Abstract
Objective:
To assess the value of preoperative imaging studies and the intraoperative assessment of perihepatic lymph nodes in patients undergoing partial hepatectomy for malignancy.
Summary Background Data:
Perihepatic lymph node status is an important prognostic factor for patients undergoing hepatic resection for 1o and metastatic cancer. The value of preoperative imaging studies and intraoperative assessment of perihepatic nodes is unknown.
Methods:
Perihepatic lymph nodes were sampled in 100 patients undergoing resection for 1o and metastatic hepatic malignancy. At the time of sampling, participating surgeons assigned a clinical suspicion score (scale, 1–5: 1 = clinically negative, 5 = clinically positive). Preoperative CT scans and PET scans were reviewed in a blinded fashion by 2 radiologists. Clinical assessment, CT, and PET scan results were analyzed in the context of the pathologic status of the lymph nodes.
Results:
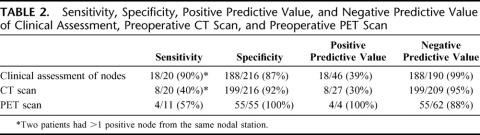
A mean of 3.2 ± 0.2 nodes were sampled per patient. Fifteen patients had metastatic disease in perihepatic lymph nodes; 13 had suggestive findings on preoperative CT or PET, and 2 were clinically positive at exploration. Clinical assessment had a high negative predictive value (NPV) = 99% but a low positive predictive value (PPV) = 39%. Similarly, CT scans had a high NPV = 95% and a low PPV = 30%. PET scans had a NPV = 88% and a PPV of 100%. Of the 48 patients with both negative preoperative CT and PET scans, only 1 (2.1%) had metastatic nodal disease, and this was suspected based on the clinical assessment. Of the patients with negative CT and PET scans and a negative clinical assessment (n = 39), none had involved perihepatic nodes.
Conclusions:
In patients with 1o and metastatic liver cancer, the incidence of truly occult metastatic disease to perihepatic lymph nodes is low. Routine sampling of perihepatic lymph nodes will therefore have a low yield in patients without some evidence of disease on preoperative CT or PET scans or at the time of exploration.
Imaging studies (CT scan and PET scan) and the intraoperative assessment of lymph nodes were analyzed in the context of the pathologic status of perihepatic lymph nodes at the time of partial hepatectomy for malignancy. These modalities can be used in combination to determine which patients in whom lymph node sampling has the greatest yield. The incidence of truly occult metastatic disease in perihepatic lymph nodes is very low.
Resection remains the most effective therapy for selected patients with primary and secondary hepatic malignancy. In general, metastatic disease to extrahepatic sites is associated with poor survival and has been a contraindication to hepatic resection. In particular, regional metastases to perihepatic lymph nodes are a significant negative prognostic factor for both primary1–3 and secondary hepatic malignancies.4–8 On the other hand, selected patients with limited regional nodal disease may benefit from resection.7 Furthermore, as improvements in systemic chemotherapy continue to evolve, particularly for metastatic colorectal cancer, regional lymph node status may not necessarily be used to determine resectability but rather to select patients for optimal postresection chemotherapeutic therapies.9
The reported rate of perihepatic lymph node positivity in patients undergoing hepatic resection varies by histology,10 ranging from 10.6%7 to 28%5 in patients with hepatic colorectal metastases, 27%3 to 45%11 for intrahepatic cholangiocarcinoma and approximately 5% for hepatocellular carcinoma.12 Many of these studies include patients with obvious nodal involvement. Clearly, some patients have microscopic disease that is beyond detection by conventional radiographic imaging or even direct palpation. As a result, some authors have advocated routine lymph node sampling or lymphadenectomy for all patients undergoing hepatic resection.10,13
Although it has been suggested that occult metastasis to perihepatic lymph nodes is relatively common in patients with malignant liver disease, the incidence of truly occult disease is difficult to determine. Furthermore, there is currently no consensus regarding the optimal method of preoperative or intraoperative assessment of perihepatic lymph nodes in patients undergoing resection for hepatic malignancy. The present study investigates and compares the relative value of preoperative imaging (CT and PET) and intraoperative assessment of perihepatic lymph nodes, and particularly aims to determine the incidence of metastatic involvement that is clinically and radiographically undetectable.
METHODS
Patients submitted to operation for complete resection of primary (hepatocellular carcinoma, intrahepatic cholangiocarcinoma) or secondary hepatic malignancy between July 2002 and June 2004 at Memorial Sloan-Kettering Cancer Center (MSKCC) comprised the study group. Patients with extrahepatic biliary malignancy (gallbladder cancer and hilar cholangiocarcinoma) and those submitted to operation to confirm extrahepatic disease were not included. Lymph nodes were sampled from up to 3 stations: portocaval, pancreaticoduodenal, and common hepatic artery at the time of hepatic resection (Fig. 1). Patients with suspicious lymph nodes at exploration underwent excisional lymph node biopsy for immediate frozen section analysis only if the results would have influenced treatment; patients were otherwise nonselected, and lymph nodes were sent for routine histology. At the time of lymph node sampling, a “clinical suspicion score” was assigned to each lymph node from each sampled station. Determination of clinical suspicion scores for lymph nodes was made by the attending hepatobiliary surgeon in all cases. The clinical suspicion score used was a Likert scale ranging from 1 to 5. Guidelines for scoring lymph nodes were as follows: score = 1, clinically negative (small, soft); score = 2, likely negative; score = 3, equivocal; score = 4, likely positive; score = 5, clinically positive (large/firm and/or necrotic). Clinical suspicion scores were recorded prospectively; lymph nodes were sent for permanent section and analyzed using routine hematoxylin and eosin staining in the Department of Pathology.
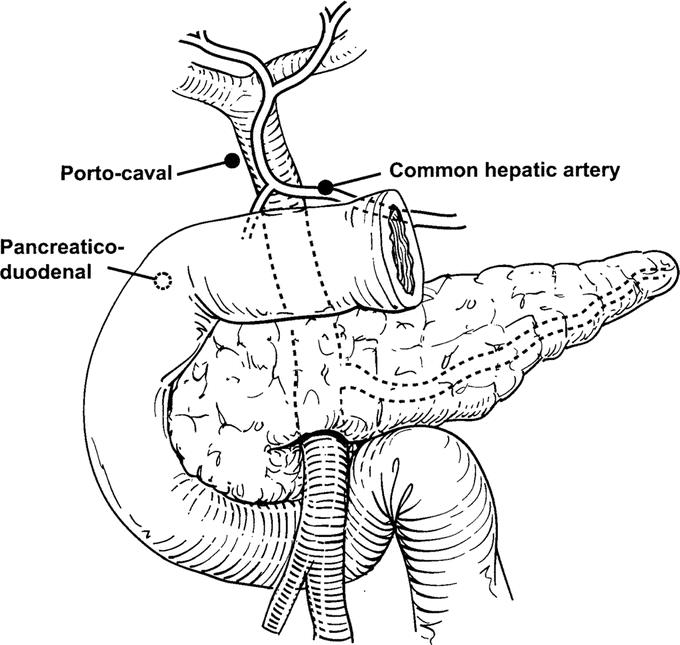
FIGURE 1. Schematic diagram showing lymph node groups sampled.
Measures of accuracy for clinical assessment, CT and PET were calculated using each nodal station as the unit of analysis and pathologic confirmation as the standard (positive or negative). For patients who had more than one node sampled from the same nodal station, the maximum value for each assessment was used. In such cases, if any node was positive histologically, then the result for that nodal station was coded as positive; likewise, if all nodes sampled from the same station were negative histologically, then the nodal station was coded as negative. Sensitivity (specificity) was calculated by dividing the number of true positive (true negative) nodal stations by the number of histologically positive (negative) nodal stations. Positive (negative) predictive value is calculated by dividing the number of true positive (true negative) nodal stations by the number of nodal stations deemed positive (negative) by the assessment (clinical or radiographic).
Receiver operating characteristic curves were used to establish thresholds for clinically “negative” and “positive” lymph nodes. This analysis resulted in a classification of clinically “negative” for lymph nodes with a score of 1 or 2 and “positive” for those with a score of 3, 4, or 5. Clinical suspicion scores were analyzed in the context of pathologic findings to determine the sensitivity, specificity, negative predictive value, and positive predictive value for intraoperative assessment.
Preoperative CT scans obtained within 1 month of operation were reexamined in a blinded fashion by 2 radiologists (L.W. and L.S.). The large majority of scans were performed at MSKCC on multidetector CT scanners (GE Medical Systems, Milwaukee, WI), ranging from 4 to 16 row detectors, with intravenous and oral contrast and with slice reconstruction of 5 mm (range, 2.5–7.5 mm). Scans from other centers were used only if similarly performed on current generation helical CT scanners and determined to be of adequate quality by the radiologists (L.W. and L.S.). Measurements of the largest lymph nodes at each of the 3 stations were made. Portocaval lymph nodes were measured in 2 dimensions. Common hepatic artery lymph nodes and pancreaticoduodenal lymph nodes were measured in their greatest dimension only.
Receiver operating characteristic curves were then used to establish thresholds for radiographically “negative” and “positive” nodes. From this analysis, it was determined that portocaval lymph nodes should be considered “positive” on CT if the cross product of dimensions was ≥0.65 cm2, while pancreaticoduodenal and hepatic artery lymph nodes should be considered “positive” if they were detectable on CT scan. The CT scan findings at each lymph node station were then compared with pathologic findings at the corresponding station to determine sensitivity, specificity, negative predictive value, and positive predictive value for CT. For purposes of this analysis, multiple nodes removed from a nodal station were combined into one data point by taking the highest clinical assessment of these nodes, as described above. Pathologic confirmation was also combined by defining a nodal station as positive if at least one of the nodes sampled from that station was positive (multiple positive nodes at a single station were counted as one).
Many patients included in the study had preoperative PET scans, which were also reexamined in a blinded fashion. The perihepatic lymph nodes were considered “positive” by PET scanning if there was increased FDG uptake seen in the subhilar region. PET findings were then compared with pathologic findings. For the purposes of this aspect of the analysis, the perihepatic nodes were considered and analyzed together as a group, since the PET scans were not uniformly of sufficient resolution to accurately discriminate between the 3 different stations. PET scan findings were compared with pathologic findings (without distinguishing among the 3 nodal groups) to determine sensitivity, specificity, negative predictive value, and positive predictive value for PET.
Approval for the study was obtained from the Institutional Review Board at MSKCC. This study did not mandate any surgical or medical treatment based on the clinical or pathologic status of the nodes. All treatment decisions were made by the attending surgeon and/or medical oncologist. In general, patients with involved extrahepatic lymph nodes confirmed intraoperatively did not undergo resection but there were some exceptions, and this decision was made by the operating surgeon.
RESULTS
Perihepatic lymph node sampling was performed in 100 patients. Metastatic colorectal cancer (n = 75) was the most common indication for partial hepatectomy, followed by hepatocellular carcinoma (n = 11) and intrahepatic cholangiocarcinoma (n = 8) (Table 1). Lymph node sampling was safe with an associated intraoperative complication rate of 2%. One patient sustained an injury to a portal vein branch and another patient had an injury of a duodenal diverticulum, both of which were successfully and uneventfully repaired. There were no postoperative complications attributable to lymph node sampling.
TABLE 1. Distribution of Histologic Diagnoses for Patients in This Series (n = 100)
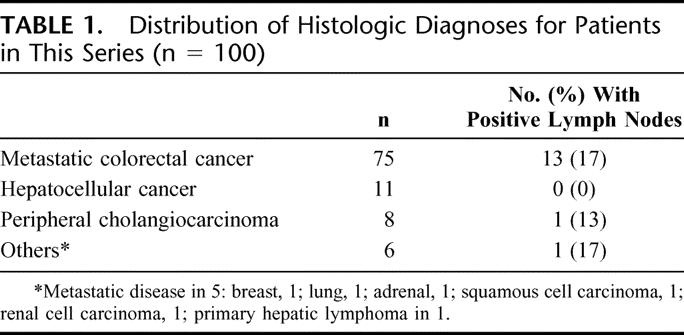
A total of 316 lymph nodes from 236 lymph node stations were analyzed histologically. The mean (± SEM) number of lymph nodes sampled per patient was 3.2 ± 0.23 (median = 3). Not all patients had lymph nodes sampled from all 3 nodal stations. Most commonly, this was because clearly positive nodal disease was identified early in the dissection and confirmed histologically. In other cases, no obvious (visible or palpable) lymph nodes were identified at the station in question, and a more extensive dissection was not considered warranted; this decision was made at the discretion of the attending surgeon. Overall, 22 lymph nodes (7.0% of all lymph nodes removed) in 15 patients (15% of the total patients) had metastatic disease. Six patients had more than one involved node: 4 had involvement of multiple nodal groups and 2 had multiple positive nodes from the same group. The percentage of patients with one or more positive lymph nodes varied by histology and was most common in patients with metastatic liver disease (14 of 80, 17.5%) compared with primary liver cancer (1 of 19, 5.3%) (Table 1). There was no difference in the incidence of lymph node involvement at each of the 3 nodal stations (no. of patients with 1, or more, positive node at the nodal station/no. of patients who had at least 1 node sampled at the station): portocaval 7/77 (9%); pancreaticoduodenal 6/72 (8%); and hepatic artery 7/87 (8%) (P = 0.97). (Two patients had more than 1 positive lymph node in the same nodal station.)
Most of the lymph nodes (82%) examined and sampled intraoperatively were not suspicious for metastatic involvement and received a clinical suspicion score of 1 or 2. The negative predictive value for the clinical assessment of lymph nodes was high (99%), while the positive predictive value was much lower (39%) (Table 2). The 2 nodes that were clinically not suspicious but pathologically positive were found at 2 different stations in 1 patient with metastatic colorectal cancer. All patients in the series had preoperative CT scans. Like intraoperative clinical assessment, CT had a high negative predictive value (95%) and a low positive predictive value (30%). PET scans were performed on 66 patients in this series. The positive predictive value for PET scanning was 100%, although there were only 4 patients in this series who had increased FDG uptake in the subhepatic area. Of note, PET scans failed to detect nodal disease in 7 patients. The sensitivities and specificities for each modality are shown listed in Table 2.
TABLE 2. Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Clinical Assessment, Preoperative CT Scan, and Preoperative PET Scan
Of the 15 patients with nodal metastases, 13 (86.7%) had some suggestion of this possibility on preoperative CT (n= 11) or PET (n = 2), while the remaining 2 patients had a positive intraoperative assessment. To determine the incidence of truly occult metastatic nodal disease, the cohort of patients (n = 48) with both negative CT scans and negative PET scans was analyzed separately. Within this group, only 1 patient (2.1%) had metastatic nodal disease. Nine other patients in the group with negative imaging studies had clinically suspicious lymph nodes that were negative on final histologic examination. Of the patients with both negative imaging studies and negative intraoperative assessments (n = 38), none had lymph node metastases.
DISCUSSION
There is no consensus regarding the optimal technique for evaluating the status of perihepatic lymph nodes at the time of liver resection for malignancy.14 Some have advocated routine nodal sampling,13 while others have advocated routine subhilar lymphadenectomy.10 Lymphatic mapping has been proposed as well, but reported success with the technique in this setting is limited.15 This study was undertaken to assess the value of intraoperative assessment, preoperative CT scan, and preoperative PET scan in selecting patients for perihepatic lymph node biopsy at the time of hepatic resection for malignancy.
The present study documents the utility of intraoperative perihepatic lymph node assessment (Table 1) by the surgeon in selecting patients for lymph node biopsy at the time of hepatic resection for malignancy. The rate of nodal involvement in those with suspicious findings on intraoperative examination (score ≥3, Table 1) was 38%, very similar to that reported by Furhman et al (39%).16 In the present study, we also document a very low rate of pathologically positive nodes among clinically nonsuspicious nodes (1%). Kokudo et al,17 in a retrospective review of their experience with perihepatic lymph nodes, similarly found that “all positive nodes were macroscopically enlarged to a certain degree and palpated as firm by the surgeon.” Our findings and those of Kokudo et al17 stand in contrast to the findings of Elias et al18 who reported a 14% rate of perihepatic nodal positivity among metastatic colorectal cancer patients with “no evidence of macroscopic lymph node involvement.” Differences in these studies may partially be related to variations between surgeons in assessment of clinically positive nodes. Of note, in the present study, only 1 of 48 patients (2.1%) without evidence of nodal involvement on CT and PET had disease seen histologically, suggesting a very low incidence of truly occult metastatic disease.
CT and PET, in addition to clinical examination of nodes at the time of operation, have utility in the assessment of perihepatic nodal status. Abnormal nodes on CT scan warrant careful scrutiny, but the low positive predictive value of CT scan suggests that enlarged perihepatic nodes on CT alone do not necessarily equate to metastatic disease, and this finding by itself should not preclude consideration for hepatic resection. It must be recognized that the results underestimate the overall positive predictive value of CT, since patients with clear-cut metastatic nodal disease (ie, bulky or necrotic) were not included and generally do not come to operation. PET scanning added important information in some patients, although the added benefit of PET beyond a high-quality CT scan remains unclear. PET positivity strongly suggests metastatic involvement; however, the low number of PET “positive” scans in the present study limits the ability to make definitive comments in this regard.
This study does not address the management of pathologically positive nodes. The therapeutic value of lymphadenectomy remains an unanswered question.18 In metastatic colorectal cancer, the studies that have suggested a therapeutic benefit are nonrandomized.19–21 Historically, many have argued that pathologically positive perihepatic nodes are a contraindication to resection;22,23 however, this tenet deserves reexamination in the current era of improved systemic therapies. In the authors’ practice, decisions regarding hepatectomy and portal lymphadenectomy in patients found to have metastasis to perihepatic nodes are handled on a case-by-case basis considering patient, tumor, and treatment specific factors.
While lymph node sampling is generally regarded a safe procedure when performed by experienced surgical teams, it is not without potential complications as demonstrated in the present series. Portal and perihepatic lymphadenectomy has been associated with lymphatic leakage18 and bleeding.10 Therefore, in addition to increased operating time associated with lymphadenectomy, the potential complications should be factored into the decision to perform the procedure.
There are limitations to the present study that should be noted. This was not a consecutive series of patients, although the patients were nonselected. In addition, 10% of patients had lymph node stations that were not sampled. Although some of these patients may have had occult nodal involvement, the likelihood of this would seem to be low in light of the overall results. Also, most patients in this series had metastatic colorectal cancer and the results may not be broadly applicable to all patients with hepatic malignancy. Finally, the number of patients with PET scan positive perihepatic disease was small, and conclusions regarding its value in this setting are therefore limited.
CONCLUSION
This study supports selective sampling of perihepatic lymph nodes at the time of hepatic resection for malignancy. Routine lymphadenectomy or routine sampling is unnecessary, particularly in patients without any clinical or radiologic evidence of disease. Instead, the decision to sample lymph nodes can be guided by information gathered from preoperative imaging studies and the intraoperative assessment of perihepatic nodes. The optimal management of those found to have positive nodes remains a question worthy of further investigation.
Footnotes
Presented in part at the International Hepato-Pancreatico-Biliary Association World Congress in Washington, D.C. on June 5, 2004.
Reprints: William R. Jarnagin, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10021. E-mail: jarnagiw@mskcc.org.
REFERENCES
- 1.Uenishi T, Hirohashi K, Shuto T, et al. The clinical significance of lymph node metastases in patients undergoing surgery for hepatocellular carcinoma. Surg Today. 2000;30:892–895. [DOI] [PubMed] [Google Scholar]
- 2.Chu KM, Lai EC, Al-Hadeedi S, et al. Intrahepatic cholangiocarcinoma. World J Surg. 1997;21:301–305. [DOI] [PubMed] [Google Scholar]
- 3.Valverde A, Bonhomme N, Farges O, et al. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122–127. [DOI] [PubMed] [Google Scholar]
- 4.Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Registry of Hepatic Metastases. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 5.Beckurts KT, Holscher AH, Thorban S, et al. Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg. 1997;84:1081–1084. [PubMed] [Google Scholar]
- 6.Rodgers MS, McCall JL. Surgery for colorectal liver metastases with hepatic lymph node involvement: a systematic review. Br J Surg. 2000;87:1142–1155. [DOI] [PubMed] [Google Scholar]
- 7.Jaeck D, Nakano H, Bachellier P, et al. Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: a prospective study. Ann Surg Oncol. 2002;9:430–438. [DOI] [PubMed] [Google Scholar]
- 8.Laurent C, Sa Cunha A, Rullier E, et al. Impact of microscopic hepatic lymph node involvement on survival after resection of colorectal liver metastasis. J Am Coll Surg. 2004;198:884–891. [DOI] [PubMed] [Google Scholar]
- 9.Elias DM, Ouellet JF. Incidence, distribution, and significance of hilar lymph node metastases in hepatic colorectal metastases. Surg Oncol Clin North Am. 2003;12:221–229. [DOI] [PubMed] [Google Scholar]
- 10.Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takasaki K, Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999;29:147–150. [DOI] [PubMed] [Google Scholar]
- 12.Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Liver Cancer Study Group of Japan. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs JF, Weber TK, Rodriguez-Bigas MA, et al. Intraoperative determinants of unresectability for patients with colorectal hepatic metastases. Cancer. 1998;82:1244–1249. [DOI] [PubMed] [Google Scholar]
- 14.Jaeck D. The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann Surg Oncol. 2003;10:1007–1011. [DOI] [PubMed] [Google Scholar]
- 15.Kane JM 3rd, Kahlenberg MS, Rodriguez-Bigas MA, et al. Intraoperative hepatic lymphatic mapping in patients with liver metastases from colorectal carcinoma. Am Surg. 2002;68:745–750. [PubMed] [Google Scholar]
- 16.Fuhrman GM, Curley SA, Hohn DC, et al. Improved survival after resection of colorectal liver metastases. Ann Surg Oncol. 1995;2:537–541. [DOI] [PubMed] [Google Scholar]
- 17.Kokudo N, Sato T, Seki M, et al. Hepatic lymph node involvement in resected cases of liver metastases from colorectal cancer. Dis Colon Rectum. 1999;42:1285–1290. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Saric J, Jaeck D, et al. Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg. 1996;83:942–945. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Yokoi Y, Suzuki S, et al. Results of extensive surgery for liver metastases in colorectal carcinoma. Br J Surg. 1992;79:35–38. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Suzuki S, Konno H. Resection of hepatic metastases of colorectal carcinoma: 20 years’ experience. J Hepatobiliary Pancreat Surg. 1999;6:16–22. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Ouellet JF, Bellon N, et al. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:567–574. [DOI] [PubMed] [Google Scholar]
- 22.August DA, Sugarbaker PH, Ottow RT, et al. Hepatic resection of colorectal metastases: influence of clinical factors and adjuvant intraperitoneal 5-fluorouracil via Tenckhoff catheter on survival. Ann Surg. 1985;201:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. [DOI] [PubMed] [Google Scholar]