Abstract
Objective:
To assess the long-term efficacy of radiofrequency ablation (RFA) and percutaneous ethanol (EtOH) injection treatment of local recurrence or focal distant metastases of well-differentiated thyroid cancer (WTC).
Background:
RFA and EtOH injection techniques are new minimally invasive surgical alternatives for treatment of recurrent WTC. We report our experience and long-term follow-up results using RFA or EtOH ablation in treating local recurrence and distant focal metastases from WTC.
Methods:
Twenty patients underwent treatment of biopsy-proven recurrent WTC in the neck. Sixteen of these patients had lesions treated by ultrasound-guided RFA (mean size, 17.0 mm; range, 8–40 mm), while 6 had ultrasound-guided EtOH injection treatment (mean size, 11.4 mm; range, 6–15 mm). Four patients underwent RFA treatment of focal distant metastases from WTC. Three of these patients had CT-guided RFA of bone metastases (mean size, 40.0 mm; range, 30–60 mm), and 1 patient underwent RFA for a solitary lung metastasis (size, 27 mm). Patients were then followed with routine ultrasound, 131I whole body scan, and/or serum thyroglobulin levels for recurrence at the treatment site.
Results:
No recurrent disease was detected at the treatment site in 14 of the 16 patients treated with RFA and in all 6 patients treated with EtOH injection at a mean follow-up of 40.7 and 18.7 months, respectively. Two of the 3 patients treated for bone metastases are free of disease at the treatment site at 44 and 53 months of follow-up, respectively. The patient who underwent RFA for a solitary lung metastasis is free of disease at the treatment site at 10 months of follow-up. No complications were experienced in the group treated by EtOH injection, while 1 minor skin burn and 1 permanent vocal cord paralysis occurred in the RFA treatment group.
Conclusions:
RFA and EtOH ablation show promise as alternatives to surgical treatment of recurrent WTC in patients with difficult reoperations. Further long-term follow-up studies are necessary to determine the precise role these therapies should play in the treatment of recurrent WTC.
Radiofrequency ablation (RFA) and percutaneous ethanol (EtOH) injection techniques are new minimally invasive alternatives to surgical treatment of local or regional recurrence of well-differentiated thyroid cancer (WTC). We report our experience and long-term follow-up results using RFA or EtOH ablation in treating local recurrence and distant focal metastases from WTC.
Thyroid cancer is the most common endocrine malignancy, accounting for an estimated 22,000 new cases in the United States in 2003 alone.1 Papillary carcinoma is the most common subtype of the well-differentiated thyroid carcinomas (WTC). It has a relative frequency ranging from 75% to 85% among all thyroid cancers, and frequently follows an indolent course, with overall 10-year survival rates reported at 90% to 98%.2–4 Treatment of WTC consists of total or subtotal thyroidectomy, with resection of suspicious lymph nodes in the central compartment. Modified neck dissection is the accepted treatment of patients with lymph node metastasis to the lateral compartments of the neck. Patients with only central compartment lymph node metastasis usually undergo central compartment node dissection only.
The overall recurrence and mortality rates for WTC have been reported at 20.5% and 8.4%, respectively, at a mean follow-up of 11.3 years.5 Patients with thyroid cancer are routinely monitored for recurrence by ultrasound examination of the central and lateral compartments of the neck, along with serum thyroglobulin testing. When thyroid cancer recurs, it is typically found within the surgical bed or in lymph nodes of the central or lateral compartments.4
Factors that have been associated with recurrence include young age at diagnosis, large size of the primary tumor, extracapsular spread, and a known distant metastasis.6 The 2 most common sites for distant metastasis of papillary carcinoma of the thyroid are to the lungs and bone.2 Cases of distant metastatic spread have been reported to occur from 5 to 47 years after initial treatment.7,8 Reported 10-year survival rates for patients with lung and bone metastases are 53% and 15%, respectively.9–11 Following diagnosis of distant metastases, overall mortality rates at 5 and 10 years are 65% and 75%, respectively.12
Recurrence in the central compartment can be either in lymph nodes or in the thyroid bed. A recurrence in the thyroid bed results in increased rates of morbidity and mortality.13 Recurrence in the lateral compartment usually occurs as lymph node metastasis. Surgery is recommended for recurrence in the central or lateral compartments of the neck that can be identified by ultrasonography (US). Reoperative surgery in the central or lateral compartments of the neck in patients who have undergone a previous neck dissection is difficult, however, due to distortion of normal tissue planes by scar tissue formation within the surgical bed, and such operations are subsequently associated with a higher rate of complications.9
Percutaneous radiofrequency ablation (RFA) and percutaneous ethanol (EtOH) injection are relatively new, minimally-invasive techniques that have been widely used as alternatives to surgical treatment in patients with hepatocellular carcinoma or liver metastasis from other malignancies.14 RFA has also shown promise as an effective treatment of metastatic malignancies in bone, lung, and kidney.15–22
RFA and EtOH injection with local anesthesia have recently been reported as alternatives to surgery in patients with local recurrence of WTC.4,15,23,24 In this study, we present our experience with treatment of local WTC recurrence by RFA or EtOH at long-term follow-up. We also report our initial experience using RFA to treat focal distant metastases of WTC.
METHODS
The study was approved by our institutional review board, and informed consent to treatment was obtained from all patients before each procedure was performed. This study followed a retrospective design; therefore, informed consent was not necessary for patient participation and was waived by the institutional review board. Patient confidentiality protocols were followed to assure compliance with Health Insurance Portability and Accountability Act regulations. All patients treated with RFA or EtOH ablation had a normal platelet count and normal coagulation parameters. No patient was taking antiplatelet medications or anticoagulants for at least 1 week before the procedure.
Radiofrequency Ablation
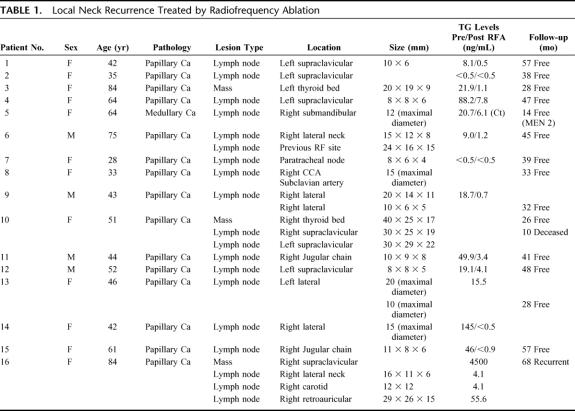
Sixteen adult patients (ages, 28–84 years; mean, 53 years), 12 women and 4 men, underwent percutaneous RFA treatment of biopsy-proven recurrent well-differentiated thyroid cancer in the neck. Three of these patients presented with a recurrent mass in the surgical bed detected by ultrasound after total thyroidectomy. The other 13 patients presented with a suspicious lymph node(s) in the lateral neck on a follow-up ultrasound examination. Twelve of the 13 patients who presented with a lateral neck recurrence previously had a modified neck dissection. The clinical information regarding the histologic findings of tumor size and location is summarized in Table 1. All patients who underwent RFA treatment of neck recurrence had a positive US-guided FNA biopsy prior to the procedure.
TABLE 1. Local Neck Recurrence Treated by Radiofrequency Ablation
All patients were premedicated with 0.625 mg of intravenous droperidol and received intravenous fentanyl citrate (100–400 ìg) and medazolam (1–4 mg) that were titrated to patient discomfort during the procedure. Two 180-cm2 grounding pads were placed horizontally on the patient's anterior or posterior chest wall and connected to the reference electrode port on the RF generator. Under ultrasound guidance with a 10 to 14 MHz linear transducer a single RF electrode (Cool-tip; Radionics Inc., Burlington, MA) with a 1- or 2-cm active tip was inserted into the mass. The RF electrode was connected to an RF generator (Cosman Coagulator-1, Radionics Inc.) and treated with the maximum allowable current for between 2 and 12 minutes. A peristaltic pump was used to perfuse chilled water (15°–20°C) at 80 mL/min through the perfusion port of the electrode to prevent tissue charring and to improve the radius of RF energy deposition. (The radius of thermocoagulation for a noninternally cooled treatment is 8 mm from the active portion of the RF electrode. This radius can be increased up to 10 to 12 mm with internal perfusion.) Internal cooling was only used for masses exceeding 1 cm in greatest dimension. The electrode tip temperature was maintained at 90°C for 2 minutes without internal cooling. Both the US-detected appearance of microbubbles and the achievement of a cytotoxic temperature of 50°C within the mass were accepted together as the endpoint of the treatment procedure.
RFA induces focal coagulative necrosis to eradicate small areas of tissue in a controlled fashion. Continuous real-time US monitoring with gray-scale and color-Doppler imaging was performed to identify proper electrode position and assess microbubble formation during the RF ablation. The presence of microbubbles results from local formation of water vapor as RF energy boils tissue within the treatment region. The temperature within the mass was measured to confirm achievement of the cytotoxic threshold temperature of 50°C. If the cytotoxic threshold was not reached, an additional RF treatment was performed. Immediate loss of the color-Doppler signal within a treated lesion that was previously hypervascular was also taken as adequate evidence of appropriate thermocoagulation in cases where microbubble formation was not observed because internal electrode cooling was not used. Continuous impedance, current delivery, power output, and RF electrode tip temperature were monitored with the RF generator. If the impedance rose 20 ohm above the baseline 3 times within 1 minute during the internally cooled RF treatments, then the treatment was stopped and the temperature within the mass recorded. At the end of a given RF treatment, the RF electrode was repositioned into the tumor mass to measure the intratumoral temperature.
Metastatic lymph node lesions ranged in size from 8 to 40 mm, with a mean diameter of 17 mm. The largest mass was 4 cm in diameter and required 5 separate treatments to ensure complete thermocoagulation. The baseline impedance of the treated areas ranged from 75 to 125 ohm. The peak current output ranged from 1000 to 1300 mA. Cytotoxic threshold temperature was reached at the conclusion of treatment in all cases but some patients required more than one treatment (range, 1–6) to reach the cytotoxic level.
A total of 4 adult patients (ages, 59–81 years; mean, 72 years), 2 women and 2 men, underwent RFA for focal distant metastases of WTC. These metastases were determined to be focal by whole-body sestamibi scan. The clinical information regarding the histologic findings of size and location of these metastases is summarized in Table 2. Three of these patients had solitary metastases to the bone, and 1 patient had a solitary metastasis to the lung. RFA of these metastatic lesions was performed as previously described, under intravenous conscious sedation and local anesthesia, using CT guidance and a 2.5-cm active tip cluster RF electrode (Cool-tip; Radionics Inc.). Four 180-cm2 grounding pads were placed on the patient's thorax, and treatments were administered to each lesion ranging in duration from 2 to 6 minutes, obtaining a maximum temperature of 52° to 75°C. Distant metastatic lesions ranged in size from 27 to 60 mm, with a mean diameter of 42 mm. The largest lesion was 6 cm in diameter and required 5 separate treatments to achieve complete thermocoagulation. The baseline impedance of the treated areas ranged from 54 to 180 ohm. The peak current output ranged from 400 to 1980 mA. Cytotoxic threshold temperature was reached at the conclusion of treatment in all cases, but some patients required more than one treatment (range, 2–6) to reach the cytotoxic level.
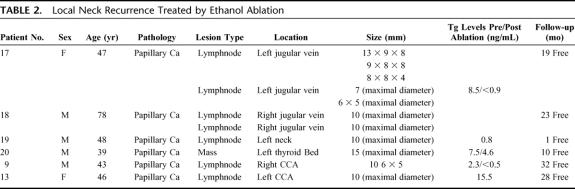
TABLE 2. Local Neck Recurrence Treated by Ethanol Ablation
Ethanol Ablation
Six adult patients (ages, 39–78 years; mean, 50 years), 2 women and 4 men, underwent ultrasound-guided percutaneous ethanol injection treatment of biopsy-proven recurrent WTC in the neck. One of these patients presented with a recurrent mass in the central compartment after a total thyroidectomy on routine US follow-up. The other 5 patients presented with a suspicious lymph node(s) in the lateral compartment of the neck on a follow-up ultrasound examination. The clinical information regarding the histologic findings of tumor size and location is summarized in Table 3.
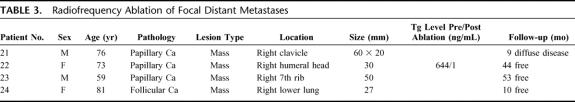
TABLE 3. Radiofrequency Ablation of Focal Distant Metastases
All patients who underwent EtOH injection treatment of a neck recurrence had a positive US-guided FNA biopsy preceding the procedure. All but 1 of the 5 patients with suspicious lymph nodes in the lateral compartment of the neck had a prior modified neck dissection on the side of the recurrence. Local anesthesia was achieved with 1.5% buffered lidocaine, and 0.2 to 2.0 mL of pure dehydrated ethanol was injected through a 22- or 25-gauge needle into each region of recurrence under real-time US guidance using a 10- to 14-MHz linear transducer. EtOH injection induces tissue necrosis as a result of cellular dehydration and protein denaturation. Real-time US monitoring is usually performed to ascertain the correct position of the needle in the lymph node or lesion being treated. Immediately following the injection, the treated lymph node becomes hyperechogenic as compared with the surrounding soft tissues in the neck and the needle becomes obscured, due to the presence of microbubbles in the ethanol solution as it disseminates throughout the tumor. After a short time (typically <1 minute), this intensely echogenic zone diminishes, thus allowing needle visualization.
Following RFA or EtOH injection patients were monitored in the radiology postprocedure recovery room for 2 to 4 hours with continuous observation of all vital signs. All patients were then discharged and given oral narcotics. Clinical follow-up consisted of a physical examination, serum thyroglobulin level testing, and a follow-up high-resolution US at 3-month intervals for the first year and then every 6 months thereafter. All patients who underwent RFA or EtOH ablation for neck recurrence were treated as outpatients, and were discharged within 6 hours of treatment.
RESULTS
Neck Recurrence
Radiofrequency Ablation
A total of 16 adult patients (age range, 28–84 years; mean, 53 years), 12 women and 4 men, underwent RFA treatment of neck recurrence of WTC. The mean size of the treated lesions was 17 mm (range, 8–40 mm). In all cases, intravenous conscious sedation was used to supplement the local anesthesia. Fifteen of the 16 patients with neck recurrence treated by RFA had histologic findings of papillary thyroid carcinoma, and 1 patient had medullary carcinoma as a component of the MEN 2 syndrome. The range of follow-up in this treated group was 10 to 68 months, with a mean follow-up of 40.7 months. All patients were followed with US-color Doppler examination, and thyroglobulin levels were available in 11 of the 16 patients. The patient with medullary carcinoma was followed by monitoring serum calcitonin.
Thirteen of the 16 patients who underwent RFA had only 1 site treated. One of these 13 patients had a new recurrence inferior to the treated site but no evidence of recurrence at the initial treatment site itself. This patient refused to have a modified neck dissection at the time that the lateral neck metastatic node was initially discovered and elected to have RFA. The patient underwent a modified neck dissection after developing a recurrence at the treated site. Subsequent follow-up (31 months) has since remained negative for recurrence in the neck. One of these 13 patients presented during follow-up with a new 10-mm lymph node recurrence that was not amenable to RFA due to its close proximity to the right common carotid artery. This patient was successfully treated with EtOH ablation, as a safer alternative, and has subsequently remained free of disease at each treatment site. Another patient within this group underwent both RFA and EtOH in the same treatment session. In this patient, RFA was performed on a 20 mm lymph node recurrence, and EtOH ablation was performed on a 10-mm lymph node recurrence in the left neck region, due to the inherent difficulty in accurately placing the RF probe in lesions of 1 cm or smaller in size.
Three of the 16 patients with neck recurrence treated by RFA had treatment of multiple sites within separate treatment sessions. One patient had a suspicious biopsy of the treated lesion after the first RFA and was subsequently retreated. Follow-up ultrasound examinations have since demonstrated no recurrence at this treatment site. The second of these 3 patients had 3 separate sites treated over 3 separate RFA sessions. This patient died of diffuse metastatic disease to the lungs 10 months after the ablation procedure, but remained free of disease in the treated sites. (This patient was referred to us for a mediastinal recurrence after a previous total thyroidectomy and modified neck dissection at an outside hospital. She underwent a partial sternotomy for removal of the mediastinal disease, and a single site of neck recurrence was treated with RFA. The histologic findings of this patient's tumor included a tall cell variant of papillary carcinoma). The third patient had 4 separate sites of recurrence that required 4 separate RFA treatment sessions; all of these treatments were done in the right neck. This patient underwent surgical treatment of papillary thyroid carcinoma in 1989 and had 2 subsequent modified neck dissections on the right side in 1992 and 1997.
US follow-up was obtained in all 16 patients with neck recurrence treated by RFA. In 15 of these patients, ultrasound evaluation at 3 months post-treatment showed loss of the hypervascular color-Doppler pattern that was initially present in the untreated lesions. In addition, pretreatment lymph nodes that were initially hypoechoic on gray-scale imaging became more echogenic (Figs. 1–4). A new 9-mm cyst with a small amount of adjacent echogenic, nonhypervascular tissue was seen in the site of a previously treated lymph node in 1 patient, but this lesion has remained unchanged throughout the duration of follow-up and has not undergone further workup. Throughout the duration of patient follow-up in this study, only 1 patient has presented with a new recurrence in the neck, but this recurrence did not occur in a previously treated lymph node. Ultrasound-guided fine-needle aspiration biopsy was performed 3 months after RFA treatment in 5 of these patients. Biopsy results demonstrated necrosis with inflammatory cells in all of these patients, but a few cells were noted to be suspicious for malignancy on pathologic analysis of the specimen in 1 patient. This patient underwent a second RF treatment of this lesion, and subsequently had the mass removed for cosmetic reasons.
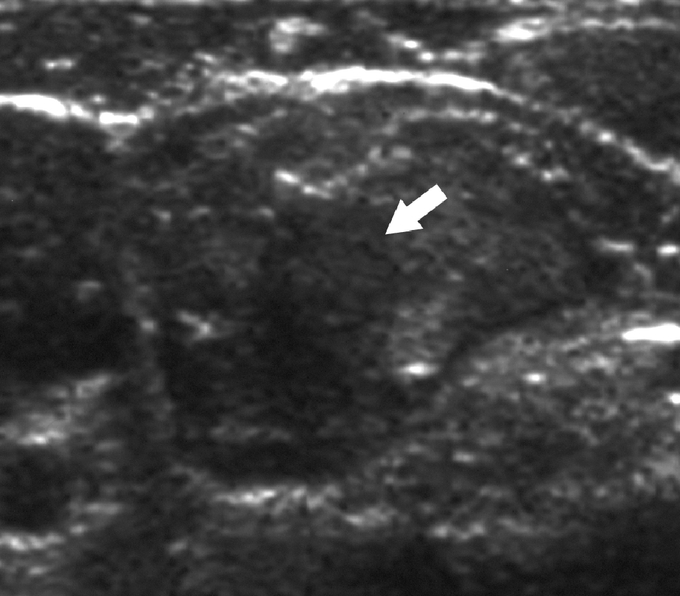
FIGURE 1. A 42-year-old woman with previous thyroidectomy for papillary carcinoma develops a 1.5-cm solitary lymph node metastasis in the right jugular chain. The patient refused surgery. Sagittal gray scale ultrasound image prior to ablation shows a rounded hypoechoic metastatic lymph node (arrow).
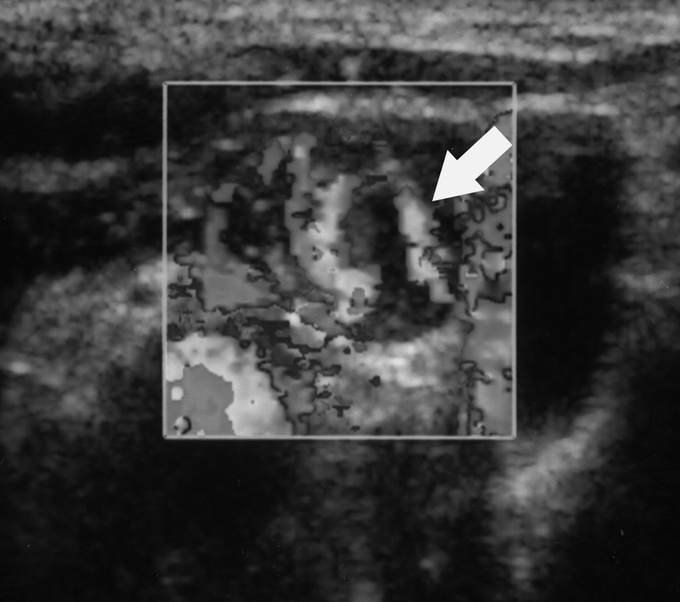
FIGURE 2. Color Doppler image of metastatic lymph node displayed in gray scale shows extensive hypervascularity (arrow).
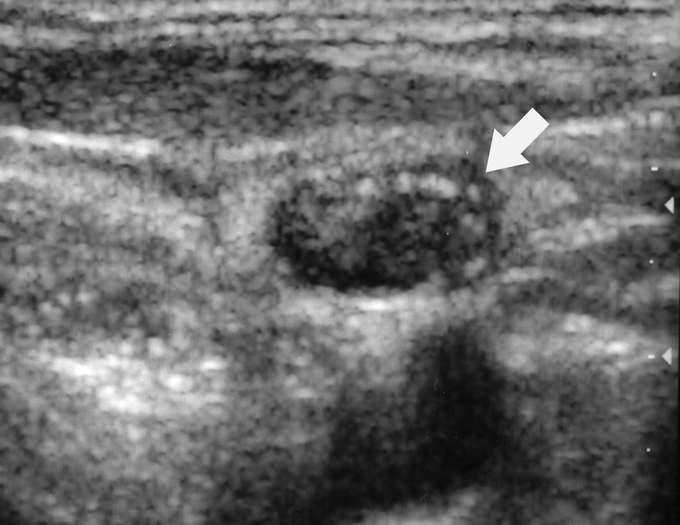
FIGURE 3. Sagittal gray scale ultrasound image of treated lymph node 3 months after RFA shows increased internal echogenicity (arrow).
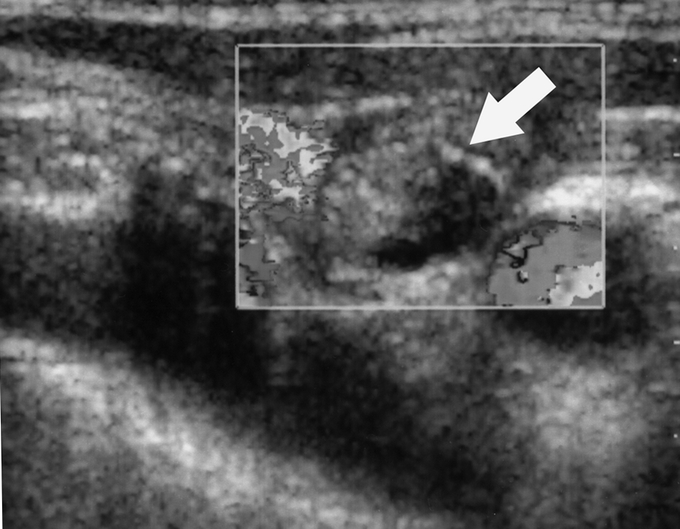
FIGURE 4. Color Doppler image of metastatic lymph node displayed in gray scale 3 months after RFA shows loss of the internal hypervascularity (arrow). At 51 months of follow-up, the patient remains disease free.
Follow-up serum thyroglobulin levels were evaluated in 11 of the 16 patients who underwent RFA ablation for neck recurrence. Thyroglobulin levels within this group showed a drop from a mean pretreatment value of 37.0 ng/mL to a mean value of 1.9 ng/mL following treatment. Our patient treated for neck recurrence of medullary thyroid carcinoma was followed by serum calcitonin levels, which demonstrated a drop from a pretreatment value of 20.7 ng/mL to a value of 6.1 ng/mL in the post-treatment period.
One patient experienced hoarseness immediately following the RFA procedure. This patient underwent treatment of recurrence of papillary carcinoma within the central compartment of the neck following total thyroidectomy. In this case, the RFA treatment most likely resulted in thermal injury to the recurrent laryngeal nerve. Although the patient's symptoms improved over the next 2 months, the right vocal cord palsy was confirmed by laryngoscopic examination. A 5-mm skin burn developed in 1 patient at the RF electrode entry site, most likely as a result of the protrusion of the proximal portion of the electrode tip through the skin during the ablation procedure. The burn healed in 2 weeks with topical antibacterial ointment and oral cephalexin (500 mg, QID for 1 week). No grounding pad burns or delayed infections were identified in any of the treated patients. Self-limited neck swelling and regional discomfort were reported in all 16 patients but resolved within 1 to 2 weeks.
Ethanol Ablation
Six adult patients (age range, 39–78 years; mean, 50 years), 2 women and 4 men, underwent US-guided percutaneous ethanol injection treatment of WTC recurrence in the neck. The mean size of the treated lesions was 11.4 mm (range, 6–15 mm). In all cases, treatment was provided using local anesthesia. All patients had a biopsy proven papillary carcinoma prior to EtOH ablation. The range of follow-up in this group was 3 to 32 months, with a mean follow-up of 18.7 months. Five of these 6 patients underwent routine ultrasound examination with color-Doppler analysis during the follow-up period, and serum thyroglobulin levels were followed for 3 of the 6 patients.
Four of the 6 patients with neck recurrence treated by EtOH ablation underwent a single treatment. One patient underwent EtOH ablation after attempts to place the RFA probe within a cystic lesion were unsuccessful. One patient underwent a repeat EtOH injection treatment of the persistent abnormal appearance of a previously treated node on follow-up ultrasound examinations over a 6-month period. The treatment site has since been free of disease on follow-up ultrasound examinations. One other patient underwent successful EtOH ablation to 3 lymph nodes in a single session. This patient underwent a second session for treatment of 2 new lymph node recurrences 1 year later, and all 5 treatment sites have been free of disease throughout subsequent follow-up. No recurrent disease has been detected to date on ultrasound examination of the treatment site in all patients who underwent EtOH ablation of recurrent WTC in the neck. US evaluation at 3 months post-treatment showed loss of the hypervascular color-Doppler pattern previously seen in untreated lesions. In addition, pretreatment lymph nodes that were initially hypoechoic on gray-scale imaging became more echogenic in these patients following EtOH injection treatment. One patient demonstrated a new 10-mm lymph node inferior to the site of a previous EtOH ablation treatment on a follow-up ultrasound examination. This patient subsequently underwent US-guided fine-needle aspiration biopsy of the lesion, which was positive for cancer, and this new metastatic lymph node was treated with EtOH injection. No other US-guided fine-needle aspiration biopsies were performed in this group.
Follow-up serum thyroglobulin levels were evaluated in 3 of the 6 patients who underwent EtOH ablation for neck recurrence. Thyroglobulin levels in these patients showed a drop from a mean pretreatment value of 6.1 ng/mL to a mean value of 2.0 ng/mL following treatment. One patient with a recurrent lesion in the central compartment of the neck treated by EtOH ablation experienced temporary hoarseness in the immediate post-treatment period, but experienced full recovery within the 3 to 4 hours preceding discharge. This group experienced no other procedure-related complications.
Distant Metastases
A total of 4 patients underwent RFA treatment of focal distant metastasis of WTC under intravenous conscious sedation and local anesthesia. Three patients (age range, 59–76 years; mean, 69.3 years), 2 men and 1 woman, were treated with CT- guided RFA for focal bone metastasis of WTC (mean size, 46.7 mm; range, 30–60 mm), and 1 patient (female, 81-year-old) underwent CT-guided RFA of a solitary lung metastasis. The tip of the RF probe can be accurately placed under CT guidance, and the treatment temperature carefully controlled by manipulation of the dose of RF energy delivered. Similar to RFA treatments for local recurrence of WTC in the neck, patients can tolerate this procedure to completion under local anesthesia and conscious sedation. All patients tolerated the RFA treatment to completion and experienced no procedure-related complications. All patients were concomitantly treated with 131I following RFA, and a follow-up whole body scan was obtained in all cases. The range of follow-up in patients with metastatic WTC to bone was 9 to 53 months, with a mean follow-up of 35.3 months, while the follow-up period for our patient treated for lung metastasis was 10 months.
Of the 3 patients that underwent RFA for bone metastases, one presented at 1 year of follow-up with persistent disease in the treatment site (right humeral head) and subsequently underwent a second CT-guided RFA treatment with concomitant systemic 131I ablation therapy. A follow-up whole-body scan demonstrated no further evidence of disease. A second patient within this group underwent RFA treatment of a metastasis to the medial aspect of the right clavicle. This patient was initially referred to us for metastatic WTC following chest wall radiation and radioactive iodine ablation therapy, for widely spread disease to bone. Nine months after RFA treatment, this patient presented with persistent disease at the previously treated site, and a new mass in the substernal region, just inferior to this site. This patient was subsequently lost to follow-up. The third and final patient of this group underwent RFA treatment and concomitant systemic 131I ablation for a solitary metastasis to the right 7th rib. A biopsy specimen obtained from the treatment site at 3 months of follow-up demonstrated absence of disease, and all subsequent whole-body scans have been negative throughout 53 months of follow-up. At this point in time, 2 of the 3 patients treated for WTC metastasis to bone have no evidence of disease at the treated site.
One patient underwent CT-guided RFA treatment of a solitary metastasis of WTC (follicular subtype) to the right lower lobe in the lung. One month post-treatment, the patient underwent a whole-body scan, which demonstrated multiple foci of increased activity in the neck, but no evidence of disease in the previously treated site within the lung. The patient subsequently underwent 131I treatment of neck recurrences. A second whole-body scan performed 3 months after this treatment showed persistent increased uptake at the sites of the reported previous activity in the neck. Systemic ablation with 131I treatment was again performed, but the patient still presented with an increased area of uptake in the right neck on follow-up whole-body scan. Several more treatments with 131I ensued, and a most recent whole-body scan demonstrated persistent uptake of radioiodine in the neck but absence of disease in the previously treated site within the lung.
DISCUSSION
Surgical treatment of patients with recurrent WTC in the neck can be challenging because of scar formation from the previous surgery in either the central or lateral compartments of the neck. Routine ultrasound follow-up in these patients can identify small lymph nodes, often less than 10 mm in maximum diameter, embedded within scar tissue surrounding the previous surgical bed. This scar tissue often causes marked distortion of the normal tissue planes within the neck, making surgical dissection difficult even for experienced surgeons. Such surgical reexploration of the neck can be associated with a high rate of morbidity in these patients, making minimally invasive US-guided ablation therapies an attractive treatment option. In this paper, we present our extended experience and follow-up with RFA for local recurrence of well-differentiated thyroid malignancy in addition to our initial experience in treating focal distant metastatic disease. We also report our initial experience with EtOH ablation for local recurrence of well-differentiated thyroid malignancy.
Both RFA and EtOH have advantages and disadvantages. RFA produces a larger zone of tumor destruction than EtOH, and this energy can be finely adjusted. RFA can, therefore, treat a larger lesion. This increased energy, however, is more likely to cause a permanent injury to an adjacent nerve. One method to avoid thermal nerve injury in these cases is to inject a bolus of 5% dextrose in water solution between the mass and the expected location of the nerve, which will serve as a protective thermal barrier to RF energy. We prefer RFA for lesions greater than 10 mm and in areas outside the neck and EtOH for lesions in the neck less than 10 mm or for lesions that are in close proximity to nerves. Therefore, size and anatomic location determine whether we use RFA or EtOH for first-line ablative therapy. Thermal damage to nerves during RFA treatment should be more of a concern than damage to blood vessels, as moving blood within the lumen of the vessel serves as an adequate means to dissipate heat emitted from the RF applicator. Extreme caution should be exercised in the treatment of lesions within the lateral aspect of the central compartment that may be in close proximity to the recurrent laryngeal nerve. In comparison to treatments within the liver, power and current used in RFA of cervical lesions are much smaller, and treatment duration is relatively short, so intralesional temperatures are the key to measuring success in these cases, unlike in some other ablative techniques that use impedance-based parameters. If the temperature of the tissue within the treated lesion (or lymph node) is maintained at just above 60°C, effective thermocoagulation can be achieved with minimal thermal damage to surrounding tissues.
Lymph nodes that are successfully treated by RFA decrease in size (by approximately 95%) and become hyperechoic.15 Assessing the adequacy of treatment of cystic lymph nodes can be more difficult because these nodes may not lose their cystic appearance following treatment. They should, however, undergo no further increase in size. Any increase in size of a previously treated lesion should suggest viable tumor which should be confirmed by US-guided FNA biopsy. Successfully ablated lymph nodes by EtOH injection should decrease in size and lose vascular flow if previously present on color-Doppler examination. A fine-needle biopsy performed in a previously treated lymph node usually yields a mixture of necrotic tissue and inflammatory cells.4
Our long-term follow-up data suggest that RFA and EtOH ablation are feasible alternative techniques in the treatment of local neck recurrence of WTC.15,25–27 We do not recommend these treatments in patients who have not had previous total thyroidectomy or modified neck dissection. However, we have treated 2 patients successfully who refused surgery. One could argue that if high resolution US only showed 1 or 2 sites of disease amenable to percutaneous ablative therapy, then ablation is a viable treatment option. However, a larger randomized series might be to prove long-term efficacy compared with surgery.
Bone metastases from WTC usually present as bone pain or pathologic fractures. Metastatic WTC to the bone usually demonstrates a poor response to 131I treatment.28,29 The treatment options for bone metastases include external beam radiation therapy, chemotherapy, and surgery.30 Several reports had shown that RFA is widely used in the treatment of distant metastatic spread of a variety of different tumors.22,31 RFA has also been shown to be an effective treatment of metastases of WTC to bone, providing patients with relief of pain within the first 24 to 48 hours without significant morbidity.22,32–35 RFA was demonstrated in our series to be effective in the treatment of bone metastases in 2 of the 3 patients with absence of previously demonstrated uptake of 131I at the treatment site in 44 and 53 months of follow-up. Thyroglobulin levels showed reduction with treatment from 644 to 1 ng/mL in 1 patient in whom the thyroglobulin follow-up was available, and disappearance of the lesion was confirmed by CT scan. We also report successful treatment with RFA of a 2.7-cm solitary metastasis to lung from WTC. This patient had no evidence of recurrence in 10 months of follow-up.
CONCLUSION
Surgery is the gold standard for treatment of recurrent WTC in the central or lateral compartments of the neck. Our results with RFA and EtOH ablation are very exciting and show promise as minimally invasive alternatives to surgical treatment in some patients. RFA also shows promise as an effective treatment modality for focal distant metastases of WTC. Further long-term follow-up studies are necessary to determine the precise role these therapies should play in the treatment of recurrent WTC and whether certain more invasive surgical procedures can be replaced.
Footnotes
Reprints: Damian E. Dupuy, MD, Department of Diagnostic Imaging, Brown Medical School, Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903. E-mail: ddupuy@lifespan.org.
REFERENCES
- 1.Landis SH, Murray T, Bolden S, et al. Cancer Statistics, 1999. CA Cancer J Clin. 1999;48:8. [DOI] [PubMed] [Google Scholar]
- 2.Cotran RS, Kumar V, Collins T. Robbins: Pathologic Basis of Disease. Philadelphia: Saunders, 1999:1142. [Google Scholar]
- 3.Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) Program 1973–1991. Cancer. 1997;79:564–572. [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Monchik JM. Radiofrequency ablation of recurrent thyroid cancer. In: Ellis, Curley, Tanabe, eds. Textbook Radiofrequency Ablation of Cancer. New York: Springer-Verlag, 2003:213–223. [Google Scholar]
- 5.Loh K, Greenspan FS, Gee L, et al. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–3561. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi S, Murakami N, Kawamoto H. Classification of papillary cancer of the thyroid based on prognosis. World J Surg. 1994;18:552–558. [DOI] [PubMed] [Google Scholar]
- 7.Schlumberger M, Tubiana M, de Vathaire F, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. [DOI] [PubMed] [Google Scholar]
- 8.Foneseca P. Thyroid lung metastasis diagnosed 47 years after thyroidectomy. Ann Thorac Surg. 1999;67:856–857. [DOI] [PubMed] [Google Scholar]
- 9.Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinoma: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. [DOI] [PubMed] [Google Scholar]
- 10.Casara D, Rubello D, Saladini G, et al. Distant metastases in differentiated thyroid cancer: long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori. 1991;77:432–436. [DOI] [PubMed] [Google Scholar]
- 11.Bernier M, Leenhardt L, Hoang C, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568–1573. [DOI] [PubMed] [Google Scholar]
- 12.Ruegemer JJ, Bergstalh EJ, Ryan JJ, et al. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67:501–508. [DOI] [PubMed] [Google Scholar]
- 13.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and longterm outcome in 2444 consecutively treated patients. World J Surg. 2002;8:879–885. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Cioni D, Crocetti L, et al. Percutaneous ablation of hepatocellular carcinoma. State of the Art Liver Transpl. 2004;10(suppl):91–97. [DOI] [PubMed] [Google Scholar]
- 15.Dupuy DE, Monchik JM, Decrea C, et al. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001;130:371–977. [DOI] [PubMed] [Google Scholar]
- 16.Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. [DOI] [PubMed] [Google Scholar]
- 17.Dupuy DE, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–1266. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;83:4195–4203. [DOI] [PubMed] [Google Scholar]
- 19.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. [DOI] [PubMed] [Google Scholar]
- 20.Gervais DA, McGovern FJ, Wood BJ, et al. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy DE, Mayo-Smith WW, Cronan JJ. Image-guided biopsy and radiofrequency ablation of renal masses. Semin Interv Radiol. 2000;17:373–379. [Google Scholar]
- 22.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities. Parts I and II. J Vasc Interv Radiol. 2001;12:1021–1032, 1135–1148. [DOI] [PubMed]
- 23.Pacini F. Role of percutaneous ethanol injection in management of nodular lesions of the thyroid gland. J Nuc Med. 2003;44:211–212. [PubMed] [Google Scholar]
- 24.Charboneau JW, Hay ID, van Heerden JA. Persistent primary hyperparathyroidism: successful ultrasound-guided percutaneous ethanol ablation of an occult adenoma. Mayo Clin Proc. 1988;63:913–917. [DOI] [PubMed] [Google Scholar]
- 25.Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol. 2002;178:699–704. [DOI] [PubMed] [Google Scholar]
- 26.Braga-Basaria M, Ringel M. Beyond radioiodine: a review of potential new therapeutic approaches for thyroid cancer. J Clin Endocrinol Metab. 2003;88:1947–1960. [DOI] [PubMed] [Google Scholar]
- 27.Solbiati L, Ierace T, Dellanoce M, et al. Percutaneous US-guided radiofrequency ablation of metastatic lymph nodes from papillary cancer of the thyroid gland: initial experience in two cases. Radiology. 1998;209:385. [Google Scholar]
- 28.Proye CA, Dromer DH, Carnaille BM, et al. Is it still worthwhile to treat bone metastasis from differentiated thyroid carcinoma with radioactive iodine? World J Surg. 1992;16:640–646. [DOI] [PubMed] [Google Scholar]
- 29.Petrich T, Widjaia A, Musholt TJ, et al. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: side effects and influence of age. Eur J Nucl Med Mol Imaging. 2001;28:203–208. [DOI] [PubMed] [Google Scholar]
- 30.Payne R, Janjan NA. Management of metastatic bone pain. In: Payne R, Patt RB, Hill CS, eds. Assessment and Treatment of Cancer Pain, vol. 12. Seattle: IASP, 1998:269–273. [Google Scholar]
- 31.Livraghi T, Solbiati L. Complication after cool-tip RF ablation of liver cancer: initial report of the Italian Multicenter Cool-tip RF study group. Radiology. 2000;217:18. [Google Scholar]
- 32.Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radiofrequency ablation. Radiology. 2002;224:97. [DOI] [PubMed] [Google Scholar]
- 33.Jain SK, Dupuy DE. Radiofrequency ablation for skeletal metastasis of papillary carcinoma of the thyroid: conjunct treatment with radioablative iodine. Endocrinologist. 2004;141:5–11. [Google Scholar]
- 34.Sugitani I, Kasai N, Fujimoto Y. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–148. [DOI] [PubMed] [Google Scholar]
- 35.Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastases. Crit Rev Oncol Hematol. 2001;40:239–250. [DOI] [PubMed] [Google Scholar]