To the Editor:
Klinkenbijl et al have previously reported the results of the European Organization for Research and Treatment of Cancer (EORTC) Trial # 40891 in Annals of Surgery.1 This trial was the second prospective randomized multicenter trial designed to evaluate the potential benefit of adjuvant chemoradiation (vs. observation) for patients with resected pancreatic cancers. The first trial was initiated in the United States by the Gastrointestinal Tumor Study Group (GITSG) in 1974, which was slow to accrue and was terminated early following an analysis of the first 43 patients that demonstrated a statistically significant survival advantage to adjuvant chemoradiation and maintenance chemotherapy in patients with resected adenocarcinoma of the pancreas.2,3 The EORTC trial was a larger-powered study designed to validate the result of the smaller GITSG trial, and adjuvant therapy was similar save for the fact that the GITSG study used maintenance chemotherapy while the EORTC trial did not.
Unlike the GITSG trial, the EORTC trial did not find a statistically significant benefit to adjuvant chemoradiation. One of several criticisms of this trial was that it allowed enrollment of nonpancreatic periampullary adenocarcinomas, which are well known to have better survival outcomes when compared with adenocarcinomas of the pancreatic head. To address these prepublication critiques, Klinkenbijl et al reported statistical analyses of not only survival in all eligible patients, but also for the subgroups of pancreatic head cancers and periampullary cancers separately. When pancreatic head cancers were analyzed as a subgroup, survival curves demonstrated consistent separation of the observation and treatment arms over time, indicative of a potential benefit to adjuvant chemoradiation (Figure 1). However, this difference did not reach statistical significance when tested with a two-sided log-rank test (P = 0.099).
FIGURE 1.
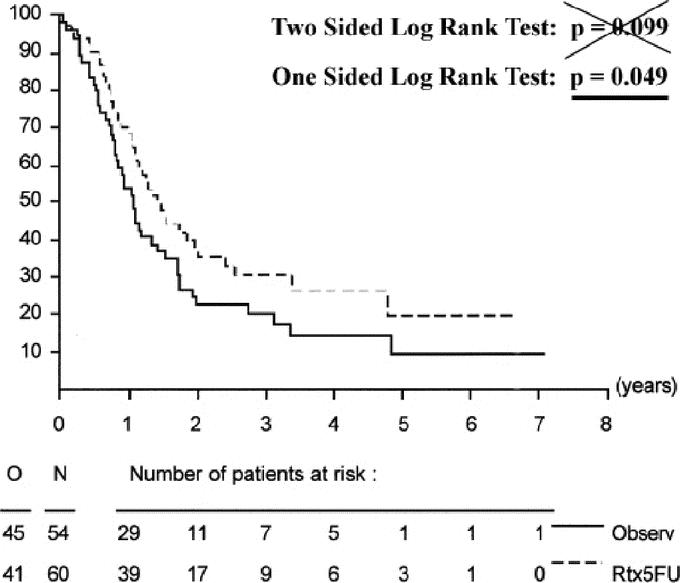
In retrospect, given the positive findings of the GITSG trial, which involved a similar treatment arm, a closer examination of the statistical design used in the EORTC trial is warranted. On reanalysis with more appropriate statistical methods, there is a statistically significant benefit to adjuvant chemoradiation for patients with pancreatic head cancers. The justification for such a reanalysis is rooted in the fact that the authors of the EORTC trial chose to use a two-sided log-rank test for statistical analysis at the 0.05 level of significance. However, there were no indications to use such a statistical design when this trial was conceived, and a one-sided log-rank test would have been most appropriate. A two-sided statistical design is only appropriate when there are data to suggest that the experimental therapy arm (adjuvant chemoradiotherapy) could be better or worse than the control arm.4 A one-sided log-rank test is appropriate for trials in which the experimental arm is being tested for improvement (not detriment) over the control arm (ie, when there is no reason to believe that the outcome of patients in the experimental arm would be significantly worse than the control arm).4
Given that the EORTC trial was designed in part to validate the GITSG study, the use of a two-sided log-rank test was inappropriate as there was no suggestion from the results of the GITSG trial that the survival of patients in the adjuvant chemoradiotherapy arm would be worse than the control arm of surgery alone; indeed, they implied a benefit. Further, in both the GITSG and the EORTC trials, chemoradiotherapy was safe and very well tolerated with no suggestions that it significantly contributed to grade 3, 4, or 5 toxicity. Therefore, a one-sided log-rank test should have been used to test for significance in the EORTC trial based on the fact that the previously published GITSG trial suggesting only a potential benefit from treatment and no suggestion of worsening/decreasing survival due to treatment and/or associated toxicity. If a one-sided log-rank test would have been used (as would have been appropriate), the 14% improvement in overall survival at 2 years (37% vs. 23%) favoring adjuvant chemoradiotherapy in patients with pancreatic head cancers would have reached statistical significance as illustrated in Figure 1 (P = 0.049).
Given the positive results of the GITSG and EORTC trials favoring adjuvant chemoradiotherapy, cooperative group protocols in the United States are conducting trials to refine adjuvant chemoradiotherapy.5 By contrast, our European colleagues have concluded that the EORTC trial and the subsequent ESPAC-1 trial were negative trials for adjuvant chemoradiotherapy.6 Consequently, present European cooperative group trials do not include radiation in any experimental adjuvant arm, which is reflective of this difference in opinion.7 It is unfortunate that this approach is currently being taken because, with the reanalysis presented above, the results of the EORTC trial demonstrate a benefit to adjuvant chemoradiotherapy. Together with the results of the GITSG trial, there is strong phase III evidence that patients may benefit from adjuvant chemoradiotherapy. In contrast, the controversial results of the ESPAC-1 trial do not support a benefit, and with the presented reanalysis of the EORTC trial, the ESPAC-1 trial now stands alone in this respect. Numerous criticisms of the ESPAC-1 trial have undermined the validity of its results and have been summarized previously in the literature.8–11
Michael C. Garofalo, MD
Department of Radiation Oncology
University of Maryland School of Medicine
Baltimore, MD
mgarofalo@umm
William F. Regine, MD
Department of Radiation Oncology, University of Maryland School of Medicine
Baltimore, MD
Ming T. Tan, PhD
University of Maryland School of Medicine
Marlene and Stewart Greenebaum Cancer Center
Division of Biostatistics
Baltimore, MD
REFERENCES
- 1.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. [DOI] [PubMed] [Google Scholar]
- 3.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. [DOI] [PubMed] [Google Scholar]
- 4.Green S, Benedetti J, Crowley J, eds. Clinical Trials in Oncology (Interdisciplinary Statistics). Boca Raton, FL: Chapman & Hall/CRC Press, 1997. [Google Scholar]
- 5.Radiation Therapy Oncology Group. RTOG 97-04. (accessible at http://rtog.org/members/protocols/97-04/97-04.pdf).
- 6.Neoptolemos JP, Stocken DD, Friess F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 7.ESPAC-3(v2) phase III adjuvant trial in pancreatic cancer comparing 5-FU and D-L-folinic acid vs gemcitibine. National Cancer Research Network Trials Portfolio (accessible at http://www.ncrn.org.uk/portfolio/data.asp?ID=669).
- 8.Choti MA. Adjuvant therapy for pancreatic cancer: the debate continues. N Engl J Med. 2004;350:1249–1251. [DOI] [PubMed] [Google Scholar]
- 9.Morris SL, Beasley M, Leslie M. Chemotherapy for pancreatic cancer [Letter to the editor]. N Engl J Med. 2004;350:2713. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Ben-Josef E, Small W. Chemotherapy for pancreatic cancer [Letter to the editor]. N Engl J Med. 2004;350(26):2713–14. [PubMed] [Google Scholar]
- 11.Bydder S, Spry N. Chemotherapy for pancreatic cancer [Letter to the editor]. N Engl J Med. 2004;350:2714. [PubMed] [Google Scholar]


