Abstract
Objective/Background:
Little is known about the epidemiology and the management of liver metastases from colorectal cancer at a population level. The aim of this population-based study was to report on the incidence, treatment, and prognosis of synchronous and metachronous liver metastases.
Methods:
Data were obtained from the population-based cancer registry of Burgundy (France).
Results:
The proportion of patients with synchronous liver metastases was 14.5%. Age-standardized incidence rates were 7.6 per 100,000 in males, 3.7 per 100,000 in females. The 5-year cumulative metachronous liver metastasis rate was 14.5%. It was 3.7% for TNM stage I tumors, 13.3% for stage II, and 30.4% for stage III (P < 0.001). The risk of liver metastasis was also associated to gross features. Resection for cure was performed in 6.3% of synchronous liver metastases and 16.9% of metachronous liver metastases. Age, presence of another site of recurrence, and period of diagnosis were independent factors associated with the performance of a resection for cure. The 1- and 5-year survival rates were 34.8% and 3.3% for synchronous liver metastases. Their corresponding rates were, respectively, 37.6% and 6.1% for metachronous liver metastases.
Conclusion:
Liver metastases from colorectal cancer remain a substantial problem. More effective treatments and mass screening represent promising approaches to decrease this problem.
The aim of this population-based study was to report on the incidence, treatment, and prognosis of synchronous and metachronous liver metastases from colorectal cancers. The age-standardized incidence rate of synchronous liver metastases was 7.6 per 100,000 in males and 3.7 per 100, 000 in females. The overall actuarial cumulative rate of metachronous liver metastasis was 4.3% at 1 year and 16.5% at 5 years. Stage at diagnosis was the most important determinant of the risk of metachronous liver metastasis. Surgical resection for cure is the only possibility to obtain long-term survival.
Relatively abundant published literature exists concerning the treatment and prognosis of liver metastases from colorectal cancer, but there is little information on their epidemiologic characteristics and on their management at a population level.1 Epidemiologic data can only be derived from figures relating to the entire population of patients with large bowel cancer within a particular area. Such studies are rare because they require accurate and detailed data collection. In particular, the active participation of the entire medical profession is needed, which is difficult to achieve for cancer registries collecting all cancer sites and covering large populations. This is the reason why data on the management and prognosis of liver metastases have mostly been provided by specialized hospital units and as such cannot be used as reference because of unavoidable selection bias. The aim of this study was to report on the incidence of synchronous and metachronous liver metastases, their management, and their prognosis, using data from a population-based series in France, covering a 25-year period.
MATERIALS AND METHODS
Population
A population-based cancer registry records all digestive tract cancers in 2 administrative areas in Burgundy (France): the Côte-d'Or (507,000 inhabitants according to the 1998 census) and the Saône-et-Loire (543,000 inhabitants). Cancer registration began in the Côte-d'Or area in 1976 and in the Saône-et-Loire area in 1982. Information is regularly obtained from pathologists, hospitals (university hospitals including the comprehensive cancer center, general hospitals), private physicians (gastroenterologists, surgeons, oncologists and radiotherapists) and general practitioners, as well as from the National Health Service and monthly review of death certificates. Because of the multiplicity of information sources, it was assumed that nearly all newly diagnosed cancers had been registered. The quality and comprehensiveness of registration are certified every 4 years by an audit of National Institutes of Health and Medical Research (INSERM) and of the National Public Health Institute (InVS).
The data routinely collected are related to the clinical features, diagnostic strategies, treatment, stage at diagnosis, and follow-up of the patients. Data on metachronous hepatic metastasis are not collected routinely. Two special surveys limited to the Côte-d'Or area were conducted. The first one was performed in 1987 and dealt with patients diagnosed between 1976 and 1984 and the second in 2003 with patients diagnosed between 1983 and 2000. Information about liver metastases was obtained from all clinicians (specialists and general practitioners) involved in the management and the follow-up of the patients.
Studied Variables
For all patients, the studied variables included sex, age at diagnosis, date of diagnosis, stage at diagnosis, and treatment. The date of diagnosis of the recurrence, its site (local recurrence, visceral metastases), as well as the treatment was recorded only for Côte-d'Or patients.
Treatment was classified into 3 categories: surgery for cure (macroscopic resection of all malignant tissue and no microscopic evidence of surgical marginal spread), palliative resection (failure to resect all disease), and other treatments. Adjuvant therapy was defined as chemotherapy given to patients resected for cure and palliative chemotherapy was defined as chemotherapy given to patients who did not undergo surgery and/or to patients with residual metastatic disease. Cancer site was classified according to the International Classification of Diseases for Oncology, 3rd revision.2 Location of the tumor was divided into right colon (cecum, ascending, hepatic flexure, and transverse) and left colon (splenic flexure, descending, sigmoid, rectosigmoid junction, and rectal ampulla). Cancer extension at the time of diagnosis was classified according to the TNM classification.3 The tumor size and gross features were also obtained from pathology reports.
Statistical Analysis
The population data used to calculate incidence rates for synchronous liver metastasis were based on population estimates by interpolation of the results of the 4 censuses (1975, 1982, 1990, and 1999), then by extrapolation. Incidence rates were calculated by sex, 5-year age groups, and 5-year periods. Rates were standardized by the direct method using the world standard population.
Crude metachronous liver metastasis rates were calculated using the actuarial method and were expressed with standard errors. The curves were compared using the log-rank test. Patients who died of undercurrent disease were censored at time of death and patients who developed a liver metastasis were censored at time of occurrence. A multivariate analysis was performed using a Cox proportional hazards regression model.
Crude survival for synchronous and metachronous liver metastases were calculated using the actuarial method. Relative survival rates were also calculated, these being defined as the ratio of the observed survival rate to the expected survival rate in a population with similar sex and age distribution derived from local mortality tables.
RESULTS
Incidence of Synchronous Liver Metastasis
Among the 13,463 patients diagnosed with a large bowel cancer over the 1976 to 2000 period, 14.5% had synchronous liver metastases identified during the diagnostic workup or in the course of treatment. Among them, 76.8% were confined to the liver and 23.2% were associated to other visceral metastases. The frequency of synchronous liver metastases was higher in males (15.9%) than in females (12.8%). Crude incidence rates were, respectively, 11.3 and 6.9 per 100,000 and age-standardized incidence rate 7.6 and 3.7 per 100,000. The sex ratio (2.1) was explained both by the higher frequency of liver metastases and the higher incidence of colon cancer in males.
The proportion of patients with synchronous liver metastasis was 19.8% before the age of 55, 16.7% between 55 and 64, 16.0% between 65 and 74, and 11.7% in patients 75 and over (P < 0.001). This proportion was 14.8% for colon cancers and 13.9% for rectal cancer. It was relatively stable over time: 12.9% for the 1976 to 1985 period, 14.1% for the 1986 to 1995 period, and 17.0% for the 1996 to 2000 period.
Incidence of Metachronous Liver Metastasis
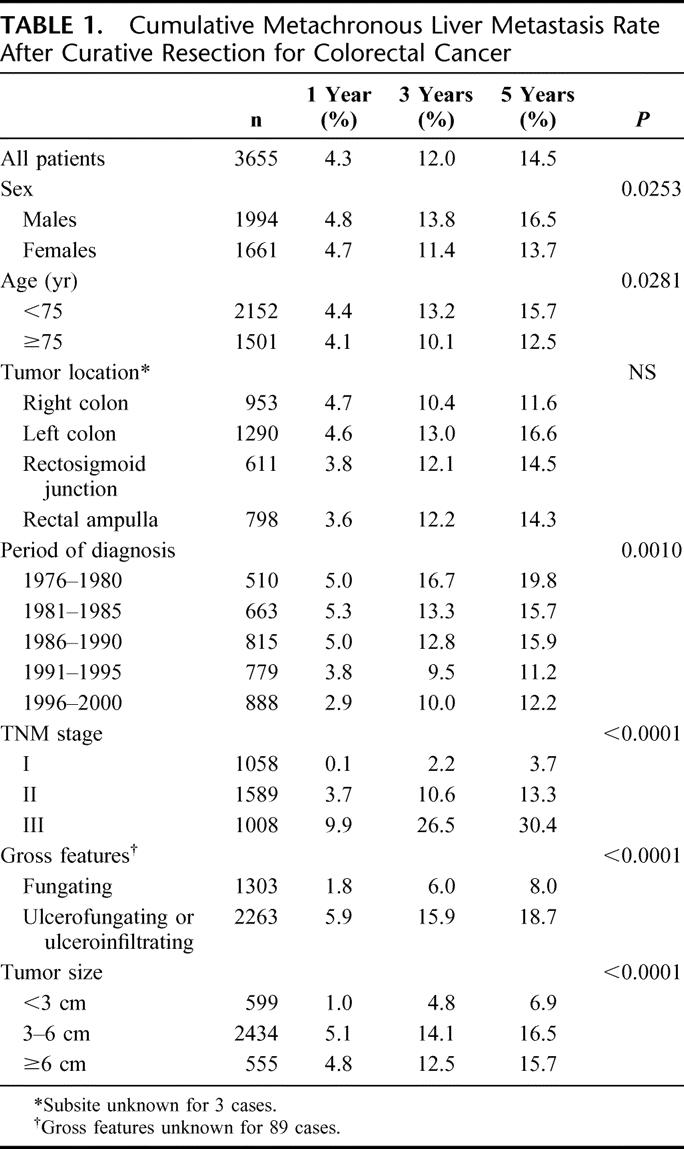
Among the 3655 patients resected for cure between 1976 and 2000 in the Côte-d'Or area (excluding operative death), 467 (12.8%) developed a metachronous liver metastasis during the 5 years following diagnosis. The overall actuarial cumulative rate was 4.3% at 1 year, 12.0% at 3 years, and 16.5% at 5 years. Table 1 shows the 1-, 3-, and 5-year actuarial cumulative rate according to the characteristics of the patient and of the tumor. Cancer site did not significantly influence the metachronous liver metastasis rate. It was lower in females (P = 0.0253), in patients aged 75 and over (P = 0.0281), and decreased over time (P = 0.001). The 5-year cumulative rate was 19.8% over the 1976 to 1980 period and 12.2% over the 1996 to 2000 period (P = 0.001). An increase in the metachronous liver metastasis rate was noted with advancing stage at diagnosis: the 5-year cumulative liver metastasis rate varied from 3.7% for TNM stage I to 30.4% for stage III (P < 0.0001). The liver metastasis rate was also influenced by gross features and the tumor size. Ulcero-infiltrating and ulcero-fungating types showed a higher incidence of liver metastasis than fungating lesions. Liver metastases were more frequent in cancers more than 3 cm in diameter than in smaller cancers. The liver metastasis rate was also higher in males than in females.
TABLE 1. Cumulative Metachronous Liver Metastasis Rate After Curative Resection for Colorectal Cancer
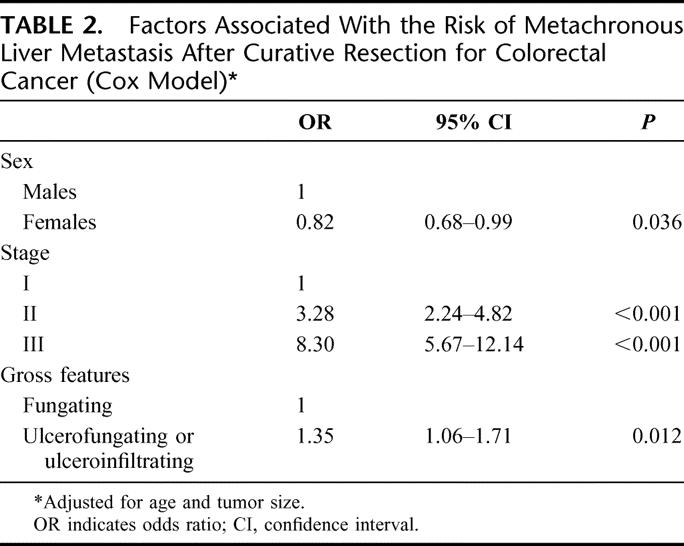
Factors with an impact on the metachronous liver metastasis risk were analyzed in a multivariate model to obtain a relative risk of liver metastasis adjusted for the other covariables (Table 2). There were 3 variables significantly and independently associated with the risk of metachronous liver metastasis. Stage at diagnosis was the strongest prognostic factor. There was a nearly 8-fold increase in the relative risk of liver metastasis for stage III lesions compared with stage I. Sex and gross features remained significant, while tumor size was no longer significant.
TABLE 2. Factors Associated With the Risk of Metachronous Liver Metastasis After Curative Resection for Colorectal Cancer (Cox Model)
Management of Synchronous and Metachronous Liver Metastases
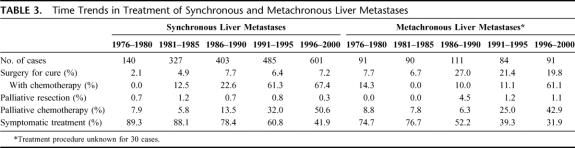
Resection for cure was less often performed in synchronous liver metastases (6.3%) than in metachronous liver metastases (16.9%; P < 0.001). The increase in the proportion of patients resected for cure over time was also more important for metachronous liver metastases than for synchronous liver metastases (Table 3). The proportion of patients treated with adjuvant and palliative chemotherapy also increased significantly, but more rapidly when there was a synchronous liver metastasis than when there was a metachronous liver metastasis. Palliative resection of the primary cancer, without resection of the liver metastases, was performed in 60.7% of the cases. This proportion increased over time from 44.3% (1976–1980) to 63.7% (1996–2000).
TABLE 3. Time Trends in Treatment of Synchronous and Metachronous Liver Metastases
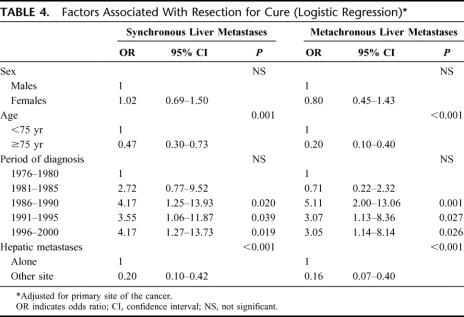
A multivariate logistic regression model was then applied to synchronous liver metastases and to metachronous liver metastases to identify factors independently associated to surgery for cure (Table 4). Sex, age, primary site of the cancer, period of diagnosis, and site of recurrence were introduced into the model. Sex and primary site of the cancer were not associated with resection for cure. Patients under 75 were 2 to 5 times more likely to be resected of their liver metastases than older patients. The presence of another site of recurrence resulted in a notable fall in the likelihood of resection of the liver metastases. The period of diagnosis was also an independent factor associated with the performance of a resection.
TABLE 4. Factors Associated With Resection for Cure (Logistic Regression)
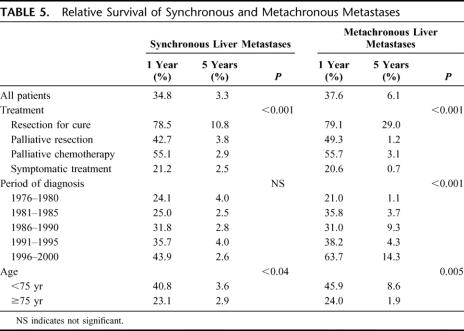
Table 5 shows the 1- and 5-year relative survival rate of synchronous and metachronous liver metastases for each studied variable. Survival rates decreased dramatically with time. For synchronous liver metastases, the 1-year relative survival rate was 34.8% and the 5-year relative survival rate was 3.3%. The corresponding rates for metachronous liver metastases were 37.6% and 6.1%. Treatment was an important determinant of prognosis. The only significant long-term survival was seen in patients resected for cure. Patients under 75 had a better survival rate than older patients. There was also a trend towards increase in survival over time.
TABLE 5. Relative Survival of Synchronous and Metachronous Metastases
DISCUSSION
This study has the advantage of providing a nonbiased and detailed view of the incidence, the management, and the prognosis of hepatic synchronous and metachronous liver metastases. Our study also suggests several interesting trends in the incidence and in the management of liver metastases.
Very little is known of the incidence of synchronous liver metastasis. The few epidemiologic studies providing data generally deal with the overall metastasis rate. It was reported to be 23% in the high resolution study conducted by EUROCARE in 6 European countries (France, Italy, the Netherlands, Poland, Spain, and the UK) in 1990.4 It was 19% in a survey conducted in 9 French administrative areas in 1995.5 Our data suggest that the liver represents 75.7% of all synchronous metastases. So our percentage of liver metastasis is in agreement with overall population-based data in Western Europe. The only similar series has been reported in Australia with a proportion of synchronous liver metastasis of 19.4%.6 The percentage of liver metastasis was slightly higher in patients under 55. There are several explanations: late stage at diagnosis in the younger age group where large bowel cancer is rare, more precise preoperative workup in the younger patients. It is lower in patients over 74, probably because of less forceful preoperative workup in the older patients. There was little change in the incidence of synchronous liver metastasis. This is disappointing but is probably due to changes in staging related to the improvement in diagnostic procedures.7 This usually results in an increase in the proportion of tumors discovered with synchronous metastases. They become more easily detected by the systematic use of ultrasonography and the development of CT scanning or MRI. This may explain the apparent stability in the proportion of liver metastasis. This is likely to have occurred as the proportion of advanced nonresectable cancer decreases, associated to an increase in stage I and an important improvement in the survival of colon and rectal cancers in France over the past 20 years.8
In this community-based study, the 5-year cumulative rate of metachronous liver metastasis was 14.5%. This finding is similar or lower to values reported in most hospital studies.1,9 To our knowledge, no similar population-based data are available. As expected, stage at diagnosis was the most important determinant of the risk of metachronous liver metastasis. The morphologic appearance was also associated with local recurrence risk after adjusting for other potential prognostic factors. Other authors have already reported that ulcerated tumors are associated with a higher metastatic risk.10,11 They may diffuse more rapidly than exophytic tumors. Tumor size was not a significant prognostic factor, according to the multivariate analysis, because it is closely correlated with stage at diagnosis.
The outlook for untreated hepatic metastasis was very poor: less than 30% of the patients were alive after 1 year and less than 5% survived 5 years. The importance of chemotherapy in non resectable liver metastases from colorectal cancer is now established as a palliative treatment even if long-term results remain poor.12 A substantial increase in the proportion of treated patients occurred over time. However, our data suggest that palliative chemotherapy, whose effectiveness was demonstrated for the first time in 1988,12 has not reached its full development. Over the 1996 to 2000 period only half of the patients with synchronous liver metastases and 43% of those with metachronous liver metastases have been treated. Our study indicates that there is a delay between the publication of scientific evidence and the complete implementation of effective treatment.
This study confirms that currently, surgical resection for cure is the only possibility to obtain long-term survival. It is generally admitted that 10% to 15% of patients with synchronous colorectal liver metastasis will benefit from hepatic resection.1 In this population-based study the proportion of resected synchronous liver metastasis was lower and disappointingly did not significantly increase over time. The situation was better for metachronous liver metastases, with one fourth of the cases resected for cure over the past 15-year period. There was a 3-fold increase in the proportion of lesions resected for cure when the first 5-year period was compared with the last 5-year period. However, only a small proportion of these patients will survive for more than 5 years. Reported survival rates in series of over 200 patients are in the range of 30% to 37%.13–18 In this nonselected series, the 5-year survival rate is lower, suggesting that prognosis may not be as good as reported in some single-center series. Surgical resection should be discussed within multidisciplinary settings in every case and should be proposed when a complete resection can be achieved. In the future, criteria suggesting a resectability of liver metastasis, like the Fong score, could be helpful.19 The presence of resectable extrahepatic disease is no longer considered as a contradiction for a liver resection.20 However, our results indicate that the possibility of resection for cure is much lower in the presence of extrahepatic disease. Not surprisingly, age is another factor influencing the resectability rate. Until recently, the effectiveness of adjuvant chemotherapy could not be demonstrated. However adjuvant chemotherapy is more and more often prescribed, probably due to the influence of borderline results in small series lacking of power.21 By their frequency and their gravity, synchronous and metachronous liver metastases from colorectal cancer remain a substantial problem. The use of new and more effective systemic chemotherapy regimens, either neoadjuvant or adjuvant, seems to be a promising approach requiring further investigation in randomized studies. Mass screening represents another promising approach to decrease the problem of the incidence of synchronous and metachronous liver metastases.22 The French health authorities have recently decided to start a pilot program covering one fourth of the population aged 50 to 74 years with biennial fecal occult blood testing.
Footnotes
Reprints: Anne-Marie Bouvier, PhD, Registre Bourguignon des Cancers Digestifs (INSERM EMI 106 and CIC-EC01), Faculté de Médecine, BP 87900 21079 DIJON Cedex, France. E-mail: anne-marie.bouvier@u-bourgogne.fr.
REFERENCES
- 1.Schlag PM, Benhidjeb T, Stroszczynski C. Resection and local therapy for liver metastases. Best Pract Res Clin Gastroenterol. 2002;16:299–317. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. International Statistical Classification of Diseases and Related Health Problem, 10th revision. Geneva: World Health Organization, 1992. [Google Scholar]
- 3.Sobin L, Wittekind C. TNM Atlas: International Union Against Cancer. New York: Wiley-Liss, 1997. [Google Scholar]
- 4.Gatta G, Capocaccia R, Sant M, et al. Understanding variations in survival for colorectal cancer in Europe: a EUROCARE high resolution study. Gut. 2000;47:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelip JM, Grosclaude P, Launoy G. Are there regional differences in the management of colon cancer in France? Eur J Cancer. 2005;131:504–510. [DOI] [PubMed] [Google Scholar]
- 6.Kune GA, Kune S, Field B, et al. Survival in patients with large-bowel cancer: a population-based investigation from the Melbourne Colorectal Cancer Study. Dis Colon Rectum. 1990;33:938–946. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. [DOI] [PubMed] [Google Scholar]
- 8.Faivre-Finn C, Bouvier-Benhamiche AM, Phelip JM, et al. Colon cancer in France: evidence for improvement in management and survival. Gut. 2002;51:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minsky BD, Mies C, Rich TA, et al. Potentially curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol. 1988;6:106–118. [DOI] [PubMed] [Google Scholar]
- 10.Michelassi F, Vannucci L, Montag A, et al. Importance of tumor morphology for the long term prognosis of rectal adenocarcinoma. Am Surg. 1988;54:376–379. [PubMed] [Google Scholar]
- 11.Steinberg SM, Barwick KW, Stablein DM. Importance of tumor pathology and morphology in patients with surgically resected colon cancer: findings from the Gastrointestinal Tumor Study Group. Cancer. 1986;58:1340–1345. [DOI] [PubMed] [Google Scholar]
- 12.Erlichman C, Fine S, Wong A, et al. A randomized trial of fluorouracil and folinic acid in patients with metastatic colorectal carcinoma. J Clin Oncol. 1988;6:469–475. [DOI] [PubMed] [Google Scholar]
- 13.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710; discussion 710–711. [PMC free article] [PubMed]
- 14.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999 230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed]
- 15.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 16.Bramhall SR, Gur U, Coldham C, et al. Liver resection for colorectal metastases. Ann R Coll Surg Engl. 2003;85:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T, Yasui K, Hirai T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(10 suppl):22–31. [DOI] [PubMed] [Google Scholar]
- 18.Cavallari A, Vivarelli M, Bellusci R, et al. Liver metastases from colorectal cancer: present surgical approach. Hepatogastroenterology. 2003;50:2067–2071. [PubMed] [Google Scholar]
- 19.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed]
- 20.Elias D, Ouellet JF, Bellon N, et al. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:567–574. [DOI] [PubMed] [Google Scholar]
- 21.Portier G, Milan C, Elias D, et al. Etude randomisée multicentrique de phase III: chimiothérapie systémique adjuvante contre observation après réduction de métastases hépatiques d'origine colo-rectale. Essai FFCD-ACHBTH-AURC 9002. Gastroenterol Clin Biol. 2002;26:A70. [Google Scholar]
- 22.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674–1680. [DOI] [PubMed] [Google Scholar]