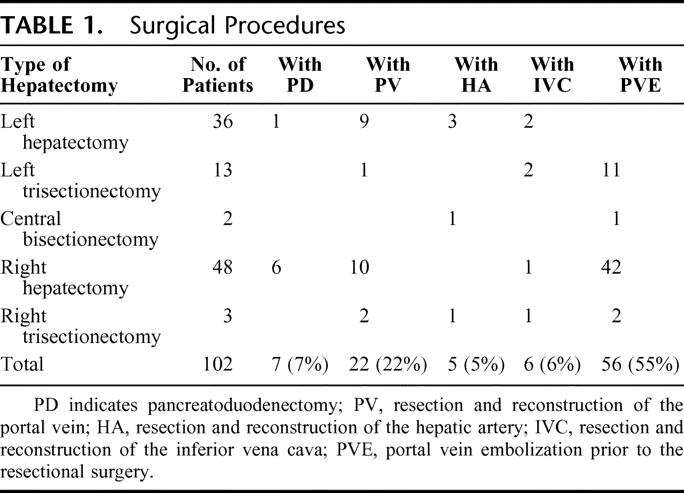
HPD was performed in 7 selected patients with extensive bile duct cancer to secure a negative distal bile duct margin. Reconstruction during HPD was conducted in one stage by end-to-side pancreaticojejunostomy using a modified Child's method. Portal vein resection and reconstruction was performed in 22 patients, including wedge resection with direct suture closure in 9 patients, segmental resection with end-to-end anastomosis in 12 patients, and segmental resection with left renal vein interposition graft in 1 patient. The right hepatic artery was resected in 4 patients who underwent left-sided hepatectomy, and the left hepatic artery was resected in 1 patient who underwent right hepatic trisectionectomy. Arterial reconstruction was performed using the right gastroepiploic artery in 3 patients and by direct end-to-end anastomosis in 2 patients. Combined IVC resection and reconstruction with direct suture closure was performed in 6 patients.
Standard Policy for Blood Product Transfusion
Red blood cell (RBC) transfusion was administered in patients with a hemoglobin concentration of less than 7.5 g/dL during the surgery, or less than 7.0 g/dL postoperatively.16 Fresh frozen plasma (FFP) was principally administered, at the discretion of the operating surgeon, in patients undergoing extensive liver resection exceeding 60% of the whole liver, poor hepatic functional reserve, or postoperative serum bilirubin concentration of more than 3.0 mg/dL.
Definition of Morbidity and Mortality
Operative mortality included all in-hospital deaths. As to morbidity, all postoperative complications that affected the outcome or lengthened the hospital stay were considered. Hepatic failure was defined as a serum total bilirubin concentration of higher than 10.0 mg/dL during the postoperative period;9 on the other hand, hyperbilirubinemia was defined as the total bilirubin level greater than 5.0 mg/dL. Bile leakage was diagnosed when the bilirubin concentration of the drainage fluid was higher than 5.0 mg/dL or higher than two-folds of the serum level in patients with hyperbilirubinemia.
Complications were defined as major when they resulted in organ failure or required another surgery or interventional radiology. On the other hand, complications such as pleural effusion necessitating thoracocentesis, wound infection, intra-abdominal infection with positive culture of the drainage fluid, delayed gastric emptying, anastomotic leakage, clinically silent pancreatic fistula with amylase-rich serous fluid or contaminated fluid with positive culture, and bile leakage from the raw surface of the liver healing spontaneously or responding to conservative management were classified as minor. Pulmonary embolism was diagnosed using lung perfusion scintigraphy or spiral CT. The postoperative course was divided into 3 categories: no morbidity, minor complications only, and major complications.
Statistics
Results are expressed as median values, with the respective ranges indicated within parentheses. The relationship between the postoperative morbidity and the dichotomous variables was evaluated by χ2 analysis or Fisher exact test, whichever was appropriate. Statistical significance of continuous variables was determined by the Mann-Whitney U test. One-way analysis of variance was used for multiple comparisons. A multivariate stepwise logistic regression analysis (backward elimination method) was performed to identify factors associated with postoperative morbidity. The survival curve for the 102 study patients was generated by the Kaplan-Meier method. Results were considered significant when the P values were less than 0.05. The statistical analyses were performed using a statistical analysis software package (SPSS 11.5, SPSS Inc., Chicago, IL).
RESULTS
All patients in this series were discharged from our hospital and could return directly to their homes in good condition, without having to be routed through other medical facilities. Patients were followed up at our outpatient clinic. Thus, 30 days, 60 days, and 90 days mortality were zero, and overall morbidity rate was 50%, with only minor complications observed in 44 patients (43.1%), and major complications in 7 patients (6.9%). None of the patients required special interventions, such as mechanical ventilation or hemodialysis, in the intensive care unit.
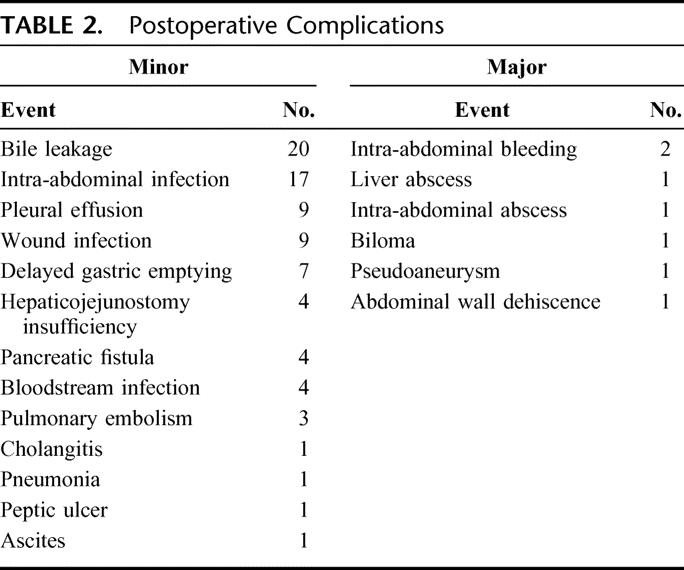
A total of 81 minor complications were documented in 51 patients (Table 2). Bile leakage from the liver transection surface, the most frequent complication, was observed in 20 (19.6%) patients. The maximum postoperative concentration of serum total bilirubin was 2.8 mg/dL (range, 0.8–9.2 mg/dL). Accordingly, there was no postoperative liver failure. Relaparotomy was required in 2 patients, due to intra-abdominal bleeding on the 6th postoperative day and abdominal dehiscence on the 9th postoperative days, respectively. In 5 patients, radiologic intervention was indicated owing to postoperative bleeding from transection surface of the liver, biloma, liver abscess, intraabdominal abscess, and pseudoaneurysm of the left hepatic artery.
TABLE 2. Postoperative Complications
Univariate Analyses
Preoperative Variables
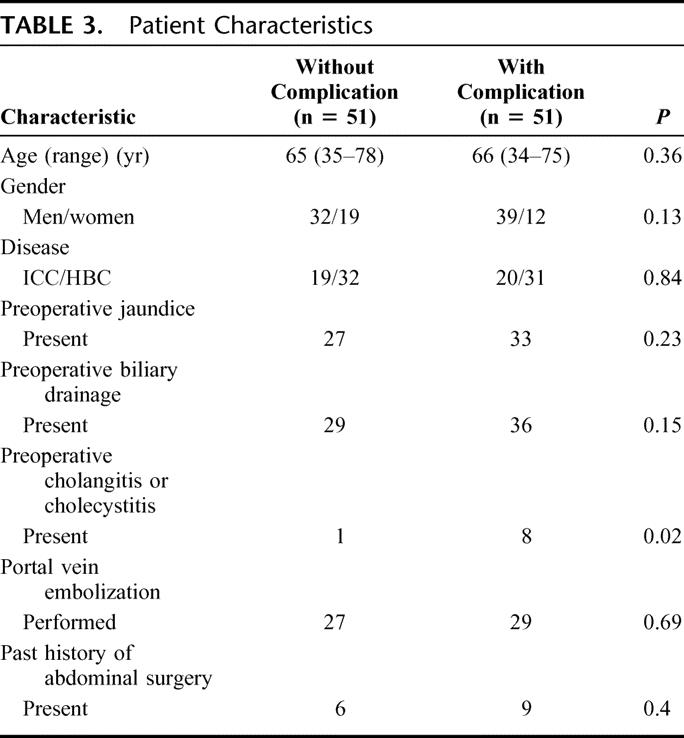
The patient characteristics are summarized in Table 3. The 8 clinicopathologic variables were compared. Preoperative cholangitis or cholecystitis requiring additional intervention was significantly frequent in patients with complications (P = 0.015). In other variables, there was no significant difference between patients with and without complication.
TABLE 3. Patient Characteristics
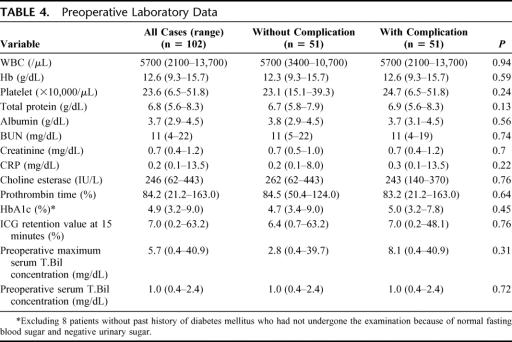
There were no significant differences in the 14 preoperative laboratory variables between patients with and without complications (Table 4).
TABLE 4. Preoperative Laboratory Data
Operative and Postoperative Variables
The median operative time was 648 minutes (range, 375–992 minutes); the median intraoperative blood loss was 1847 mL (range, 670–7350 mL); the total clamping time of the hepatic pedicle was 64.5 minutes (range, 25–174 minutes). Packed RBC transfusion was required intraoperatively in 34 patients (33%) (at a median of 4 units; range, 2–16 units), and FFP transfusion was administered perioperatively in 80 patients (78%) (at a median of 48 units; range, 6–398 units).
Histologically, the proximal bile duct, distal bile duct, and excisional margin were positive for cancer in 27, 15, and 16 patients, respectively. Consequently, R0 (curative resection) with histologically negative margins was achieved in 62 patients (61%).
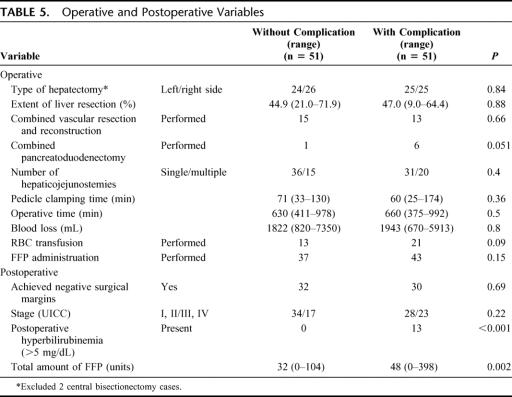
The 15 operative and postoperative variables were compared (Table 5). Postoperative hyperbilirubinemia greater than 5.0 mg/dL was more frequently documented in patients with complications (P < 0.001). Complications were more frequently found in patients who underwent HPD, although the difference did not reach statistical significance (P = 0.051). The total volume of FFP transfused was significantly higher in patients with complications than in those without (32 units; range, 0–104 units vs. 48 units; range, 0–398 units; P = 0.002).
TABLE 5. Operative and Postoperative Variables
Multivariate Analysis
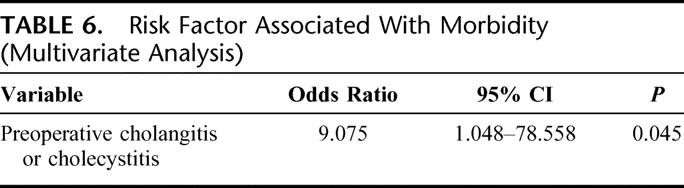
Multivariate analysis selected 22 preoperative and 10 intraoperative variables using a stepwise logistic regression model identified only an independent predictive factor for postoperative morbidity, preoperative cholangitis, or cholecystitis (Table 6).
TABLE 6. Risk Factor Associated With Morbidity (Multivariate Analysis)
Postoperative Hospital Stay and Survival
The overall median length of postoperative hospital stay was 26 days (range, 13–119 days) in all; it was 21 days (range, 13–33 days) in patients without complications, 36 days (range, 20–119 days) in those with only minor complications, and 58 days (range, 19–84 days) in those with major complications. The differences among the 3 groups were significant (patients with vs. without complications, P < 0.001; patients with minor versus, major complications, P = 0.031).
The overall 1-, 2-, 3-, and 5- year survival rates were 80.4%, 60.4%, 47.7%, and 44.0%, respectively (Fig. 2). Median survival and follow-up time was 34 months (range, 4–62 months) and 19 months (range, 4–62 months), respectively. Forty patients died of tumor recurrence, and the remaining 62 patients are now alive with recurrence (n = 20) or without any sign of recurrence (n = 42) at this writing.
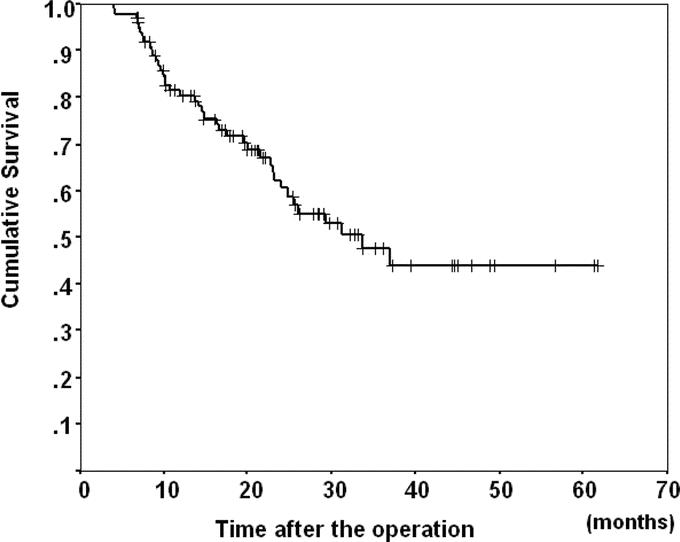
FIGURE 2. The survival curve for the 102 study patients. The overall 1-, 2-, 3-, and 5-year survival rates were 80.4%, 60.4%, 47.7%, and 44.0%, respectively.
DISCUSSION
Surgical treatment of hilar cholangiocarcinoma still remains a challenge for the surgeon, and the most striking result of this study was successful performance of hepatobiliary resection in more than 100 consecutive cases with zero mortality. The results of this study were accomplished by 6 attending surgeons, but not by 1 or 2 principal surgeons; therefore, our strategy (Fig. 1) can be used flexibly by hepatobiliary surgeons devoted to the surgical treatment of perihilar cholangiocarcinoma. Implementation of a standard approach by all team members played a vital role in the achievement of zero mortality in this study.
In 1999, we reported our surgical result for hilar cholangiocarcinoma in which 65 resected patients were involved with mortality of 9.2%.9 Thereafter, our protocol has been revised step by step, reaching to the present treatment strategy (Fig. 1). And also, we had refined the treatment protocol as is written in the manuscript, for example, routine preoperative check of bile culture for appropriate use of antibiotics. Perioperative septic complications considerably influence surgical outcome. To prevent severe septic complications, appropriate choice of antibiotics as well as effective drainage is required. Actually, our success might depend on a large volume of practice to some extent; however, we think that this success depends considerably upon refinement of treatment strategy.
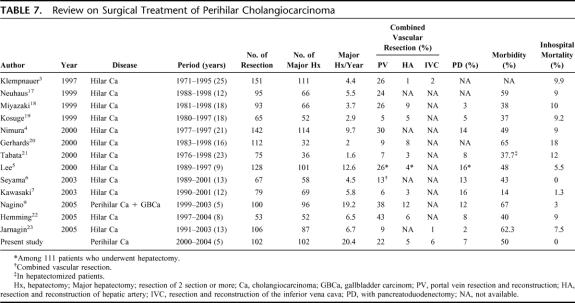
The results of recent literature review of a large series of hilar cholangiocarcinoma treated surgically (50 or more resection cases were analyzed) are summarized in Table 7. 3–7,9,17–23 The results revealed that institutes performing 4 or more major hepatectomies per year or having experience of greater than 50 major hepatectomies appeared to report mortality rates of less than 10%. These results are compatible with the reported impact of hospital surgical volume on the operative mortality for major surgery, that is, high-volume centers report low mortality rates.24 There has been no report until now of major hepatobiliary resection conducted in more than 100 cases with zero mortality. Our resection rate of 82.2% was by no means low when compared with previously reported resection rates for perihilar cholangiocarcinoma, ranging from 49.2% to 95%.3–5,7–9,17,19,21 The postoperative morbidity rate in our series was just 50%, which seems to be comparable to that reported in recent series, ranging 14% to 67%.
TABLE 7. Review on Surgical Treatment of Perihilar Cholangiocarcinoma
Most of the studies published until now on the prognosis of perihilar cholangiocarcinoma have analyzed various clinicopathologic variables, focusing on their influence on the long-term survival. In this study, we paid special attention to the short-term outcome because reduced postoperative mortality would clearly be expected to influence the overall survival. Factors affecting the postoperative mortality could not be identified because of the zero mortality in our series. However, one factor, namely, preoperative cholangitis or cholecystitis, was found by multivariate analysis to be independently associated with the postoperative morbidity. While the definition or severity of segmental cholangitis might not be uniform, our results were consistent with the observations of Kanai et al, who reported that preoperative segmental cholangitis was a significant prognostic factor affecting the postoperative morbidity and mortality in patients with malignant hilar obstruction.25
The role of preoperative biliary drainage is still under debate.26,27 Endoscopic biliary drainage is a useful method for stenting to counter middle or lower bile duct stricture prior to Whipple operation or bile duct resection; however, it entails the risk of catheter obstruction and ascending cholangitis, especially in patients with malignant biliary obstruction dividing the liver into multiple segments, as shown in our series. Cherqui et al28 reported the surgical results of major hepatobiliary resection without preoperative biliary drainage in 20 patients with biliary cancer; the postoperative liver failure rate was 5%, and mortality was documented in the same patients. The postoperative morbidity and transfusion requirement were also significantly higher in the patients with jaundice in that series. The ideal and ultimate biliary drainage method in patients with perihilar cholangiocarcinoma might be resection of the tumor followed by bilio-enteric anastomosis. However, we consider that the prime concern in the surgical treatment of perihilar cholangiocarcinoma should be an ideal balance between radicality and safety. From this point of view, PTBD, but not ERBD, for avoiding future cholestatic liver injury in the remnant lobe, would be more advisable in patients undergoing hepatobiliary resection, to reduce the potential risk of postoperative morbidity
Major hepatectomy with vascular resection and reconstruction might be considered as a high-risk surgery, and the mortality of this surgery with portal vein resection and reconstruction for hilar cholangiocarcinoma performed in series of 10 or more cases has been reported in the range of 9.6% to 40.0%.3,5,17,18,20,29 In our series, we did not hesitate to perform vascular resection and reconstruction in patients with potentially resectable tumors; thus, portal vein resection and reconstruction was performed in 22 patients with zero mortality and no significant increase of morbidity.
To secure negative bile duct margins and increase the curative resection rate, more extensive surgery, such as trisectionectomy or HPD, may seem to be ideal; however, the safety, efficacy, and indications of HPD in cases of biliary cancer remain controversial. Additional resection of liver volume beyond hemihepatectomy would be a burden, especially for patients with impaired liver function. As substantiated by several reports,2,30 the morbidity and mortality associated with HPD for biliary cancer still remains considerably high. In this study, the number of HPD cases was small, but the morbidity rate was higher in these cases than in those undergoing only hepatectomy, although the difference did not reach statistical significance (morbidity rate 86%; P = 0.051). We selected one-stage reconstruction of pancreaticojejunostomy (P-J) during HPD. Taking into consideration the invasiveness of the procedure, 2-stage P-J might be an alternative in the high-risk patients.31
Only a few papers have described the use of FFP for hepatectomy;9,10,12,16,23,32 the majority of reports indicate the use of packed RBC or whole blood as the blood products used during hepatectomy. We had routinely used FFP during hepatectomy for cirrhotic livers or hepatobiliary resection for biliary malignancies. The main purpose of using FFP is to induce volume expansion without homologous RBC transfusion,16 which is associated with the risk of postoperative hyperbilirubinemia.9,16 However, liberal use of FFP may increase the perioperative cost and entail a risk of transmission of infectious diseases. Recently, Nagino et al9 reported a large series of hepatobiliary resection where they sought preoperative blood donation by the patients. In that series, the mean operative blood loss was 1850 mL, comparable to our result, and the frequency of homologous transfusion was 35%. It is essential to achieve a balance between safety of surgery and changing policy for curtailment of blood products, such as FFP.
CONCLUSION
Despite the relatively high morbidity, hepatobiliary resection was accomplished in 102 consecutive patients of perihilar cholangiocarcinoma with zero mortality with our strategy. Our aggressive surgical approach for cases of perihilar cholangiocarcinoma, involving PTBD, PVE, and major hepatectomy, has been established as a safe management strategy; however, there is room for further refinements to achieve even better short-term outcomes, especially in terms of the postoperative morbidity and blood product requirements.
ACKNOWLEDGMENTS
The authors thank Professor Tadatoshi Takayama (Department of Digestive Surgery, Nihon University School of Medicine, Tokyo) for his critical review of the manuscript.
Footnotes
Supported in part by a Grant-in-Aid for scientific research from the Ministry of Health and Welfare of Japan.
Reprints: Tsuyoshi Sano, MD, Hepato-Biliary and Pancreatic Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045 Japan. E-mail: tsano@ncc.go.jp.
REFERENCES
- 1.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543. [DOI] [PubMed] [Google Scholar]
- 2.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology. 1991;38:170–175. [PubMed] [Google Scholar]
- 3.Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. [DOI] [PubMed] [Google Scholar]
- 4.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–62. [DOI] [PubMed] [Google Scholar]
- 5.Lee SG, Lee YJ, Park KM, et al. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–141. [DOI] [PubMed] [Google Scholar]
- 6.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagino M, Kamiya J, Arai T, et al. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148–155. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 13.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 14.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 15.Nagino M, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434–439. [PubMed] [Google Scholar]
- 16.Makuuchi M, Takayama T, Gunven P, et al. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644–648. [DOI] [PubMed] [Google Scholar]
- 17.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki M, Ito H, Nakagawa K, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg. 1999;189:575–583. [DOI] [PubMed] [Google Scholar]
- 19.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma: a single center experience. Surgery. 2000;127:395–404. [DOI] [PubMed] [Google Scholar]
- 21.Tabata M, Kawarada Y, Yokoi H, et al. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:148–154. [DOI] [PubMed] [Google Scholar]
- 22.Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 25.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- 26.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. [DOI] [PubMed] [Google Scholar]
- 28.Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. [DOI] [PubMed] [Google Scholar]
- 29.Ebata T, Nagino M, Kamiya J, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Angelica M, Martin RC 2nd, Jarnagin WR, et al. Major hepatectomy with simultaneous pancreatectomy for advanced hepatobiliary cancer. J Am Coll Surg. 2004;198:570–576. [DOI] [PubMed] [Google Scholar]
- 31.Miyagawa S, Makuuchi M, Kawasaki S, et al. Second-stage pancreatojejunostomy following pancreatoduodenectomy in high-risk patients. Am J Surg. 1994;168:66–68. [DOI] [PubMed] [Google Scholar]
- 32.Ekberg H, Tranberg KG, Andersson R, et al. Major liver resection: perioperative course and management. Surgery. 1986;100:1–8. [PubMed] [Google Scholar]