Abstract
Summary Background Data:
High rate of complications has been reported following revascularization for acute limb ischemia (ALI). No adjuvant pharmacologic treatment, apart from anticoagulation and standard perioperative care, has been shown clinically effective.
Objective:
Aim of this study was to evaluate the effects of the prostacyclin analog iloprost as adjuvant to surgery for ALI.
Methods:
A total of 300 patients were randomly assigned to receive perioperative iloprost (intra-arterial, intraoperative bolus of 3000 ng, plus intravenous infusion of 0.5–2.0 ng/kg/min for 6 hours/day for 4–7 days following surgery), or placebo. The primary endpoint was the combined incidence of death and amputation at 3-month follow-up. Secondary endpoints were the incidence of each single major complication, total event rate, symptomatology, and tolerability.
Results:
The combined incidence of death and amputation was 19.9% in the placebo and 14.1% in the iloprost group (relative risk, 1.56; 95% confidence interval, 0.89–2.75, P = 0.12, Cox regression analysis). A statistically significant lower mortality (4.7%) was reported in patients receiving iloprost, compared with controls (10.6%; relative risk, 2.61; 95% confidence interval, 1.07–6.37, P = 0.03). The overall incidence of fatal plus major cardiovascular events was 33.1% and 22.8% in placebo and iloprost groups, respectively (relative risk, 1.61; 95% confidence interval, 1.04–2.49, P = 0.03). No serious adverse reactions occurred after iloprost administration, nor differences in the incidence of bleeding or hypotension between treatment groups.
Conclusions:
Although at lower levels than previously reported, our results confirm the severity of ALI. Iloprost as adjuvant to surgery significantly reduced mortality and overall major event rate. Further data are needed to support this finding, and to face a still open medical issue.
Acute limb ischemia is a medical emergency leading to high rate of complications. In this randomized, double-blind, placebo-controlled study in 300 patients with acute ischemia of lower limbs, iloprost administered as adjuvant treatment to surgery significantly reduced 3-month mortality and overall event rate.
Acute limb ischemia (ALI) is a serious medical emergency leading to high rate of complications, being not only limb- but even life-threatening, often despite early successful revascularization.1 Improvements in surgical techniques and perioperative patient care may have reduced the incidence of major complications in ALI patients over the years, but the results of trials published recently seem to document a persistent high risk, with reported 30-day amputation rate of 5% to 12%, mortality risk at 10% to 38%, combined incidence of amputation and death of 25% to 37.5%, at 1- to 6-month follow-up.2–7
Concomitant underlying diseases, the metabolic derangement that seems as a result of the acute insult, and a possible reperfusion injury following revascularization may account for this severe prognosis.8 Only anticoagulation, fasciotomy (when indicated), and perioperative supportive treatment are established strategies in ALI patients.1,8,9 Possible benefit from cardiovascular active therapies has recently been suggested in patients undergoing peripheral revascularization or noncardiac major surgical intervention.10,11 Moreover, several categories of compounds, potentially acting on pathobiological mechanisms of ischemia-reperfusion syndrome, have been tested in experimental models, but none of them has as yet been proven effective in clinical studies in patients with ALI.12–18
Because of their pharmacologic profile, prostanoids represent a potentially interesting category as adjuvant treatment of ALI patients.19 Several ischemia-reperfusion studies described the use of prostaglandins for reduction of postischemic tissue injuries, and even recently both PGE1 and PGI2 appeared as potent inhibitors of reflow-paradox in a preclinical model of reperfusion injury.20
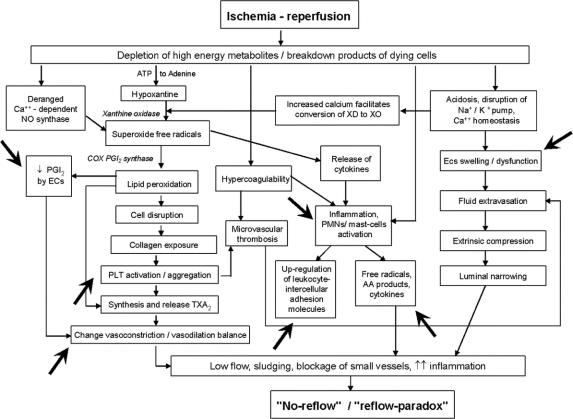
Iloprost is a widely studied synthetic analogue of prostacyclin, with a 10-fold higher half-life than the native compound, and indicated in the treatment of severe chronic limb ischemia.1,21–23 Results from pilot studies and case reports also described the positive effects of iloprost in the management of acute ischemia secondary to various causes, particularly after accidental intra-arterial administration of drugs or toxic agents.24–26 Several preclinical studies have assessed the effects of iloprost in experimental ischemia-reperfusion injury and documented the actions of the compound on different pathophysiologic mechanisms potentially relevant for damage following ALI.27–32 A diagram indicating where iloprost can interfere in the mechanisms, leading from ischemia and reperfusion, to the development of no-reflow and reflow-paradox, is reported in Figure 1. 33
FIGURE 1. Pathobiological mechanisms leading from ischemia-reperfusion, to “no-reflow”/“reflow-paradox.” Points where iloprost can act are indicated (from de Donato et al33).
Some years ago, we performed a placebo-controlled, double-blind pilot study in 30 patients with ALI undergoing Fogarty's thromboembolectomy. Encouraging results were obtained with the use of intraoperative and postoperative iloprost (lower incidence of major clinical events, more evident metabolic improvement by means of transcutaneous tensiometry).34
In this paper, we report the results of ILoprost in Acute Ischemia of Lower Limbs (ILAILL) study, a larger, multicenter trial including patients undergoing all types of surgical revascularization, who received iloprost or placebo administration during intervention and therefore for 4 to 7 days, and were observed for a 3-month postoperative period.
METHODS
Protocol
ILAILL was a multicenter, randomized, placebo-controlled, double-blind study performed between December 2000 and December 2003 in 22 Italian departments of vascular surgery (see list in the Appendix), under the endorsement of Italian Society for Vascular and Endovascular Surgery.
The aim of this study was to evaluate the effects on clinical outcome of perioperative treatment with iloprost in patients with acute ischemia of lower limbs undergoing surgical revascularization. Patients were considered for inclusion if they were at least 18 years of age and presenting acute rising (<14 days) of the symptomatology suggestive for acute ischemia of lower limbs (native vessels and/or graft occlusions), to be treated with surgical revascularization. The cutoff of 14 days to define acute ischemia was chosen on the basis of Trans-Atlantic Inter-Society Consensus1 indications and of what reported by previous studies in this setting.2,3
Patients were excluded if symptomatology persisted for more than 14 days, and in case of arterial occlusion due to crushing trauma. Women were not eligible if they were pregnant or breast-feeding. Other main reasons for exclusion were: acute myocardial infarction or ictus cerebri within the last 6 months prior to enrolment; cardiac failure (NYHA class >I); unstable angina; angina pectoris (Canadian classification >II); hyperkinetic ventricular arrhythmias; severe hypertension (sitting systolic blood pressure ≥180 mm Hg or sitting diastolic blood pressure ≥110 mm Hg) or hypotension (systolic blood pressure <90 mm Hg); hemorrhagic diathesis; concomitant clinical conditions in which iloprost might increase the risk of bleeding (ie, peptic ulcer in active phase, trauma, cerebral hemorrhage); thrombocytopenia (<80,000/mm3) or thrombocytosis (>500,000/mm3); severe hepatic failure (cirrhosis); renal failure requiring dialysis treatment.
Immediately before surgery patients were randomly assigned (in a ratio of 1:1 in blocks of four, stratified according to center) to receive iloprost or placebo.
Iloprost (Endoprost, Italfarmaco S.p.A., Milan, Italy, under license of Schering AG, Berlin, Germany) or placebo were administered as an intra-arterial bolus of 3000 ng over 1 to 3 minutes immediately after revascularization and in the affected artery, according to previous clinical experiences.34–36 Starting from the first day after surgery, a daily 6-hour intravenous infusion of iloprost (or placebo) at doses recommended for chronic critical limb ischemia was performed for 4 to 7 days (7 days recommended), depending on the length of hospital stay. Initial intravenous infusion rate corresponded to 0.5 ng/kg/min for 30 minutes. At 30-minute intervals, it was possible to increase infusion rate (by 0.5 ng/kg/min) up to 2.0 ng/kg/min, or to the maximum tolerated dose. This phase of dose adjustment occurred during the first 3 days after surgery; subsequently, the individual dose was maintained throughout the remaining treatment period. During infusion of experimental drug, strict monitoring of blood pressure and heart rate was requested.
Doppler examinations were performed before and after revascularization to assess the early outcome of surgery. Clinical assessment of patients' conditions occurred on the day of revascularization, before and after surgery, and thereafter on the first postoperative day, on the day of end of experimental treatment, and at 2 scheduled follow-up visits (at 30 ± 3 and 90 ± 5 days after surgery, respectively). During clinical controls, pain (by VAS scale, 0–10), paresthesia, motility, pallor, arterial peripheral pulses (by specific scores), and Ankle Brachial Index (ABI) at the affected limb were assessed. In details, a 4-point scale was adopted for paresthesia, indicating sensory loss (1 = none, 2 = minimal [toes], 3 = more than toes, associated with rest pain, 4 = profound, anesthetic), a 4-point scale for pallor (1 = absent, 2 = slight, 3 = moderate, 4 = marked), a 3-point scale for motility based on muscle weakness (1 = none, 2 = mild-moderate, 3 = profound, paralysis [rigor], and a 3-point scale for peripheral pulses (1 = present, 2 = reduced, 3 = absent).
Routine laboratory tests for safety reasons were performed before surgery, at the end of experimental treatment, and at the closure of follow-up.
Patients were instructed not to take any additional medication throughout the study, unless it was first reviewed by the investigators. The concomitant use of drugs potentially affecting blood coagulation and pressure was allowed with caution; in the case of bleeding or hypotension, discontinuation or dose reduction of study drug was required.
The study was conducted according to the ethical principles stated in the Declaration of Helsinki and local regulations. The protocol was approved by the Ethic Committee of each participating center, and written informed consent was obtained by all patients before randomization.
The primary efficacy outcome of ILAILL was assessed by the combined incidence of death and amputation, in the 2 treatment groups, during a 3-month follow-up postoperative period. Secondary efficacy endpoints were the incidence of each single major clinical event (death, amputation, acute myocardial infarction, stroke, peripheral embolism, pulmonary embolism, other major cardiovascular events) during the study, and the total event rate (fatal plus other major events). Events were considered “major” if they were fatal, or caused permanent disability, or hospitalization or increased duration of hospital stay. Evolution of symptomatology and ABI in the 2 groups were also evaluated.
Safety assessments consisted of recording of all the adverse events observed as well as spontaneously reported by patients during the trial, of the results of routine laboratory tests (red blood cell, white blood cell, platelet count, hemoglobin, tests for renal and liver function, cholesterol and triglycerides, glycemia, sodium, potassium, prothrombin time) and of monitoring of blood pressure and heart rate throughout study drug infusion and immediately after its interruption.
An independent Safety Committee reviewed all serious adverse events registered during the study, at 3 steps (after 50%, 75% of patients completed, and at the end of the trial).
Statistical Analysis
According to the available data concerning incidence of death and amputation, to the results of the above-mentioned pilot study,34 and on the basis of clinical experience of study Steering Committee, it was calculated that the sample size required to demonstrate a reduction in composite endpoint from 35% to 20%, by means of a 2-sided test with an alpha error of 0.05 and a beta error of 0.2, was 150 patients per group. Baseline numeric variables were compared between the treatment groups by unpaired t test, categorical variables were compared by χ2 test or Fisher exact test, as appropriate. The cumulative incidence of the outcomes was compared between groups by χ2 or Fisher exact test. The relative risks and their 95% confidence intervals (CI) were also calculated. The incidence of the outcomes was also analyzed by computing Kaplan-Meier event-free curves, and compared between groups by the log-rank test. Multivariable analysis, adjusting for potential confounders, was performed using the Cox proportional hazard regression model. The following variables were selected for the multivariable analysis, on the basis of their clinical plausibility and to assess study hypothesis: age (over 70 years vs. under or equal to 70 years); previous cardiovascular event(s) (acute myocardial ischemia, ictus cerebri, peripheral revascularization); duration of ischemia (lasting over 24 hours vs. less than or equal to 24 hours); class of ischemia (IIb–III vs. I–IIa); type of surgery (thromboembolectomy vs. other modalities); and experimental treatment (placebo vs. iloprost). Proportional hazard assumption was tested for all the covariates included and no relevant violations were found. Longitudinal clinical and laboratory data were analyzed by repeated-measures analysis of variance. All statistical tests were two-sided and P values below 0.05 were considered as significant. Statistical analyses were performed by using SAS software (version 8.2, SAS Institute, Cary, NC), on the basis of the intention-to-treat principle, ie, the primary analysis included all patients randomized to one of the 2 treatments regardless of the treatment actually received.
RESULTS
Study Populations
Three-hundred patients (151 in the placebo and 149 in the iloprost group) were enrolled in ILAILL study, received at least one dose of experimental treatment, and were included in the efficacy and safety analyses.
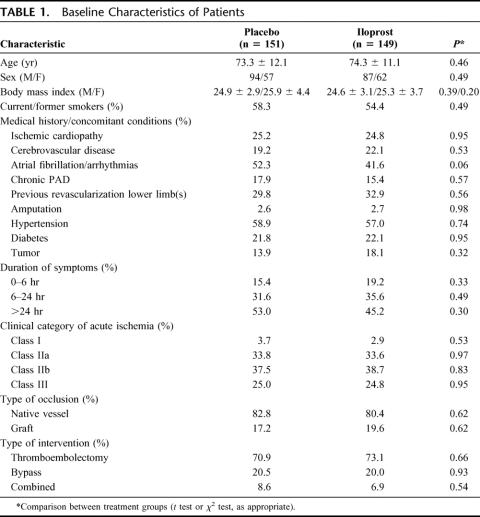
Baseline characteristics were similar between the 2 groups of patients (Table 1). To note, a high proportion of patients had symptoms lasting for more than 24 hours (53.0% and 45.2% in the placebo and iloprost group, respectively). Moreover, on the basis of clinical symptomatology (grade of sensory loss and muscle weakness) and arterial and venous Doppler signals, patients were classified at baseline in 4 categories (I, IIa, IIb, III) according to SVS/ISCVS–Trans-Atlantic Inter-Society Consensus criteria1,37: the majority of ILAILL patients (62.5% and 63.5% in the placebo and iloprost group, respectively) were globally classified in the 2 most severe categories (IIb or “immediately threatened,” and III or “irreversible”).
TABLE 1. Baseline Characteristics of Patients
Heparin (unfractionated or low-molecular weight) was administered postoperatively in 84.8% of patients receiving placebo and in 83.2% of those receiving iloprost; treatment with oral anticoagulants occurred in 28.5% and 31.5% of patients assigned to placebo or iloprost, respectively. Furthermore, the 2 groups did not differ with regard to the use of other therapies.
The duration of experimental treatment was similar between study groups: 81.7% of patients in the placebo group and 86.3% in the iloprost group received at least 4 intravenous infusions, and the maximum duration of treatment (7 days) was reached by 35.1% and 34.9% of patients assigned to placebo and iloprost, respectively.
Withdrawal from study before completion of 3-month follow-up and without occurrence of major clinical events requiring study discontinuation (see below) occurred in 13.2% of patients in the placebo and 13.4% of patients in the iloprost group, mainly due to patient noncompliance/patient lost to follow-up.
Efficacy Outcomes
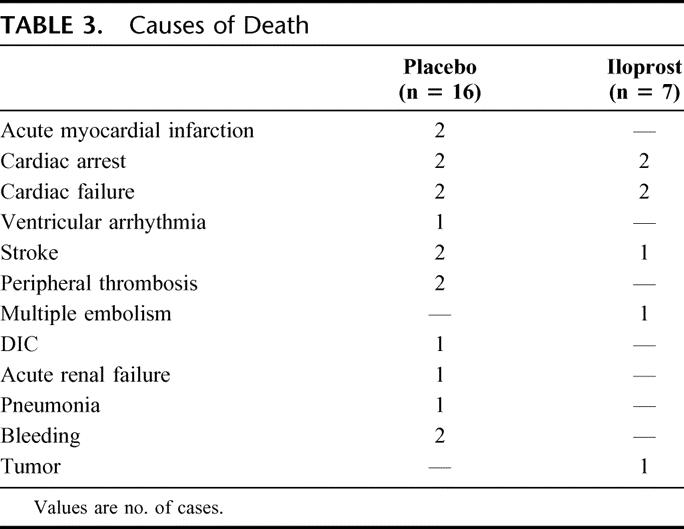
In Table 2, the incidence of major events in the 2 study groups is reported. The combined incidence of death + amputation (primary study endpoint) at the 3-month follow-up was 19.9% in the placebo and 14.1% in iloprost group. A lower mortality was reported in patients receiving iloprost: death occurred in 16 (10.6%) and 7 (4.7%) patients in placebo and iloprost group, respectively. Causes of death as reported by investigators are listed in Table 3. Amputation occurred in the same number of cases in placebo and iloprost patients (14 cases), whereas the overall incidence of fatal plus other major events (cardiovascular) was 33.1% in patients treated with placebo and 22.8% in those receiving iloprost.
TABLE 2. Occurrence of Major Clinical Events in the 2 Study Groups
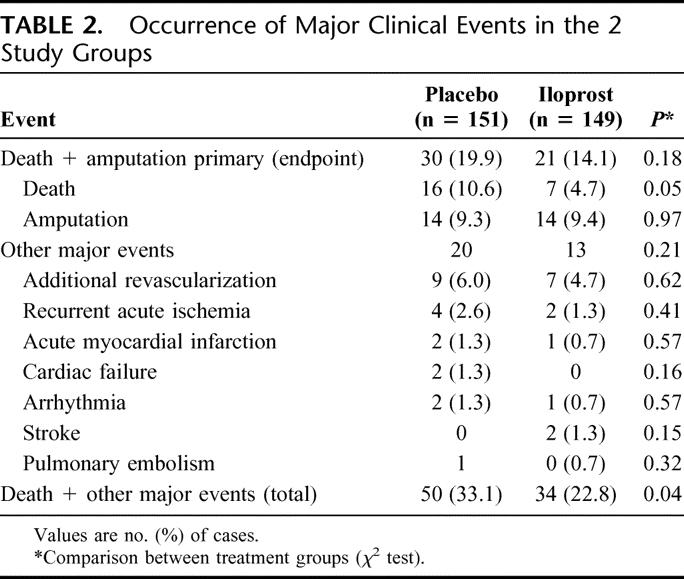
TABLE 3. Causes of Death
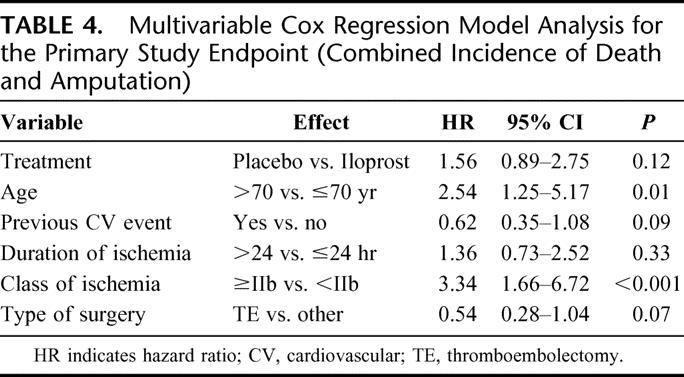
Multivariable analysis by means of the Cox proportional hazard regression model were performed for composite incidence of death + amputation (primary study endpoint), death alone, and combined event rate (fatal plus major cardiovascular events). In the case of primary study endpoint, the hazard ratio between placebo and iloprost treatment was estimated 1.56 (95% CI, 0.89–2.75, P = 0.12). Age >70 years was significantly related with a higher risk. In this category of patients the incidence of death + amputation appeared significantly reduced in those treated with iloprost in a posthoc analysis (hazard ratio, 1.99; 95% CI, 1.05–3.76, P = 0.03). A higher risk of death or amputation was reported in patients with class of ischemia ≥IIb, while a trend for a lower risk was documented for patients undergoing thromboembolectomy (vs. other surgical procedures), and for those experiencing a previous cardiovascular event (Table 4).
TABLE 4. Multivariable Cox Regression Model Analysis for the Primary Study Endpoint (Combined Incidence of Death and Amputation)
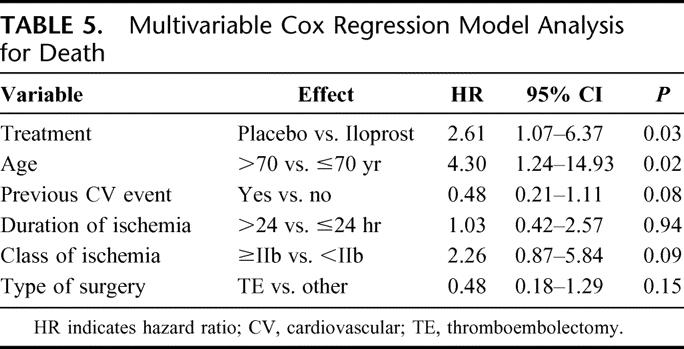
In the same multivariable analysis model, mortality appeared to be significantly reduced by the use of iloprost (hazard ratio, 2.61; 95% CI, 1.07–6.37, P = 0.03) (Table 5). Death was significantly more frequent in elderly patients (more than 70 years old), and a trend for fatal outcome was reported for patients with class of ischemia equal or higher than IIb. Similar to the combined endpoint, patients with previous cardiovascular events showed a trend for lower risk of death.
TABLE 5. Multivariable Cox Regression Model Analysis for Death
By means of multivariable Cox regression analysis, in the case of the combined incidence of fatal plus major cardiovascular events, a statistically significant lower rate occurred in the group of patients treated with iloprost compared with control (hazard ratio, 1.61; 95% CI, 1.04–2.49, P = 0.03).
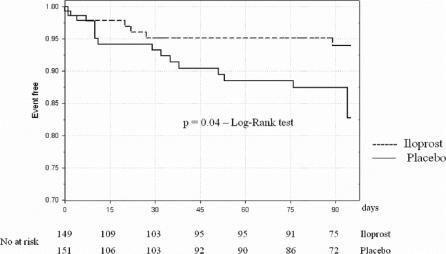
In Figure 2, the Kaplan-Meier curve for survival is shown; it can be noted how the difference between the placebo and iloprost groups is evident mainly starting from 3 to 4 weeks after surgery.
FIGURE 2. Kaplan-Meier estimate for survival in the 2 treatment groups.
In 5 patients of the placebo group and in 2 patients treated with iloprost, study was interrupted following the investigator's decision due to “lack of effect” and patients assigned to open treatments not allowed by study design.
Symptoms and signs of ALI (pain, motility, pallor, paresthesia), peripheral pulses and ABI were significantly improved immediately following surgical revascularization, with no difference between study groups. Moreover, not significant further improvements occurred over the time for patients completing the 3-month follow-up period, at similar extent for both patients receiving placebo and iloprost.
Safety Outcomes
Adverse events were reported in 76 patients (50.3%) in the placebo and in 78 (52.3%) patients in the iloprost group (not significant). Study interruption due to adverse events occurred in 2 patients treated with placebo (1.3%) and in 3 (2.0%) of those receiving iloprost. Figure 3 shows the incidence of the more frequent types of event. Headache and flushing were reported in more patients treated with iloprost than in the control group (P < 0.001 and P = 0.04, respectively). No different effects on blood pressure (neither hypotension nor hypertension) were associated to the use of iloprost, if compared with placebo. Bleeding has been reported in 6 patients of iloprost group, and in 5 patients receiving placebo. All the cases in iloprost group (2 intraoperative, 1 case each of wound bleeding, inguinal hematoma, urinary bleeding, epistaxis) were considered as minor episodes, while 2 cases in the placebo group were fatal (Table 3) and occurred in patients receiving oral anticoagulants. No clinically relevant changes in routine laboratory tests considered to be related to study treatment were detected.
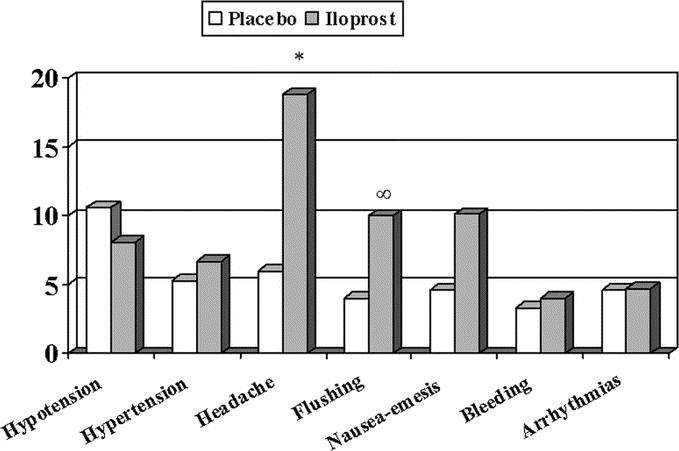
FIGURE 3. Incidence of the more frequent adverse events in the placebo and iloprost groups. *P < 0.001. ∞P = 0.04, between treatments.
DISCUSSION
A high level of major complications following surgery for acute limb ischemia has been shown in many papers in the past few years, and even recently ALI was reported to be affected by a worst outcome (higher incidence of above-knee amputation, trend toward higher mortality following surgery) than chronic limb ischemia, a condition with a well-known severe prognosis38.
In this paper, we describe the results of a 3-month follow-up in a population of 300 patients with ALI undergoing surgical revascularization and randomly assigned to perioperative adjuvant treatment with iloprost or placebo.
As a first evidence, in our study overall amputation free-survival was higher than expected on the basis of available data. Possible explanations for this fact are the development of further recent improvements in surgical techniques and patient care and the setting of centers participating to this study, made of a restricted number of highly specialized vascular surgery units. This fact may have affected statistical power as for primary endpoint: in the iloprost group a lower combined incidence of death and amputation was reported (relative risk reduction, 35.9%; 95% CI, 12.7%–63.6%), but this difference did not reach statistical significance. The reduction of death and amputation incidence in patients treated with iloprost was however statistically significant in higher risk, elderly patients.
No difference between iloprost and placebo group occurred in the incidence of amputation, while a small trend in favor of patients receiving iloprost was documented as for other peripheral complications (additional revascularization, recurrence of acute ischemia). This result may conflict with study rationale, and the hypothesis that iloprost could reduce peripheral major complications by improving microcirculation. The timing of occurrence of amputations, quite early after revascularization (median, 8.5 and 13.5 days after placebo and iloprost, respectively), seems to suggest a fundamental role for surgery (and grade of ischemia) in determining possible limb salvage, and a need for more aggressive and optimized pharmacologic support to improve peripheral outcome.
On the other side, the major result of our study is a significant reduction of 3-month mortality in patients treated with iloprost, in comparison to control group (relative risk reduction, 61.7%; 95% CI, 6.5%–84.3%). Such an indication, waiting for further data on this topic, seems to us of particular interest and deserves possible explanations. It is well known how there are 2 components to the reperfusion syndrome, a local one that can result in increasing the regional damage from ischemia and a systemic component potentially leading to secondary failure of organs remote from ischemic tissues. Breakdown products of dead and dying cells, oxygen-free radical production, hypercoagulability, release of cytokines, activation of neutrophils, and endothelial cell dysfunction are mechanisms following ischemia and reperfusion that contribute to the development of an inflammatory response and set up a vicious cycle that may remain primarily local or be active both at regional and systemic level.8 In our study, causes of death are related, in high proportion, to failure of organs (heart, kidney, lung) known as typical target of reperfusion syndrome. Similar to what has been reported for myocardial ischemia,39 it can be speculated that mortality following skeletal muscle damage could be related to a significant increase of inflammatory markers primarily produced at the level of ischemic site.
Iloprost is known to modulate many of the mechanisms involved in inflammatory response and systemic damage following ischemia and reperfusion (Fig. 1). The effects on platelet activation and blood clotting, the reduction of free radicals and cytokines production, and lower expression of intercellular adhesion molecules have been described in different patient populations.21,30,32,40 A cytoprotective effect of iloprost toward peripheral ischemic damage has been previously described,41 and interesting data on this topic were obtained in a pilot study we performed some years ago in patients undergoing surgical reperfusion for ALI.34 In that experience, a statistically significant more evident reduction of transcutaneous pCO2 (an index of tissue resistance to ischemia42) was documented in patients treated with iloprost in comparison to controls. Moreover, of particular potential interest is the neutrophil-target activity of iloprost, since these cells have been recently claimed to be specifically involved in the mediation of remote organ injury following ischemia and reperfusion.16,43,44
A further interesting point is that reduction of mortality in the iloprost group versus control patients becomes evident starting from 3 to 4 weeks after revascularization. This finding may result hard to explain for a treatment limited to 4 to 7 days after surgery, but long-lasting effects of iloprost have been reported both on pharmacologic and clinical side,22,45 and early modulation of inflammatory self-perpetuating response may produce later benefit in patients' outcome.
In our study, the use of iloprost was associated to a quite good tolerability. No serious adverse events were judged to be related to study treatment, by an independent Safety Committee. This appears to us of relevance, since in our study iloprost was used in emergency conditions, and not in patients with chronic diseases, or as adjuvant to elective surgery, as previously reported.39 Moreover, in a large proportion of our patients, iloprost was administered in combination with anticoagulant treatments (heparin, oral anticoagulants). Adverse events like headache, flushing, and nausea-emesis are well known in the pharmacologic profile of iloprost, and not surprisingly, they occurred more frequently in patients receiving the prostacyclin analog. Of interest, no difference in comparison to placebo was reported in patients receiving iloprost, neither for the occurrence of alterations in blood pressure (hypotension, hypertension) nor for bleeding events.
From a general point of view, a finding of potential clinical interest is the absence of a relationship between duration of ischemia and patients' outcome. To assess this variable, we tested a cutoff of 24 hours, but even a more rigid one (6 hours, by means of a Cox regression hazard model), and we obtained the same qualitative result. Duration of ischemia has been reported as a predictor of the occurrence of major complications in patients with ALI,1 and data from our study, since it was not powered to address this point, can not hardly conflict with this indication. However, this finding, together with the evidence that in our study amputation-free survival of patients classified as class III (“irreversible”) was 70.3% (and 59.6% of class III patients were free of serious events during the observation period), suggests the opportunity of considering a more aggressive treatment strategy even in patients with severe conditions or late presentation, particularly if they are admitted to specialized care units.
Another finding of interest, and apparently surprising, is a trend for a minor occurrence of complications in patients experiencing a previous major cardiovascular event (acute myocardial infarction, ictus cerebri, peripheral revascularization). We can't give precise indications on this topic because of the different objectives of the study, but some speculations can be made to interpret these data, suggesting the hypothesis of a minor intense inflammatory response to acute ischemic insult in atherosclerotic “stabilized” patients,46,47 and the value of a more frequent use of concomitant therapies active at cardiovascular and metabolic level. Furthermore, a speculative supportive interpretation to this finding may derive from the evidence of protective effects from ischemia-reperfusion damage by ischemic preconditioning,48 although short-lasting. Interesting clinical data reported more favorable neurologic recovery and the development of smaller-volume cerebral infarcts in patients who experienced transient ischemic attacks prior to sustaining stroke.49,50
CONCLUSION
Our study confirms that, although at levels lower than previously reported, and even when treated in specialized vascular surgery departments, acute limb ischemia is still a medical emergency with high rate of complications. The use of iloprost as adjuvant to surgical revascularization did not affect amputation rate at a 3-month follow-up, but it significantly reduced all-cause mortality, and the overall incidence of death and other major events. This finding needs further evidence, but it seems encouraging to increase the efforts of basic researchers and clinicians to improve the present severe prognosis of patients with acute limb ischemia.
ACKNOWLEDGMENTS
The authors thank the Centres of Vascular Surgery (see list below) participating to the ILAILL Study; to Carlo Bianchini for his scientific advice; to Patrizia Ronchetti and Luisa Siori for secretarial assistance; to Evelina Curatolo, Laura Filiberti, Agostino Lazzaro, Milena Meo, Marzia Martusciello, Matteo Mondellini, Laura Omoboni, Giuseppe Portera, Rossella Rivolta, Elisa Robberto, Antonella Stroppolo, actually or formerly at MDS Pharma Services, Milan, Italy, for monitoring of the study and data management; to Georg Groetzbach (Schering AG, Berlin, Germany) and Laura Ottolenghi (Schering Italy, Milan) for kindly providing study drug; and to the patients included in this study, for their trust and support.
Appendix
The Members of the ILAILL Study Group were as follows: Steering Committee, G. de Donato (Chairman), G. Paroni, C. Setacci, P. Settembrini; Writing Committee, G. M. Andreozzi, G. de Donato, G. de Donato, G. Gussoni, R. Martini, A. Mazzone, F. Setacci; Safety Committee, E. Bonizzoni, A. Mazzone, A. Odero; Data Analysis, F. Veglia; Endorsement, Italian Society for Vascular and Endovascular Surgery; Clinical Centers (at the time of ILAILL study), Department of Vascular Surgery, Hospital “San Giovanni Bosco,” Naples: G. de Donato, R. de Laurentiis, G. Bianco; Unit and Chair of Vascular Surgery, Policlinico “Le Scotte,” University of Siena: C. Setacci, I. Baldi, G. de Donato; Unit and Chair of Vascular Surgery, Hospital “Careggi,” University of Florence: C. Pratesi, R. Pulli, E. Romano; Unit of Vascular Surgery, Hospital “Civico,” Palermo: A. Martino, G. La Marca; Vascular Surgery, Hospital “San Maurizio,” Bolzano: H. Ebner, P. Sbraga, F. Zaraca; Department of Vascular Surgery, University of Messina: F. Spinelli, T. Mandolfino, F. Benedetto, D. Baccellieri; Vascular Surgery Unit, Hospital “Cisanello,” Pisa: M. Ferrari, D. Adami, A. Del Corso; Department of Vascular Surgery, Hospital “Casa Sollievo della Sofferenza,” S. Giovanni Rotondo: G. Paroni, M. Ruggieri; Department of Vascular Surgery, Hospital “S. Croce e Carle,” Cuneo: C. Novali, B. Mangiacotti; Unit of Vascular Surgery 1, Hospital “San Giovanni Battista,” Turin: F. Ponzio, G. Capaldi; Department of Vascular Surgery, University of Perugia: P. Cao, B. Parente, G. Parlani; Chair of Vascular Surgery, University of Milan, Hospital “S. Carlo”: P. Settembrini, P. Maltempi; Chair of Vascular Surgery, Hospital “S. Martino,” Genoa: S. Ferrero, P. Colotto, L. Nardella, S. Pastorino, G. Rauti; Chair of Vascular Surgery, “Vita-Salute” University, Scientific Institute H. San Raffaele, Milan: R. Chiesa, E.M. Marone, F. Setacci; Deparment of Surgery, Unit of Vascular Surgery, ASL 1, Imperia: C. Bertoglio, A.M. Cristiani, T. Carissimi; Chair of Vascular Surgery, University of Padua: G. Deriu, M. Antonello; Department of Vascular Surgery, Hospital “Mauriziano,” Turin: D. Palombo, F. Nessi, P. Cumbo, E. Ferrero; Department of Vascular Surgery, Hospital “G. Salvini,” Garbagnate Milanese: R. Mattassi, E. Callini; Chair of Vascular Surgery, University Tor Vergata, Rome: A. Ippoliti, A. Ascoli Marchetti, L. Di Giulio; Department of Vascular Surgery, University of L'Aquila: C. Spartera, C. Petrassi, G. Saracino; Chair of Vascular Surgery, University of Milan, Hospital “Bassini,” Cinisello Balsamo: G. Biasi, P. Mingazzini, Y. Thsomba; Division of Vascular and Endovascular Surgery, University Hospital Policlinico, Bari: G. Regina, G. Impedovo, A. Lillo, D. Angiletta, V. Marotta.
Footnotes
Supported by a grant from Italfarmaco S.p.A., Milan, Italy. The study drug was kindly provided by Schering AG, Berlin.
Reprints: Gianmarco de Donato, MD, Unità Operativa di Chirurgia Vascolare ed Endovascolare, Università di Siena-Policlinico “Le Scotte,” Viale Bracci, 53100 Siena, Italy. E-mail: dedonato@unisi.it.
REFERENCES
- 1.Management of Peripheral Arterial Disease (PAD). Trans-Atlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31(suppl):1–296. [PubMed] [Google Scholar]
- 2.STILE Investigators. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischaemia of the lower extremity. Ann Surg. 1994;220:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouriel K, Veith FJ, Sasahara AA, et al. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. N Engl J Med. 1998;338:1105–1111. [DOI] [PubMed] [Google Scholar]
- 4.Aune S, Trippestad A. Operative mortality and long-term survival of patients operated on for acute lower extremity ischemia. Eur J Vasc Endovasc Surg. 1998;15:143–146. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite BD, Davies B, Birch PA, et al. Management of acute leg ischemia in the elderly. Br J Surg. 1998;85:217–220. [DOI] [PubMed] [Google Scholar]
- 6.Nypaver TJ, White BR, Endean ED, et al. Non traumatic lower-extremity acute arterial ischemia. Am J Surg. 1998;176:147–152. [DOI] [PubMed] [Google Scholar]
- 7.Pemberton M, Varty K, Nydahl S, et al. The surgical management of acute limb ischaemia due to native vessel occlusion. Eur J Vasc Endovasc Surg. 1999;17:72–76. [DOI] [PubMed] [Google Scholar]
- 8.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630. [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Sacks D, Patel RI, et al. SCVIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Intervent Radiol. 2001;12:559–570. [DOI] [PubMed] [Google Scholar]
- 10.Poldermans D, Bax J, Kertai M, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major non-cardiac vascular surgery. Circulation. 2003;107:1848–1851. [DOI] [PubMed] [Google Scholar]
- 11.Henke PK, Blackburn S, Proctor MC, et al. Patients undergoing infrainguinal by-pass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage and mortality. J Vasc Surg. 2004;39:357–365. [DOI] [PubMed] [Google Scholar]
- 12.Walker PM, Lindsay TF, Labbe R, et al. Salvage of skeletal muscle with free radical scavengers. J Vasc Surg. 1987;5:68–75. [DOI] [PubMed] [Google Scholar]
- 13.Randomized trial of ridogrel, a combined thromboxane A2 synthase inhibitor and thromboxane A2/prostaglandin endoperoxide receptor antagonist, versus aspirin as adjunct to thrombolysis in patients with acute myocardial infarction: the Ridogrel versus Aspirin Patency Trial (RAPT). Circulation. 1994;89:588–595. [DOI] [PubMed] [Google Scholar]
- 14.Adams JG, Dhar A, Shukla SD, et al. Effect of pentoxifylline on tissue injury and platelet-activating factor production during ischemia-reperfusion injury. J Vasc Surg. 1995;21:742–748. [DOI] [PubMed] [Google Scholar]
- 15.Faxon DP, Gibbons RJ, Chronos NA, et al. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–1204. [DOI] [PubMed] [Google Scholar]
- 16.Chan RK, Ibrahim SI, Verna N, et al. Ischaemia-reperfusion is an event triggered by immune complexes and complement. Br J Surg. 2003;90:1470–1478. [DOI] [PubMed] [Google Scholar]
- 17.Kearns SR, Daly AF, Sheehan K, et al. Oral vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004;86:906–911. [DOI] [PubMed] [Google Scholar]
- 18.Nanobashvili J, Neumayer C, Fuegl A, et al. Combined L-arginine and antioxidative vitamin mollifies ischemia-reperfusion injury of skeletal muscle. J Vasc Surg. 2004;39:868–877. [DOI] [PubMed] [Google Scholar]
- 19.Rowlands TE, Gough MJ, Homer-Vanniasinkam S. Do prostaglandins have a salutary role in skeletal muscle ischemia-reperfusion injury? Eur J Vasc Endovasc Surg. 1999;18:439–444. [DOI] [PubMed] [Google Scholar]
- 20.Tauber S, Menger MD, Lehr HA. Microvascular in vivo assessment of reperfusion injury: significance of prostaglandin E1 and I2 on post-ischemic ‘no-reflow' and ‘reflow-paradox'. J Surg Res. 2004;120:1–11. [DOI] [PubMed] [Google Scholar]
- 21.Grant SM, Goa KL. Iloprost: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs. 1992;43:889–924. [DOI] [PubMed] [Google Scholar]
- 22.Andreozzi G, Allegra C, Biasi G, et al. Evaluation of a conservative treatment with iloprost in severe peripheral occlusive disease (POAD): GISAP Study. Int Angiol. 1994;13:70–74. [PubMed] [Google Scholar]
- 23.Loosemore TM, Chalmers TC, Dormandy JA. A meta-analysis of randomised placebo control trials in Fontaine stages III and IV peripheral occlusive arterial disease. Int Angiol. 1994;13:133–142. [PubMed] [Google Scholar]
- 24.Tait IS, Holdsworth RJ, Belch JJ, et al. Management of intra-arterial injection injury with iloprost. Lancet. 1994;343:419. [DOI] [PubMed] [Google Scholar]
- 25.Andreev A, Kavrakov T, Petkov D, et al. Severe acute hand ischaemia following an accidental intraarterial drug injection, successfully treated with thrombolysis and intraarterial iloprost infusion: case report. Angiology. 1995;46:963–967. [DOI] [PubMed] [Google Scholar]
- 26.Motz E, Gasparini D, Basadonna P, et al. Intra-arterial iloprost for limb ischaemia. Lancet. 1995;346:1295. [DOI] [PubMed] [Google Scholar]
- 27.Belkin M, Wright JG, Hobson RW II. Iloprost infusion decreases skeletal muscle ischemia-reperfusion injury. J Vasc Surg. 1990;11:77–82. [DOI] [PubMed] [Google Scholar]
- 28.Thomson IA, Egginton S, Hudlicka O, et al. Iloprost reduces leukocyte adhesion in skeletal muscle venules following ischaemia in a rat model of femorodistal bypass. Eur J Vasc Surg. 1994;8:335–341. [DOI] [PubMed] [Google Scholar]
- 29.Thomson IA, Egginton S, Simms MH, et al. Effect of muscle ischaemia and iloprost during femorodistal reconstruction on capillary endothelial swelling. Int J Microcirc Clin Exp. 1996;16:284–290. [DOI] [PubMed] [Google Scholar]
- 30.Mazzone A, Mazzucchelli I, Fossati G, et al. Iloprost effects on phagocytes in patients suffering from ischaemic diseases: in vivo evidence for down-regulation of αMβ2 integrin. Eur J Clin Invest. 1996;26:860–866. [DOI] [PubMed] [Google Scholar]
- 31.Bozkurt AK. Alpha-tocopherol (Vitamin E) and iloprost attenuate reperfusion injury in skeletal muscle ischemia/reperfusion injury. J Cardiovasc Surg. 2002;43:693–696. [PubMed] [Google Scholar]
- 32.Mazzone A, Faggioli P, Cusa C, et al. Effects of iloprost on adhesion molecules and F1+2 in peripheral ischemia. Eur J Clin Invest. 2002;32:882–888. [DOI] [PubMed] [Google Scholar]
- 33.de Donato G, Gussoni G, de Donato G. Is it possible to improve outcome in patients undergoing surgery for acute limb ischemia? Can iloprost, a prostacyclin analogue, be helpful? Chir Ital. 2004;56:769–780. [PubMed] [Google Scholar]
- 34.de Donato G, Sangiuolo P, Abbonizio C, et al. Embolie arteriose: embolectomia e prostanoidi. Quad Med Chir. 1995;11:2–6. [Google Scholar]
- 35.Hickey NC, Shearman CP, Crowson MC, et al. Iloprost improves femoro-distal graft flow after a single bolus injection. Eur J Vasc Surg. 1991;5:19–22. [DOI] [PubMed] [Google Scholar]
- 36.Iloprost Bypass International Study Group. Effects of perioperative iloprost on patency of femorodistal bypass grafts. Eur J Vasc Endovasc Surg. 1996;12:363–371. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischaemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 38.Campbell WB, Marriott S, Eve R, et al. Amputation for acute ischemia is associated with increased comorbidity and higher amputation level. Cardiovasc Surg. 2003;11:121–123. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopal V, Gurm HS, Bhatt DL, et al. Relation of an elevated white blood cell count after percutaneous coronary intervention to long-term mortality. Am J Cardiol. 2004;94:190–192. [DOI] [PubMed] [Google Scholar]
- 40.Della Bella S, Molteni M, Mascagni B, et al. Cytokine production in sclerodermia patients: effects of therapy with either iloprost or nifedipine. Clin Exp Rheumatol. 1997;15:135–141. [PubMed] [Google Scholar]
- 41.Andreozzi GM, Di Pino L, Li Pira M, et al. Iloprost, stable analogue of the prostacyclin, is able to improve the tissue resistance to ischaemia. Intern Angiol. 1994;13:68–69. [PubMed] [Google Scholar]
- 42.Andreozzi GM, Riggio F, Buttò G, et al. Transcutaneous pCO2 as an index of tissue resistance to ischaemia. Angiology. 1995;46:1097–1102. [DOI] [PubMed] [Google Scholar]
- 43.Hill J, Lindsay T, Valeri CR, et al. A CD18 antibody prevents lung injury but not hypotension after intestinal ischemia-reperfusion. J Appl Physiol. 1993;74:659–664. [DOI] [PubMed] [Google Scholar]
- 44.Baxter GF. The neutrophil as a mediator of myocardial ischemia-reperfusion injury: time to move on. Basic Res Cardiol. 2002;97:268–275. [DOI] [PubMed] [Google Scholar]
- 45.Rademaker M, Thomas RH, Provost G, et al. Prolonged increase in digital blood flow following iloprost infusion in patients with systemic sclerosis. Postgrad Med J. 1987;63:617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreozzi GM, Martini R. The fate of the claudicant limb. Eur Heart J. 2002;4(suppl. B):41–45. [Google Scholar]
- 47.Cordova R, Martini R, D'Eri A, et al. Flogistic arterial activity or own inflammatory attitude: what acts on PAD evolution? Int Angiol. 2003;22(suppl 1):21–22. [Google Scholar]
- 48.Pasupathy S, Homer-Vanniasinkam S. Ischaemic pre-conditioning protects against ischemia-reperfusion injury: emerging concepts. Eur J Vasc Endovasc Surg. 2005;29:106–115. [DOI] [PubMed] [Google Scholar]
- 49.Weih M, Kallenberg K, Bergk A, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain. Stroke. 1999;30:1851–1854. [DOI] [PubMed] [Google Scholar]
- 50.Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. [DOI] [PubMed] [Google Scholar]