Abstract
Objective:
To provide evidence that iNOS expression solely in leukocytes plays a role in postoperative ileus.
Summary Background Data:
Intestinal handling initiates a molecular and cellular muscularis inflammation that has been associated with iNOS expression and ileus. The specific cellular source of iNOS is a matter of speculation.
Methods:
Chimeric mice were constructed that selectively express the iNOS gene only in their leukocytes or only in their parenchymal cells by lethal radiation and reconstitution with reciprocal bone marrow. Mild intestinal manipulation was used to induce postoperative ileus.
Results:
Intestinal manipulation caused a significant leukocyte extravasation into the muscularis of all groups. Postoperative iNOS mRNA expression was evident in iNOS+/+ and transplanted iNOS−/− mice with iNOS+/+ bone marrow but not in iNOS−/− animals. The loss of the iNOS gene in leukocytes of iNOS+/+ mice reduced iNOS mRNA expression by 59%. iNOS-deficient mice and iNOS+/+ animals with iNOS−/− leukocytes presented with a significant improvement in postoperative intestinal transit and in vitro smooth muscle contractility, whereas the replacement with iNOS+/+ bone marrow in iNOS−/− mice completely reversed this improvement.
Conclusion:
These results clearly show that iNOS expressed in leukocytes within the intestinal muscularis plays a major role in mediating smooth muscle dysfunction and subsequently postoperative ileus.
Nitric oxide released postoperatively plays a seminal role in postoperative ileus, but the cellular source is unknown. Using bone marrow chimeric mice constructed from iNOS+/+ and iNOS −/− mice, we demonstrate that iNOS selectively in leukocytes within the muscularis plays a major role in mediating postoperative intestinal smooth muscle dysfunction.
Postoperative ileus causes considerable patient discomfort and distress with a significant delay in the resumption of enteral nutrition.1,2 Despite these obvious problems, postoperative ileus has been traditionally accepted as a near inevitable consequence of intra-abdominal surgery. The advantages of laparoscopic techniques with regard to decreased duration of ileus and faster recovery have again called attention to the significance of postoperative intestinal dysmotility. The rediscovered interest in and need for ileus research have led to new insights into the underlying molecular events contributing to postoperative ileus.3,4
In this context, it has recently been proposed that the muscularis externa itself acts as an immunologically active participant in the development of postoperative ileus.5 It is known that the gastrointestinal muscularis is populated with an extraordinarily dense network of macrophages.5–7 We have previously shown that an intestinal insult such as intraoperative handling of the bowel results in a significant activation of these resident macrophages.5 This activation in turn was associated with the initiation of a cascade of events that includes inflammatory gene expression and leukocyte extravasation.8–12 The observed correlation between the inflammatory response and postoperative dysmotility implies the associated expression of mediators that are capable of inhibiting smooth muscle motor function.10,13
NO is the principle inhibitory neurotransmitter of the nonadrenergic, noncholinergic enteric nervous system.14–16 The inducible isoform of the nitric oxide synthase (iNOS) is a potent NO generator and is typically expressed in response to tissue trauma and inflammation.17,18 Moreover, NO liberation has been associated with postoperative ileus,19,20 and we have previously demonstrated that intestinal manipulation of rodents induces iNOS expression within the intestinal muscularis that the subsequent generation of NO participates in causing postoperative smooth muscle dysfunction in vitro.13 However, the specific cellular source of postoperative NO liberation remained a matter of speculation. Therefore, we designed experiments to selectively test the hypothesis that leukocyte-derived iNOS plays a central role in ileus. Our objectives were: 1) to determine the contribution of leukocytes to the postoperative iNOS expression within the intestinal muscularis, 2) to define the functional significance of postoperative iNOS in vivo, and 3) to determine conclusively the significance of leukocyte iNOS in the development of postoperative intestinal dysfunction.
METHODS
Animals and Model of Postoperative Ileus
Female C57BL/6-iNOS+/+ and C57BL/6-iNOS−/− mice (age, 6–10 weeks) were generously provided by the Department of Surgery, University of Pittsburgh. All knockout mice were inbred into a C57BL/6 background for more than 8 generations. The experimental design was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). All animals were kept in a pathogen-free facility that is accredited by the American Association for Accreditation of Laboratory Animal Care and complies with the requirements of humane animal care as stipulated by the U.S. Department of Agriculture and the Department of Health and Human Services.
The selective deletion or addition of the iNOS gene in leukocytes in C57BL/6-iNOS+/+ or C57BL/6-iNOS−/− mice, respectively, was performed by lethal radiation of the host followed by rescue bone marrow transplantation (BMTX). The mice were irradiated in a Nordion Gammacell 40 Irradiator (MDS Nordion, Ottawa, Ontario, Canada). They received 500 cGy of total body irradiation initially and then 500 more cGy 4 to 6 hours later. Lethally radiated C57BL/6-iNOS+/+ (or C57BL/6-iNOS−/−) mice were then reconstituted with 9.5 × 106 bone marrow cells from C57BL/6-iNOS−/− (or C57BL/6-iNOS+/+) mice, followed by 3 months of recovery. The reconstituted inbred C57BL/6-iNOS+/+ mice were deficient for iNOS only in their leukocytes (iNOS+/+ BMTX−/−) and the reconstituted C57BL/6-iNOS−/− mice expressed the iNOS gene selectively in the transplanted iNOS+/+ bone marrow derived cells (iNOS−/− BMTX +/+). Animals were divided into five experimental groups: one unmanipulated control group (iNOS+/+) and four groups that underwent intestinal manipulation (IM): IM iNOS+/+, IM iNOS−/−, IM iNOS+/+ BMTX−/−, and iNOS−/− BMTX +/+.
The animals were subjected to a standardized surgical manipulation of the small intestine using sterile moist cotton applicators as described previously.5 To define the postoperative iNOS gene expression among the genetically different groups, animals were killed at 12 hours postoperatively (n = 4 each). Mice were killed 24 hours postoperatively for functional and immunohistochemical studies (n = 5–6 each). Age-matched unoperated C57BL/6-iNOS+/+ mice served as controls. The resident macrophage network was characterized using phagocytosis experiments (n = 4 each).
iNOS mRNA Expression
Total mRNA extraction was performed as previously described using the guanidinium-thiocyanate phenol-chloroform extraction method.21 Equal aliquots (5 μg) of total RNA from each sample quantified by spectrophotometer (at 260 nm) were processed for complimentary DNA (cDNA) synthesis. Amplification of synthesized cDNA from each sample was carried out using polymerase chain reaction with 32P-labeled primers. GAPDH was used as the housekeeping gene. Primers were designed according to published sequences22,23 (GenBank accession numbers M87039 [iNOS] and M32599 [GAPDH]), using the web accessible Primer3 computer program.24 Sequences of the PCR primers were as follows: iNOS sense 5′-CTCACTGGGACAGCACAGAA-3′ and antisense 5′-TGGTCAAACTCTTGGGGTTC-3′ (product size, 200 bp); GAPDH sense 5′-GGCATTGCTCTCAATGACAA-3′ and antisense 5′-TGTGAGGGAGATGCTCAGTG-3′ (product size, 199 bp). iNOS and GAPDH amplification was carried out for 30 cycles (92°C for 1 minute, 62°C for 1 minute, 72°C for 1 minute). RAW cells (RAW 264.7 murine macrophage cell line) stimulated in vitro with LPS served as positive controls, water added instead of DNA template as a negative control; 20 μL of the iNOS-RT-PCR amplification product and a 123-bp DNA ladder were separated using polyacrylamide gel electrophoresis. The gel was dried on a gel dryer and the PCR product bands were visualized by autoradiography. PhosphorImager (Molecular Dynamics, Sunnyvale, CA) was used to quantify the bands (Molecular Dynamics). To assure equal cDNA synthesis, data from all samples were normalized against their respective control counts. To assure equal reaction conditions, all samples received pooled reagents, and comparisons were made only with experiments performed simultaneously.
Histochemistry and Immunohistochemistry
Histochemical and immunohistochemical analysis was performed on whole mounts of the intestinal muscularis as described previously.5–7 Myeloperoxidase (MPO) positive cells were detected using Hanker-Yates reagent (Polysciences, Warrington, PA). For iNOS immunohistochemistry, whole mounts were incubated overnight at 4°C in rabbit-antimouse-iNOS antibodies (1:100), followed by 3× washes in 0.05 mol/L phosphate-buffered saline solution (PBS). Each specimen was then incubated with a Cy3 goat-antirabbit secondary antibody (1:500) for 4 hours at 4°C and washed again 3 times in 0.05 mol/L PBS. Secondary antibodies without iNOS antibody preincubation were used in parallel in all staining procedures to ensure specificity. Fluorescein Isothiocyanate Isomer I (FITC)-labeled F4/80 antibodies (rat-antimouse) were used to stain infiltrating monocytes. Whole mounts were incubated in FITC-labeled F4/80 antibodies (1:10) overnight at 4°C and washed 3 times in 0.05 mol/L PBS. Resident muscularis macrophages were visualized using fluorescein-labeled dextran as a phagocytotic marker.25 Animals received an intraperitoneal injection of fluorescein-labeled dextran (12.5 mg in 0.2 mL sterile 0.9% saline) and were killed after 24 hours. Muscularis whole mounts were washed 3 times in 0.05 mol/L PBS. Leukocytes were counted in 5 randomly chosen areas in each specimen (3 specimens per animal) at a magnification of 200×.
Tissue Culture Preparation and Determination of Nitrite Production
The small bowel of control and manipulated mice 24 hours postoperatively was processed for tissue culture as described previously.13 To determine the generation of NO from the intestinal muscularis, nitrite production was measured by the Griess reaction.26 Supernatant from the tissue culture was mixed with equal amount of Griess reagent and incubated at room temperature for 10 minutes. The absorbance at 550 nm was measured with a microplate reader and compared with standard prepared from serial dilutions of sodium nitrite. Each standard and supernatant sample was analyzed in duplicate, and the mean value of nitrite production was normalized to the tissue weight.
Functional Studies
Intestinal transit was measured in unoperated iNOS+/+ mice and in manipulated, iNOS-genetically different mice (iNOS+/+, iNOS+/+ BMTX−/−, iNOS−/−, iNOS−/− BMTX+/+) at 24 hours postoperatively by evaluating the intestinal distribution of orally given fluorescein-labeled dextran as previously described.27 The data were expressed as the percentage of activity per segment and plotted in a median histogram. Gastrointestinal transit was calculated as the geometric center (GC) of distribution of fluorescein-labeled dextran as described before.11 Mechanical activity of the circular muscularis was measured in vitro as described earlier.21 After recording spontaneous contractility for 30 minutes, dose-response curves were generated using increasing doses of the muscarinic agonist bethanechol (1–300 μmol/L) for 10 minutes and intervening wash periods (KRB) of 10 minutes. The contractile response was recorded and analyzed as grams/mm2 of tissue.
Drugs and Solutions
A standard KRB was used as described previously.28 KRB constituents and bethanechol were purchased from Sigma Chemical Co. (St. Louis, MO). For immunohistochemistry, antibodies were diluted in 0.05 mol/L PBS containing 0.2% bovine serum albumin (Sigma Chemical Co.), 1000 U/mL penicillin G, and 1 mg/mL streptomycin (Sigma Chemical Co.). Rabbit-antimouse iNOS antibodies (1:100) were purchased from Chemicon International Inc. (Temecula, CA) and indocarbocyanine (Cy3)-conjugated goat-antirabbit secondary antibodies (1:500) were obtained from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). FITC-labeled F4/80 antibody (rat-antimouse) (1:10) was purchased from Serotec Inc. (Raleigh, NC). Fluorescein-labeled dextran (molecular weight = 70,000), used for the phagocytosis experiment and the transit measurements was purchased from Molecular Probes, Eugene, OR.
Data Analysis
Data are expressed as means ± standard error of the mean (SEM). Data were statistically analyzed using an unpaired Student t test. A probability level of P < 0.05 was considered significant.
RESULTS
Cellular Inflammatory Events Following Intestinal Manipulation
We have previously shown that intestinal manipulation rapidly activates the resident muscularis macrophage network and recruits a significant and diverse population of circulating leukocytes into the intestinal muscularis.8,27 To demonstrate the existence of a similar cellular leukocytic inflammatory response in each genetically different mouse model of postoperative ileus, we quantified muscularis leukocyte infiltrates postoperatively 24 hours after compression of the small intestine. This time point was chosen according to previous observations in rats that leukocyte extravasation begins around 4 hours postoperatively and reaches a maximum at 24 hours.8,27 We focused on polymorphonuclear neutrophils (PMN) and monocytes because they both possess the ability to express iNOS and are known to infiltrate the intestinal muscularis following surgical manipulation.13,29 As depicted in Figure 1B, intestinal manipulation resulted in a significant extravasation of MPO+ neutrophils into the intestinal muscularis (37 ± 2 cells/field), whereas control whole mounts presented only with occasional PMNs (0.5 ± 0.1 cells/field; Fig. 1A). After surgery, there were no significant differences in postoperative PMN infiltration between the genetically homogeneous animals and their chimeric variants (iNOS+/+, 42 ± 2.1; iNOS+/+BMTX−/−, 35 ± 5.5; iNOS−/−, 36 ± 4.5; iNOS−/− BMTX+/+, 33 ± 2.6 cells/200× field). In addition to the dark brown-stained neutrophils, small round and lightly stained cells were visualized with the Hanker Yates reagent. Using an antibody directed against the macrophage/blood monocyte marker F4/80 these cells were identified as monocytes. As illustrated in Figure 1C, monocytes were virtually absent in control whole mounts (0.9 ± 0.2 cells/field). Intestinal manipulation caused a dense monocytic infiltration into muscularis whole mounts (45 ± 2 cells/field; Fig. 1D). As with the PMNs, there were no significant differences in monocyte infiltration among the genetically different iNOS animals (iNOS+/+, 42 ± 3.2; iNOS+/+BMTX−/−, 45 ± 4.9; iNOS−/−, 50 ± 5.4; iNOS−/− BMTX+/+, 43 ± 2.1 cells/200× field). Thus, in this intestinal compression model, leukocytic infiltrates were quantified to be similar between the various groups of mice.
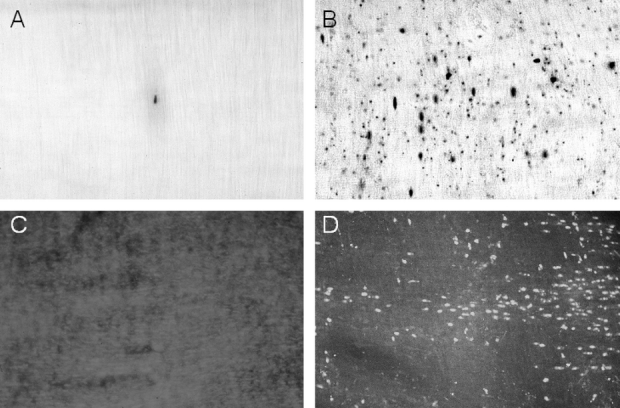
FIGURE 1. MPO staining and F4/80 immunohistochemistry of jejunal muscularis whole mounts demonstrating leukocyte recruitment after intestinal manipulation. A, Occasionally, a few MPO-positive cells could be observed within control whole mounts from iNOS+/+ mice. B, Infiltrating MPO-positive cells 24 hours after intestinal manipulation (iNOS−/− mouse with iNOS+/+ bone marrow). In addition to darkly stained neutrophils, numerous smaller and lighter stained cells, representing monocytes, were observed by light microscopy. F4/80 immunohistochemistry was used to stain monocytes (C and D). C, The near absence of monocytes in whole mounts from control iNOS+/+ mice. D, A typical whole mount containing numerous F4/80-positive monocytes after intestinal manipulation (iNOS+/+ mouse). Original magnifications: A–D, ×200.
Enhanced iNOS mRNA Expression in the Small Bowel Muscularis After Intestinal Manipulation
Previously, we have demonstrated that surgical manipulation of the rodent intestine results in a late muscularis iNOS mRNA expression, which peaks between 6 and 12 hours postoperatively.13 To specifically define the role of inflammatory cell iNOS expression, we investigated iNOS mRNA in iNOS wild-type, knockout mice and mice with chimerically different iNOS bone marrow reconstitutions. As illustrated in Figure 2, intestinal compression induced a distinct up-regulation in total muscularis extract iNOS mRNA expression in iNOS+/+ animals (173 fold up-regulation), whereas this signal was not significantly up-regulated by bowel compression in iNOS-deficient mice (iNOS−/−) compared with controls. Selective deletion of the iNOS gene in leukocytes of the chimeric iNOS+/+ mice significantly reduced this molecular response by 59% (iNOS+/+BMTX−/−, 71-fold up-regulation). On the other hand, the selective addition of iNOS+/+ bone marrow into iNOS−/− mice resulted in a distinct postoperative iNOS mRNA signal that was not significantly different from the wild-type iNOS+/+ mice (iNOS−/− BMTX+/+, 161-fold up-regulation).

FIGURE 2. RT-PCR analysis of iNOS mRNA expression 12 hours postoperatively, comparing the genetically different chimeric groups (IM, intestinal manipulation; PC, positive control; NC, negative control). A, Gel bands of muscularis extracts. B, Results of gel band analysis using phosphor imaging. The iNOS signal is absent in control iNOS+/+ mice and in manipulated iNOS−/− animals. Levels of iNOS mRNA expression were significantly up-regulated in manipulated iNOS+/+ mice. The selective deletion of the iNOS gene in leukocytes (iNOS+/+ BMTX−/−) resulted in less expression, whereas the addition of the iNOS gene in iNOS−/− animals (iNOS−/− BMTX+/+) caused a distinct postoperative iNOS mRNA up-regulation.
Localization of iNOS Protein
Recruited leukocytes as well as resident macrophages are known to express iNOS in the inflamed intestinal muscularis.13,29 Thus, the existence of a similar network of resident macrophages among the genetically different mice, particularly the irradiated animals, had to be assured for an accurate interpretation of the iNOS expression experiments above. Resident macrophages in each group were visualized following their phagocytosis of intraperitoneal injected fluorescein-labeled dextran. As illustrated in Figure 3, muscularis whole mounts presented with a dense network of equally distributed macrophages (47 ± 0.7 cells per field). The pattern and the quantity of the resident muscularis macrophage network were not significantly different between the various genetically altered groups (iNOS+/+, 47 ± 0.7; iNOS+/+ BMTX−/−, 47 ± 1.9; iNOS−/−, 48 ± 1.6; iNOS−/− BMTX+/+, 48 ± 2 cells/200× field). No iNOS positively stained structures were observed in control whole mounts or specimens from manipulated iNOS−/− animals (Fig. 4A). Manipulated iNOS+/+ and iNOS−/− BMTX+/+ mice presented with numerous iNOS-positive small round cells with a distribution and morphology resembled those of infiltrating leukocytes (Fig. 4B). A weaker but noticeable iNOS signal was also present on the stellate resident macrophages (Fig. 4C, D). iNOS positive infiltrating leukocytes or resident macrophages were not seen in manipulated iNOS+/+ BMTX−/− mice. Compared with iNOS−/− mice, whole mounts from these animals presented with a light immunohistochemical signal, possibly attributed to microvasculature or connective tissue. However, we were not able to distinguish a specific cellular source for postoperative iNOS expression in iNOS+/+ BMTX−/− animals, even though a significant up-regulation in iNOS was quantified by RT-PCR.

FIGURE 3. Fluorescence micrographs of jejunal muscularis whole mounts from an iNOS−/− mouse (A) and from an iNOS−/− BMTX+/+ mouse (B, both unmanipulated), 1 day after fluorescein-dextran injection. Both panels show an equal distribution of dendritic-appearing resident macrophages within the intestinal muscularis containing fluorescein-labeled dextran. Original magnifications: A, ×150; B, ×400.
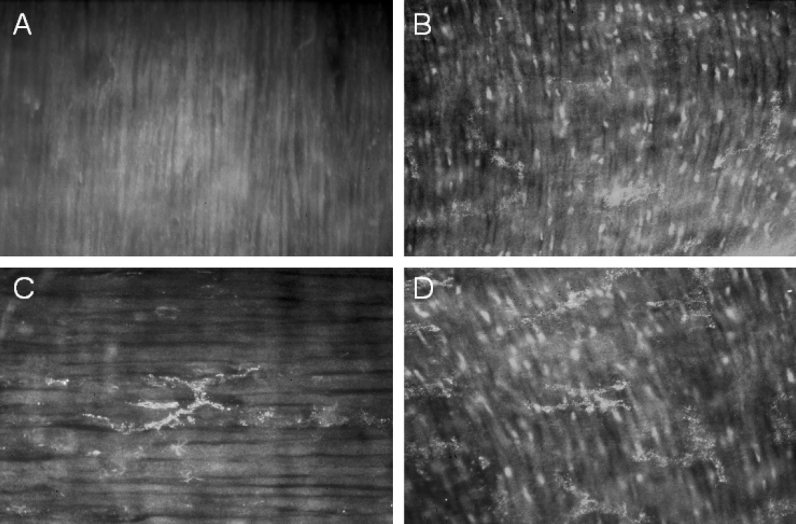
FIGURE 4. Fluorescence micrographs of muscularis whole mounts 24 hours after intestinal manipulation, stained with antibodies against iNOS. A, The absence of iNOS immunoreactivity in the muscularis from a manipulated iNOS−/− animal. B, The occurrence of iNOS-positive infiltrating cells in a manipulated iNOS−/− animal with iNOS+/+ bone marrow. C, Postoperative iNOS expression in a muscularis macrophage. D, The concurrent appearance of strong iNOS immunoreactivity in recruited leukocytes and a weaker but noticeable signal in macrophages (c, d: manipulated iNOS−/− BMTX+/+ mice). Original magnifications: A, B, ×200; C, ×400; D, ×300.
Nitric Oxide Production by the Inflamed Intestinal Muscularis
We have previously shown that the postsurgical manipulated intestinal muscularis of rats is able to release significant amounts of nitric oxide into the supernatant of the cultured muscularis externa.13 This release of nitric oxide could be blocked by the addition of the selective iNOS inhibitor l-NIL. In the present study, we next sought to determine the impact of the various genetic backgrounds on the release of nitric oxide after intestinal manipulation. Nitric oxide produced within the intestinal muscularis, as measure by the Griess reaction in the supernatant of the cultured muscularis isolate 24 hours after surgery, was significantly (4.2-fold) increased in manipulated wild-type mice compared with control wild-type mice (260 ± 44 vs. 62 ± 2 nmol/100 g tissue/24 hours), while the muscularis of manipulated iNOS knockout mice released significantly less nitric oxide (26 ± 1 nmol/100 g tissue/24 hours). Second, we investigated the specific contribution of iNOS from bone marrow-derived leukocytes following surgery. The selective deletion of the iNOS gene in leukocytes of wild-type mice (iNOS+/+ BMTX−/−) resulted in a 90% reduction in postoperative nitric oxide release compared with wild types, whereas the addition of the iNOS gene to iNOS knockout animals (iNOS−/− BMTX+/+) caused a 273% increase in postoperative nitric oxide production by the manipulated muscularis compared with iNOS knockout mice.
Postoperative Impairment of In Vivo Gastrointestinal Transit
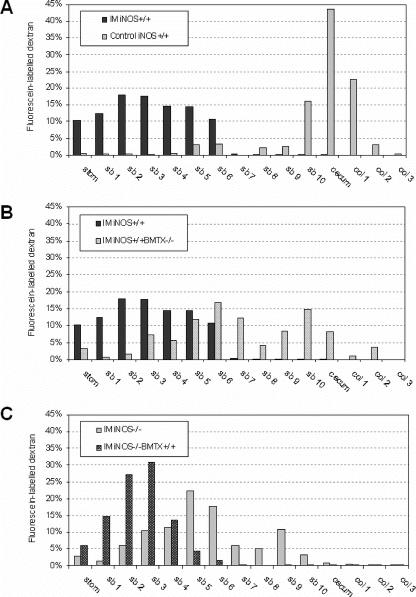
Gastrointestinal transit was investigated to determine the significance of leukocyte iNOS in postoperative in vivo motility changes. As depicted in the histogram of Figure 5A, intestinal manipulation caused a significant delay in intestinal transit in the iNOS+/+ animals. In the control wild-type mice, the fluorescein-labeled dextran moved through the stomach and down the small intestine and was located in the distal small bowel and colon (GC, 11.4 ± 0.2) after 90 minutes, whereas the marker was retained within the proximal bowel in the manipulated iNOS+/+ mice (GC, 4.1 ± 0.4). As shown in Figure 5B, the selective loss of the iNOS gene in leukocytes resulted in a significant prevention of the postoperative delay in intestinal transit (iNOS+/+ BMTX−/−; GC, 8 ± 0.9), which was not significantly different from the manipulated iNOS−/− mice (GC, 6.5 ± 0.8). On the other hand, iNOS−/− mice reconstituted with iNOS+/+ bone marrow again demonstrated a significant delay in gastrointestinal transit (Fig. 5C; iNOS−/− BMTX+/+; GC, 3.6 ± 0.4). There were no significant differences between the manipulated iNOS−/− BMTX+/+ and the iNOS+/+ wild-type mice.
FIGURE 5. Transit histograms for the distribution of fluorescein-labeled dextran along the gastrointestinal tract 90 minutes after oral administration (IM, intestinal manipulation; stom, stomach; sb, small bowel; col, colon). In control animals, the nonabsorbable marker accumulated in the cecum. Intestinal manipulation in iNOS+/+ mice caused a significant delay in intestinal transit (A). The loss of the iNOS gene in leukocytes ameliorated the delay caused by manipulation (B). iNOS−/− mice showed an improvement of postoperative transit similar to the iNOS+/+ BMTX−/−, which was completely reversed in iNOS+/+ bone marrow-transplanted animals (iNOS−/− BMTX+/+) (C).
Inhibition of In Vitro Smooth Muscle Contractile Activity
The inhibition of intestinal transit after surgical manipulation reflects altered smooth muscle contractile activity, as well as alterations in enteric neural reflexes.30 To examine the role of immune cells in iNOS-mediated postoperative dysmotility, independent of neuronal influences, spontaneous and bethanechol-stimulated circular smooth muscle contractility was studied in vitro. Circular smooth muscle strips from control iNOS+/+ mice generated rhythmic spontaneous contractions with a mean contractile area of 0.81 ± 0.12 g/mm2/s (Fig. 6A). Basal spontaneous circular muscle contractions from the various groups of unmanipulated mice were not different compared with wild-type controls. Intestinal manipulation of wild-type mice caused a significant 71% reduction in spontaneous contractile activity to 0.3 ± 0.03 g/mm2/s (Fig. 6B). The manipulated spontaneous contractility of circular muscle strips of iNOS-deficient mice (iNOS−/−, 0.61 ± 0.16 g/mm2/s) (Fig. 6C), as well as spontaneous contractions from wild-type mice with iNOS−/− bone marrow (iNOS+/+BMTX−/−, 0.46 ± 0.06 g/mm2/s) was significantly improved compared with the manipulated wild-type iNOS+/+ mice (Fig. 6D). Indeed, spontaneous contractility recorded from muscle strips harvested from manipulated iNOS−/− mice was not significantly different from unoperated iNOS+/+ control mice. In contrast, chimeric iNOS−/− mice with transplantation of iNOS+/+ bone marrow (iNOS−/− BMTX+/+) lost the protection seen in pure iNOS−/− mice and again demonstrated a significant postoperative suppression in spontaneous contractility (0.35 ± 0.08 g/mm2/s) (Fig. 6E). This magnitude of suppression was statistically similar to that observed in iNOS+/+ mice.
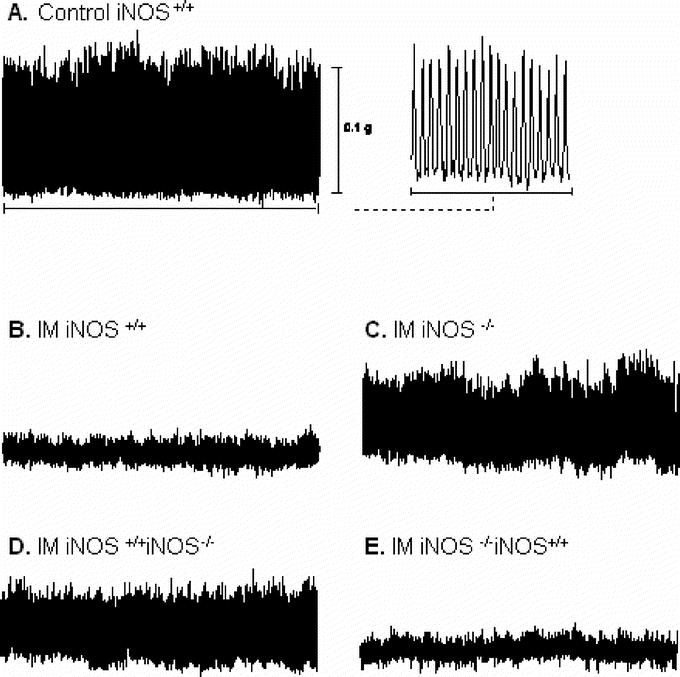
FIGURE 6. Representative traces of spontaneous contractile activity recorded from jejunal circular smooth muscle strips. A, Trace from a control iNOS+/+ mouse. The last portion of the trace is shown to the right on an expanded time scale, demonstrating characteristic rhythmic and robust contractions. B, Intestinal manipulation results in a marked decrease in spontaneous contractile activity. Traces in C and D demonstrate the amelioration of postoperative in vitro contractility in complete iNOS-deficient mice and iNOS−/− bone marrow transplanted mice (iNOS+/+ BMTX−/−). E, Significant inhibition of spontaneous contractile activity in manipulated iNOS−/− BMTX+/+ mice.
The capacity of the intestinal muscularis to respond to the cholinergic agonist bethanechol is illustrated in Figure 7. The stimulated contractile force generated by circular muscle strips from manipulated iNOS+/+ mice in response to 100 μmol/L bethanechol was only 55% (1.2 ± 0.18 g/mm2/s) of that observed in unoperated control mice (2.2 ± 0.21 g/mm2/s). In contrast, the stimulated contractile activity of manipulated iNOS+/+ BMTX−/− was significantly improved, with only a 14% reduction in bethanechol-stimulated contractions (1.89 ± 0.23 g/mm2/s) (Fig. 7A). As depicted in Figure 7B, 100 μmol/L bethanechol-stimulated contractions from manipulated iNOS−/− mice (2.07 ± 0.18 g/mm2/s) were similar in magnitude to those recorded from unoperated iNOS+/+ controls. Transplantation of wild-type iNOS+ bone marrow into the knockouts (iNOS−/− BMTX+/+) again resulted in a marked suppress in postoperative circular muscle contractility (1.05 ± 0.09 g/mm2/s with bethanechol 100 μmol/L). We also performed contractility measurements on iNOS+/+ BMTX iNOS+/+ mice to evaluate the effect of bone marrow transplantation itself. There was no statistical difference in spontaneous or bethanechol-stimulated contractile activity between control wild-type and iNOS+/+ BMTX+/+ mice after transplantation (spontaneous activity: control = 0.81 ± 0.12 vs. iNOS+/+ BMTX+/+ = 0.76 ± 0.19 g/mm2/s and bethanechol [100 μmol/L]: control = 2.2 ± 0.18 vs. iNOS+/+ BMTX+/+ = 2.09 ± 0.19 g/mm2/s). Thus, bone marrow transplantation alone had no direct effect on contractility.
FIGURE 7. Bethanechol dose response curves of jejunal circular smooth muscle contractile activity in response to surgical manipulation. A, Significant improvement of contractility in iNOS+/+ mice with selective loss of the iNOS gene in leukocytes (iNOS+/+ BMTX−/−). The improved response to bethanechol in iNOS−/− animals is completely reversible through an iNOS+/+ bone marrow replacement (B).
DISCUSSION
The occurrence of inflammatory events within the intestinal muscularis has recently been recognized as a substantial pathophysiologic mechanism for the development of postoperative ileus.4,10,27,30 The previous observation that the magnitude of muscularis inflammation is proportional to the extend of postoperative intestinal smooth muscle dysfunction indicates that the expression of inflammatory and kinetically active mediators is capable of impairing intestinal motility.10,13 Among these potentially liberated agents, NO synthesized by the inducible form of NO synthase (iNOS) has been identified as one of the important suppressors of rodent postoperative intestinal smooth muscle function.13 A multitude of cell types could potentially be involved in the observed postoperative iNOS expression, including leukocytes, smooth muscle, endothelium, or neuronal tissue.31 Based on our previous immunohistochemical studies, leukocytes have been emphasized as the primary source of iNOS protein in the manipulated intestinal muscularis.13 However, it remains a matter of speculation if this cell population presents the exclusive and/or functionally most significant cellular source of postoperative iNOS expression within the intestinal muscularis. Therefore, we studied postoperative intestinal smooth muscle function in chimeric mice with iNOS expression limited either to bone marrow-derived cells or to intestinal parenchymal cells, and demonstrated conclusively for the first time that iNOS selectively expressed in activated resident and recruited leukocytes is a major mediator of postoperative murine ileus.
Interestingly, we detected a reduced but significant up-regulation of iNOS mRNA in manipulated mice that were deficient for iNOS only in their leukocytes. This result could be due to residual/incomplete bone marrow cell replacement and/or that cells that are not derived from the bone marrow also participate in the postoperative iNOS mRNA expression within the intestinal muscularis. In this context, it has been reported that cells outside the classic immune system, such as vascular smooth muscle cells, may participate during inflammation by expressing iNOS.32 Nevertheless, the results derived from our functional and tissue culture experiments clearly indicate that potential iNOS sources outside the classic immune system do not have the capability to release a significant amount of NO with functional impact on postoperative smooth muscle contractile activity and as well as on intestinal transit.
We have previously reported that elevated iNOS expression within the intestinal muscularis exhibited a temporal correlation with the infiltration of massive numbers of inflammatory cells and the development of postoperative smooth muscle dysfunction.13 But the functional relevance of leukocyte-derived iNOS expression within the intestinal muscularis and smooth muscle dysfunction on postoperative in vivo motility had not yet been defined. The current study extends our previous observations by demonstrating that intestinal smooth muscle dysfunction, associated with intestinal manipulation, was dependent upon the ability of leukocytes to express iNOS. Indeed, the selective deletion of the iNOS gene in wild-type mice significantly ameliorated postoperative intestinal smooth muscle contractile activity and reciprocal replacement with wild-type bone marrow in knockout mice completely reversed the improvement seen in iNOS-knockout mice. In the present study, we investigated postoperative intestinal transit and confirmed for the first time in an in vivo model of postoperative ileus the significance of iNOS, particularly expressed in leukocytes. However, iNOS gene deletion did not result in complete amelioration of postoperative ileus. Manipulated iNOS-deficient mice (iNOS−/−) as well as mice that were deficient for iNOS only in their leukocytes (iNOS+/+ BMTX−/−) both presented with improved but still delayed postoperative transit compared with controls. This observation suggests the involvement of additional inhibitors of motility. In this context, activated inflammatory cells are known to be potent secretors of other kinetically active substances such as prostaglandins and reactive oxygen intermediates that have been shown to inhibit smooth muscle contractility and to interact with neurons of the enteric nervous system.10,33,34 In particular, the postoperative liberation of prostaglandins has recently been associated with the development of ileus.10,35 Furthermore, there is evidence that the constitutive form of NOS (cNOS) may also participate in the inflammatory process and subsequent development of ileus.19
It was questionable whether recruited or resident leukocytes contributed more to the postoperative iNOS expression. In this context, it has been previously demonstrated by immunohistochemistry that iNOS protein after intestinal manipulation in rats is predominantly expressed in infiltrating leukocytes.13 In the present study, using mice we were able to detect iNOS protein also in resident macrophages, however, with much less intensity than in the infiltrating cells. Together with the previous finding of the late postoperative iNOS mRNA peak expression 12 hours postoperatively and the observation that adhesion molecule blockade reduces leukocyte extravasation and iNOS mRNA expression,13 we conclude that infiltrating cells are more contributing to iNOS expression than resident macrophages.
CONCLUSION
The results confirm the functional significance of local inflammatory events, particularly leukocyte recruitment within the intestinal muscularis after surgical manipulation. Furthermore, the data provide conclusive evidence that the kinetically active mediator iNOS specifically expressed in recruited as well as resident leukocytes directly modulates the contractile activity of the circular muscularis, which contributes to an inhibition of gastrointestinal transit and postoperative ileus. The recognition of postoperative ileus as a consequence of inflammatory events within the intestinal muscularis opens up the potential for new therapeutic strategies in the prevention of this surgical complication. According to our data, the perioperative treatment with iNOS inhibitors might be an encouraging way to prevent postoperative dysmotility.13 However, it has been reported that iNOS inhibition results in impaired anastomotic healing.36,37 Thus, this therapeutic approach might have too adverse effects in abdominal surgery. Furthermore, other kinetically active substances, such as prostaglandins, appear to be also involved in causing inflammatory dysmotility and should be addressed in this context.10 We think that the initiation of the inflammatory cascade within the intestinal muscularis might be a better target for the pharmacologic prevention of postoperative ileus. Further investigations are needed to identify and characterize this initial pathway.
ACKNOWLEDGMENTS
The authors thank Amanda Wolf, Sandra Tögel, and Esther Türler for their technical help.
Footnotes
Supported by National Institutes of Health grants R01-GM-58241 and P50-GM-53789 and a grant from the Deutsche Forschungsgemeinschaft (TU 116/2-1).
Reprints: Anthony J. Bauer, PhD, Department of Medicine/Gastroenterology, University of Pittsburgh Medical School, S849 Scaife Hall, 3550 Terrace Street, Pittsburgh, PA 15261. E-mail: tbauer@pitt.edu.
REFERENCES
- 1.Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480–1493. [DOI] [PubMed] [Google Scholar]
- 2.Graber JN, Schulte WJ, Condon RE, et al. Relationship of duration of postoperative ileus to extent and site of operative dissection. Surgery. 1982;92:87–92. [PubMed] [Google Scholar]
- 3.Bauer AJ, Schwarz NT, Moore BA, et al. Ileus in critical illness: mechanisms and management. Curr Opin Crit Care. 2002;8:152–157. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge WJ, van den Wijngaard RM, The FO, et al. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterology. 2003;125:1137–1147. [DOI] [PubMed] [Google Scholar]
- 5.Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228:652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995;10:719–736. [PubMed] [Google Scholar]
- 7.Kalff JC, Schwarz NT, Walgenbach KJ, et al. Leukocytes of the intestinal muscularis: their phenotype and isolation. J Leukoc Biol. 1998;63:683–691. [DOI] [PubMed] [Google Scholar]
- 8.Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117:378–387. [DOI] [PubMed] [Google Scholar]
- 9.Hierholzer C, Kalff JC, Chakraborty A, et al. Impaired gut contractility following hemorrhagic shock is accompanied by IL-6 and G-CSF production and neutrophil infiltration. Dig Dis Sci. 2001;46:230–241. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz NT, Kalff JC, Türler A, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. [DOI] [PubMed] [Google Scholar]
- 11.Türler A, Moore BA, Pezzone MA, et al. Colonic postoperative inflammatory ileus in the rat. Ann Surg. 2002;236:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Türler A, Kalff JC, Heeckt P, et al. Molecular and functional observations on the donor intestinal muscularis during human small bowel transplantation. Gastroenterology. 2002;122:1886–1897. [DOI] [PubMed] [Google Scholar]
- 13.Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. [DOI] [PubMed] [Google Scholar]
- 14.Salzman AL. Nitric oxide in the gut. New Horiz. 1995;3:352–364. [PubMed] [Google Scholar]
- 15.Christinck F, Jury J, Cayabyab F, et al. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 1991;69:1448–1458. [DOI] [PubMed] [Google Scholar]
- 16.Stark ME, Bauer AJ, Sarr MG, et al. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology. 1993;104:398–409. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Deitch EA, Mishima S, et al. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia-reperfusion injury. Crit Care Med. 2000;28:3692–3696. [DOI] [PubMed] [Google Scholar]
- 18.Kawachi S, Cockrell A, Laroux FS, et al. Role of inducible nitric oxide synthase in the regulation of VCAM-1 expression in gut inflammation. Am J Physiol. 1999;277:G572–G576. [DOI] [PubMed] [Google Scholar]
- 19.De Winter BY, Boeckxstaens GE, De Man JG, et al. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br J Pharmacol. 1997;120:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moojen TM, Van Gulik TM, Hoek FJ, et al. Possible role of nitric oxide in postoperative ileus: a comparative study. Neurogastroenterol Motil. 1999;11:403–408. [DOI] [PubMed] [Google Scholar]
- 21.Eskandari MK, Kalff JC, Billiar TR, et al. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol. 1997;273:G727–G734. [DOI] [PubMed] [Google Scholar]
- 22.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. [DOI] [PubMed] [Google Scholar]
- 23.Sabath DE, Broome HE, Prystowsky MB. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. [DOI] [PubMed] [Google Scholar]
- 24.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. [DOI] [PubMed] [Google Scholar]
- 25.Mikkelsen HB, Thuneberg L. Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res. 1999;295:485–493. [DOI] [PubMed] [Google Scholar]
- 26.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. [DOI] [PubMed] [Google Scholar]
- 27.Kalff JC, Buchholz BM, Eskandari MK, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126:498–509. [PubMed] [Google Scholar]
- 28.Bauer AJ, Sarr MG, Szurszewski JH. Opioids inhibit neuromuscular transmission in circular muscle of human and baboon jejunum. Gastroenterology. 1991;101:970–976. [DOI] [PubMed] [Google Scholar]
- 29.Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol. 1999;277:G478–G486. [DOI] [PubMed] [Google Scholar]
- 30.Kreiss C, Birder LA, Kiss S, et al. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. [DOI] [PubMed] [Google Scholar]
- 32.Billiar TR. Nitric oxide. Novel biology with clinical relevance. Ann Surg. 1995;221:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285–301. [DOI] [PubMed] [Google Scholar]
- 35.Josephs MD, Cheng G, Ksontini R, et al. Products of cyclooxygenase-2 catalysis regulate postoperative bowel motility. J Surg Res. 1999;86:50–54. [DOI] [PubMed] [Google Scholar]
- 36.Efron DT, Most D, Shi HP, et al. Modulation of growth factor and cytokine expression by nitric oxide during rat colon anastomotic healing. J Gastrointest Surg. 2003;7:393–399. [DOI] [PubMed] [Google Scholar]
- 37.Efron DT, Thornton FJ, Steulten C, et al. Expression and function of inducible nitric oxide synthase during rat colon anastomotic healing. J Gastrointest Surg. 1999;3:592–601. [DOI] [PubMed] [Google Scholar]