Abstract
Objective:
To evaluate whether major right hepatectomy using the anterior approach technique for large hepatocellular carcinoma (HCC) results in better operative and long-term survival outcomes when compared with the conventional approach technique.
Summary Background Data:
The anterior approach technique has been advocated recently for large right liver tumors. However, its beneficial effects on the operative and survival outcomes of the patients have not been evaluated prospectively.
Methods:
A prospective randomized controlled study was performed on 120 patients who had large (≥5 cm) right liver HCC and underwent curative major right hepatic resection during a 57-month period. The patients were randomized to undergo resection of the tumor using the anterior approach technique (AA group, n = 60) or the conventional approach technique (CA group, n = 60). The anterior approach technique involved initial vascular inflow control, completion of parenchymal transection, and complete venous outflow control before the right liver was mobilized. Operative and long-term survival outcomes of the two groups were analyzed. Quantitative assessments of markers of circulating tumor cells at various stages of surgery of the two techniques were also assessed by plasma albumin-mRNA.
Results:
The overall operative blood loss, morbidity, and duration of hospital stay were comparable in both groups. Major operative blood loss of ≥2 L occurred less frequently in the AA group (8.3% vs. 28.3%, P = 0.005). As a result, blood transfusion requirement and number of patients requiring blood transfusion were significantly lower in the AA group. Hospital mortality occurred in 1 patient in the AA group and 6 patients in the CA group (P = 0.114). Median disease-free survival was 15.5 months in the AA group and 13.9 months in the CA group (P = 0.882). Overall survival was significantly better in the AA group (median >68.1 months) than in the CA group (median = 22.6 months, P = 0.006). The survival benefit appeared more obvious in patients with stage II disease and patients with lymphovascular permeation of the tumor. The anterior approach was also found to associate with significantly lower plasma albumin-mRNA levels at various stages of surgery compared with the CA technique. On multivariate analysis, tumor staging, anterior approach hepatic resection, and resection margin involved by the tumor were independent factors affecting overall survival.
Conclusion:
The anterior approach results in better operative and survival outcomes compared with the conventional approach. It is the preferred technique for major right hepatic resection for large HCC.
A prospective randomized controlled study on 120 patients with large hepatocellular carcinoma showed that anterior approach hepatectomy resulted in better operative and survival outcomes compared with the conventional approach. The survival benefit appeared most significant in patients with stage II disease and patients with lymphovascular permeation of the tumor.
Complete mobilization of the right liver with the right hepatic vein controlled outside the liver before parenchymal transection has been a standard or the conventional approach during major right hepatectomy.1–3 This conventional approach was considered essential in reducing blood loss.4 However, injudicious mobilization of the right liver may lead to excessive bleeding caused by avulsion of the hepatic veins, prolonged ischemia of the liver remnant from rotation of the hepatoduodenal ligament, iatrogenic tumor rupture, and spillage of cancer cells into the systemic circulation. To avoid the aforementioned disadvantages, the anterior approach can be adopted. The technique involves initial vascular inflow control, completion of parenchymal transection, and complete venous outflow control, before the right liver is mobilized.5,6 Our previous retrospective analysis on 160 patients with large right liver hepatocellular carcinoma (HCC) suggested that the anterior approach technique was associated with significantly better outcome.6 However, the theoretical advantages of the anterior approach over the conventional approach have not been documented in a prospective manner. A prospective randomized study was therefore performed to evaluate the potential benefits of the anterior approach compared with the conventional approach in major right hepatectomy for large HCC.
METHODS
Patients and Flow of Study
From September 1999 to May 2004, 191 consecutive patients with a clinical diagnosis of HCC ≥5 cm in diameter on preoperative imaging were considered amenable for resection by a major right hepatectomy. Sixteen patients were excluded from the study because of the various reasons stated in Figure 1. The study protocol was approved by the Institutional Review Board of Queen Mary Hospital, and informed consent for the study was obtained from the patients. Upon exploration with laparoscopy or laparotomy and intraoperative ultrasonography, 39 patients were diagnosed to have unresectable disease and were excluded from the study (Fig. 1). A total of 136 patients were randomized initially to have either anterior approach hepatectomy (AA group) or conventional approach resection (CA group) by drawing consecutive sealed envelops. The randomization was made known to the operating surgeon only when the disease was deemed suitable for curative resection. The patients were randomly assigned to all surgeons involved in the study to minimize the surgeons’ effect on the operative outcomes. Although all patients were diagnosed to have HCC on preoperative investigations, 8 patients were diagnosed to have other pathologies on histologic examination of the specimens and were excluded. Seven patients were found to have very advanced disease after randomization, and only palliative resection was performed with gross residual tumors left behind. Segment 5 and 6 resection was performed for 1 patient instead of a major right hepatectomy. As a result, 16 patients were excluded after randomization. The remaining 120 patients, including 60 patients in the AA group and 60 patients in the CA group, were the subjects of the present study (Fig. 1).
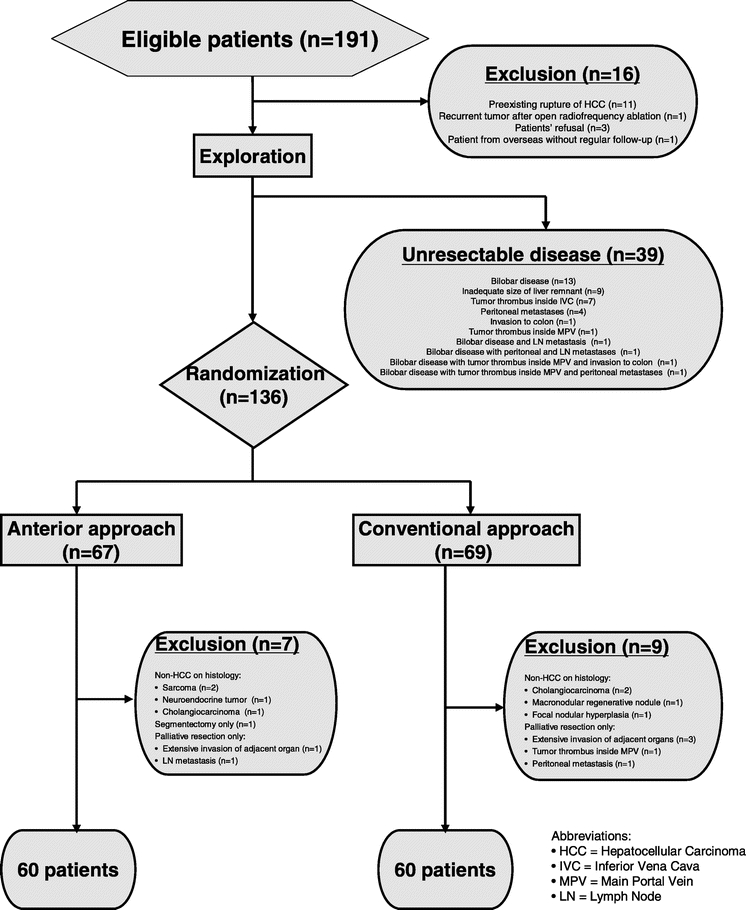
FIGURE 1. Flow chart and randomization of patients with large HCC (≥5 cm), which was considered suitable for major right hepatectomy.
Preoperative Management
Preoperative investigation of the patients included blood biochemistry, alpha-fetoprotein assay, chest x-ray, percutaneous ultrasonography, computed tomography (CT), and hepatic angiography in selected patients.7 Liver function was assessed by both the Child's-Pugh grading8 and indocyanine green clearance test.9 Major right hepatic resection was defined as resection of 4 or more Couinaud's segments including segments 5 to 8.10
Operative Technique
For patients who were randomized to the CA group, liver hilar dissection was performed to divide the right hepatic artery and portal vein. The right liver together with the tumor were then completely mobilized from the posterior abdominal wall and rotated anteriorly and to the left side to allow separation of the liver from the inferior vena cava (IVC). All the small caval branches were individually ligated and divided. The right hepatic vein was then isolated, divided, and sutured. When difficulty was encountered during right liver mobilization due to huge tumor or tumor infiltration to posterior abdominal structures, the abdominal incision was extended into the right thoracic cavity. Hepatic parenchymal transection was then performed by an ultrasonic dissector.
For patients who were randomized to the AA group, mobilization of the tumor and the right liver was not performed. After hilar dissection, the plane of parenchymal transection, depending on the extent of hepatic resection, was marked on Glisson capsule with the help of intraoperative ultrasonography. The transection was performed using an ultrasonic dissector from the anterior surface of the liver to the right liver hilum, and down to the anterior surface of the IVC, which was completely exposed. If concomitant caudate lobectomy was performed, the entire caudate lobe was completely mobilized from the IVC, and retracted toward the right side to be resected together with the right liver. All the small caval branches were then individually ligated, and the right hepatic vein was isolated and divided extrahepatically. When the right liver was completely disconnected from the IVC, the triangular ligament was divided to allow delivery of the specimen.6
Postoperative Care and Follow-up
All patients received the same postoperative care by the same team of surgeons in the intensive care unit during the early postoperative course. All the patients had postoperative follow-up by the same team of surgeons every month for the first year, and every 3 months thereafter. Serum alpha-fetoprotein levels and liver biochemistry were monitored at each follow-up. CT scan was performed 1 month after hepatectomy, and every 3 months thereafter for surveillance of recurrence. Chest radiology was performed every 3 months, which was followed by CT thorax when suspicious lesion was detected. None of the patients was lost to follow-up during the study period, and the minimal duration of follow-up of the surviving patients was 10 months. Disease-free survival time was calculated from the date of hepatectomy to the date when recurrence was diagnosed. Treatment of intrahepatic recurrences included reresection, radiofrequency ablation, transarterial chemoembolization (TACE), or no treatment, depending on the size, location, and number of recurrent tumors, liver function status, presence of extrahepatic recurrences, and presence of tumor thrombus in the portal vein, hepatic vein, or IVC. Treatment of extrahepatic recurrences included local excision (such as excision of solitary lung metastasis), systemic chemotherapy, tamoxifen, or no treatment.
Cell-Free Circulating Albumin-mRNA in Plasma
Plasma albumin-mRNA was assayed for evidence of circulation of liver cells during liver mobilization and surgery. Three blood samples were collected for all patients in both groups during the operation through a central venous catheter, including just before skin incision, just before parenchymal transection (after hilar dissection in the AA group and after mobilization of the liver in the CA group), and after delivery of the tumor. Plasma specimens were stored at −70°C until use. Total RNA was extracted using TRIZOL LS reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Real-time quantitative RT-PCR for albumin was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The first strand cDNA was synthesized with 4.5 μL of total RNA in 50-μL reaction using the High Capacity cDNA Archive kit (Applied Biosystems) following the manufacturer's instruction. The first strand cDNA of 5 μL was then used in each 25 μL quantitative assay with 1 × PCR buffer II, 5.5 mmol/L MgCl2, 0.2 mmol/L dATP, dCTP, dGTP, 0.4 mmol/L dUTP, and 0.625 unit of AmpliTaq Gold. Primers and probe for albumin were ALB-F (5′-TCT GCT TGA ATG TGC TGA TGA C-3′), ALB-R (5′-GGT TTT TCA CAG CAT TCC TTC A-3′), and ALB-P (5′-ATC AGG ATT CGA TCT C-3′). Primer and probe reagents for control 18s were ready-made reagents (Pre-Developed TaqMan Assay Reagents, Applied Biosystems) and served as quality control for all samples in all the assays. Transcript quantification was performed at least duplicate for each sample. The PCR profile for the quantitative assay included incubation of 10 minutes at 95°C and then 40 cycles comprised at 95°C for 15 seconds and 60°C for 1 minute. The amplification plots of the PCR reaction were used to determine the threshold cycle (CT). The CT value represented the PCR cycle at which an increase in reporter fluorescence above a baseline signal can first be detected. The relative amount of albumin, after normalization with calibrator and adjustment for plate-to-plate variation, was presented as the fold difference (log 2 base) relative to the baseline level. The relative amount of albumin released was expressed as ΔCT, where
ΔCT (mobilization) = [CT (preincision) − CT (pretransection)], and
ΔCT (liver surgery) = [CT (preincision) − CT (post-transection)].
Sample Size and Statistical Analysis
Assuming that 50% of the patients who underwent major right hepatectomy using the conventional approach developed recurrent disease in 2 years,6 that the use of the anterior approach technique reduces the incidence to 25%, and that a level of statistical significance of 0.05 and a power of 0.8 were required, it was estimated that 60 patients should be recruited in each arm of the study to document the advantages of the anterior approach hepatectomy. Primary outcome measures included hospital mortality, disease-free survival duration, and overall survival duration. Secondary outcome measures included operative blood loss, transfusion requirement, duration of intensive care unit stay and hospital stay, and operative morbidity. Continuous data were expressed as medians with their interquartile ranges unless otherwise stated. Proportions were given as number and percentage. Differences in clinical parameters between the two groups were assessed with either the Mann-Whitney U test for continuous data, or the χ2 test or Fisher exact test where appropriate for proportions. Survival analysis, including cumulative overall survival and disease-free survival, was estimated by the Kaplan-Meier survival method. Statistical comparison of survival distributions was analyzed by log-rank tests. Multivariate analysis by the Cox proportional hazard regression model was used to identify independent prognostic factors in predicting overall cumulative survival. Circulating plasma albumin-mRNA levels at various stages of surgery of the two techniques were compared by the Mann-Whitney U test. All P values less than 0.05 were considered to indicate statistical significance. Statistical analyses were made with the help of SPSS for Windows computer software (SPSS Inc., Chicago, IL).
RESULTS
Clinical and Pathologic Data
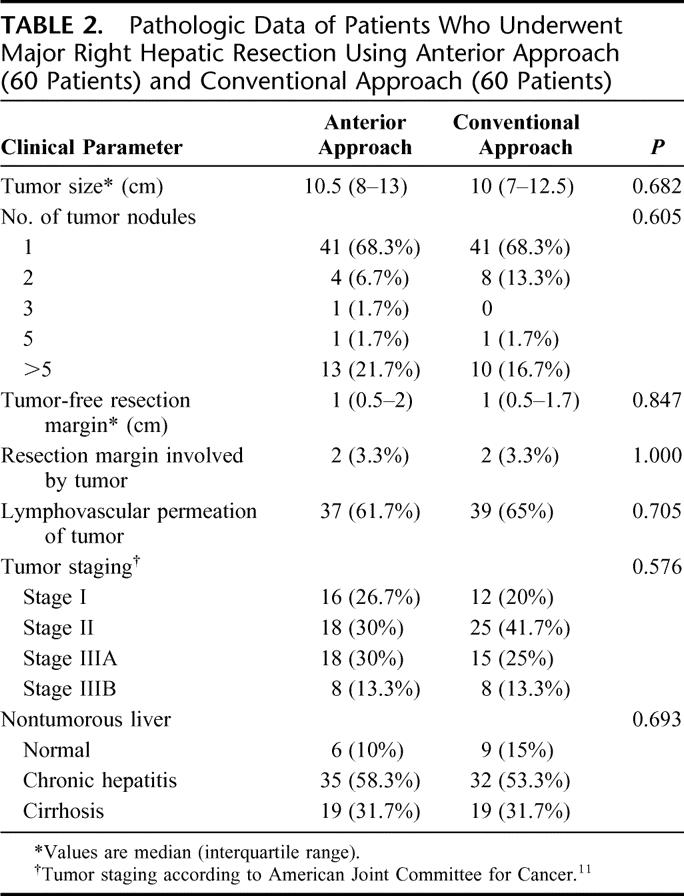
The preoperative clinical and laboratory parameters were comparable in both groups of patients (Table 1). The median size of the tumors was 10.5 cm in the AA group, which was comparable to that in the CA group (10 cm, P = 0.682). The pathologic data including tumor staging according to the American Joint Committee on Cancer classification11 were comparable between the two groups (Table 2). A total of 105 (88%) patients had histologic evidence of chronic liver diseases, including 19 (32%) patients with liver cirrhosis in both groups. Histopathology of the HCC confirmed lymphovascular permeation in 37 (62%) patients and 39 (65%) patients in the AA group and CA group, respectively.
TABLE 1. Clinical and Laboratory Data of Patients Who Underwent Major Right Hepatic Resection for Large Hepatocellular Carcinoma Using Anterior Approach (60 Patients) and Conventional Approach (60 Patients)
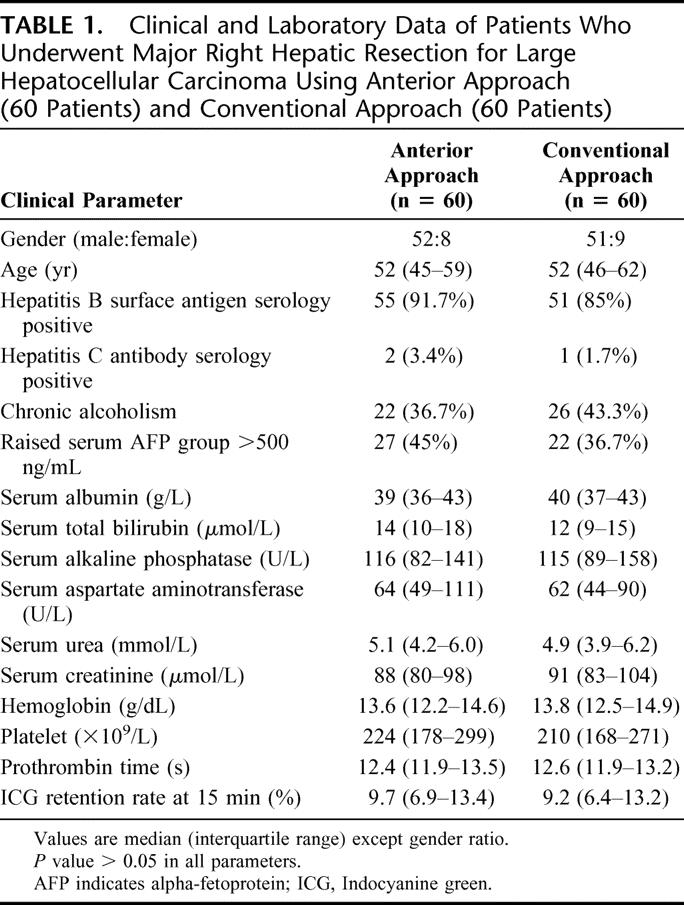
TABLE 2. Pathologic Data of Patients Who Underwent Major Right Hepatic Resection Using Anterior Approach (60 Patients) and Conventional Approach (60 Patients)
Operative Outcomes
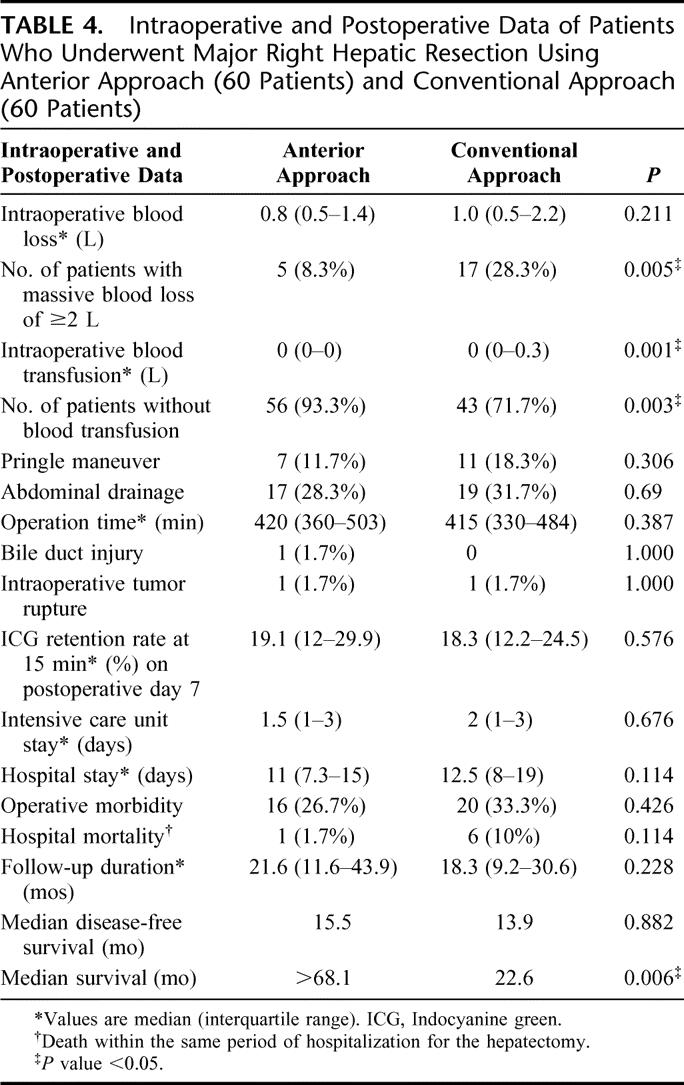
The extent of hepatic resection12 was comparable between the two groups of patients (Table 3). Thoracic extension was required in 14 (23.3%) patients in the CA group and in 11 (18.3%) patients in the AA group. Duration of the operation was comparable in the two groups (Table 4). Although there was no significant difference in the overall operative blood loss between the two groups, more patients in the CA group had massive blood loss of ≥2 L (28% in CA group vs. 8% in AA group, P = 0.005). As a result, significantly more patients in the CA group required blood transfusion (28% vs. 7%, P = 0.003, Table 4). Intraoperative iatrogenic tumor rupture during mobilization of the right liver occurred in 1 patient in each group, and 1 patient in the AA group had an intraoperative complication of common bile duct injury and required hepaticojejunostomy. The overall operative morbidity was comparable in both groups of patients. Hospital mortality occurred in 1 (1.7%) patient in the AA group and 6 (10%) patients in the CA group (P = 0.114). The only mortality in the AA group was a female patient with ruptured cerebral aneurysm on postoperative day 4. The causes of death for the 6 patients in the CA group included liver failure (3 patients), chest infection and multiorgan failure (2 patients), and intraabdominal bleeding and multiorgan failure (1 patient).
TABLE 3. Extent of Hepatic Resection of the Anterior Approach and Conventional Approach Groups of Patients
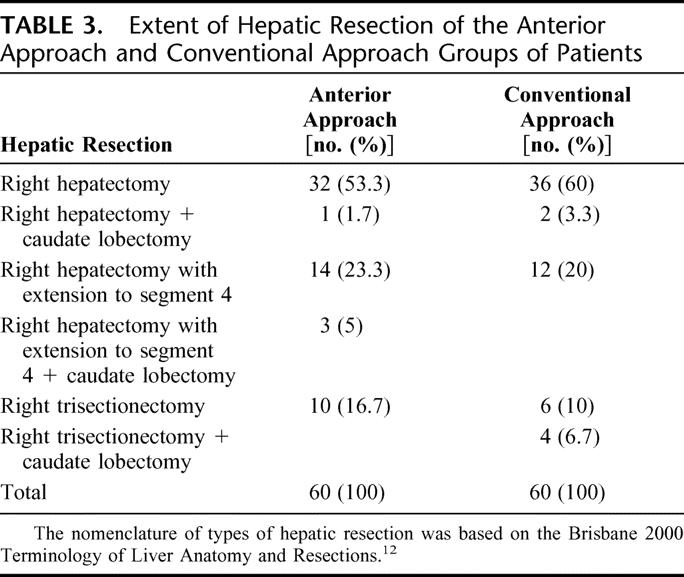
TABLE 4. Intraoperative and Postoperative Data of Patients Who Underwent Major Right Hepatic Resection Using Anterior Approach (60 Patients) and Conventional Approach (60 Patients)
Tumor Recurrence and Treatment
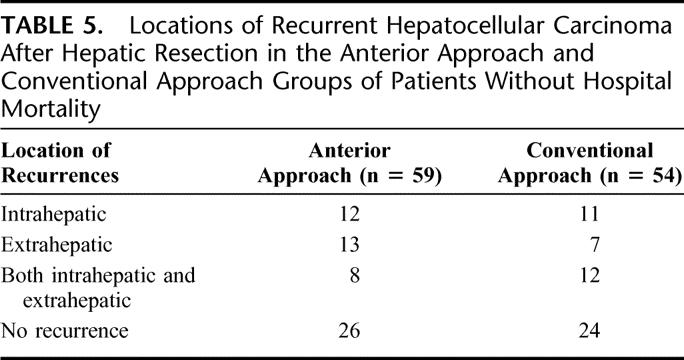
On follow-up, 33 (56%) patients and 30 (56%) patients developed tumor recurrences in the AA group and CA group, respectively (Table 5). Effective treatment of the recurrences was feasible more often in the AA group, especially in patients with stage II disease. This was because AA group patients had localized or solitary recurrent tumors more often that the CA group. Among the 10 stage II HCC patients in the AA group with recurrence, 8 (80%) had localized or solitary tumors, which were amenable to effective treatment such as radiofrequency ablation (n = 2) for solitary intrahepatic recurrence, TACE for intrahepatic recurrences (n = 2), or resection of solitary pulmonary metastasis (n = 4). On the contrary, in the CA group, among the 12 stage II disease patients with recurrent HCC, only 2 (17%) were suitable for effective treatment (P = 0.008). These included TACE for intrahepatic recurrence (n = 1) and resection of pulmonary metastases (n = 1). The remaining patients had multiple and bilateral pulmonary metastases (n = 6), intrahepatic recurrences with decompensated liver function (n = 1), intrahepatic recurrences with left portal vein thrombosis (n = 1), intrahepatic recurrences with tumor thrombus inside the IVC (n = 1), and bone metastases (n = 1). Significant difference in the pattern of recurrent HCC was not observed between the two groups with stage I and III diseases.
TABLE 5. Locations of Recurrent Hepatocellular Carcinoma After Hepatic Resection in the Anterior Approach and Conventional Approach Groups of Patients Without Hospital Mortality
Survival Outcomes
The median disease-free survival of the AA group was 15.5 months, which was not statistically different from that of the CA group (13.9 months, P = 0.882, Fig. 2a). However, the overall cumulative survival of the AA group was significantly better than that of the CA group (median >68.1 months vs. 22.6 months, P = 0.006, Fig. 2b). Significantly better survival outcome of the AA group was observed in patients with stage II disease. Although there was no significant difference in disease-free survival between the two groups of patients with stage II disease (P = 0.718, Fig. 3a), the median overall cumulative survival of patients with stage II disease was >68.1 months in the AA group, which was significantly better than that of 23.7 months in the CA group (Fig. 3b, P = 0.0009). This difference was attributed to the availability of effective treatment of localized or solitary recurrences in 8 (80%) of 10 patients with recurrent disease in the AA group. On the contrary, effective treatment was only suitable for 2 of 12 patients with recurrent disease in the CA group (17%, P = 0.008). As a result, there was no mortality in 18 patients with stage II disease in the AA group with a median follow-up of 29.3 months. There were 12 deaths in 25 patients with stage II disease in the CA group with a median follow-up of 20 months. In addition, better overall cumulative survival outcome of the AA group was also observed in a subgroup of patients with lymphovascular permeation of the tumor when compared with the CA group (Fig. 4b). The median overall cumulative survival of patients with lymphovascular permeation of the tumor was >68.1 months in the AA group, which was significantly better than that of 20 months in the CA group (P = 0.034). However, there was no significant difference in disease-free survival between the two groups of patients with lymphovascular permeation of the tumor (P = 0.890, Fig. 4a). In patients without lymphovascular permeation of the tumor, there was no significant difference in overall survival between the two groups (P = 0.104).
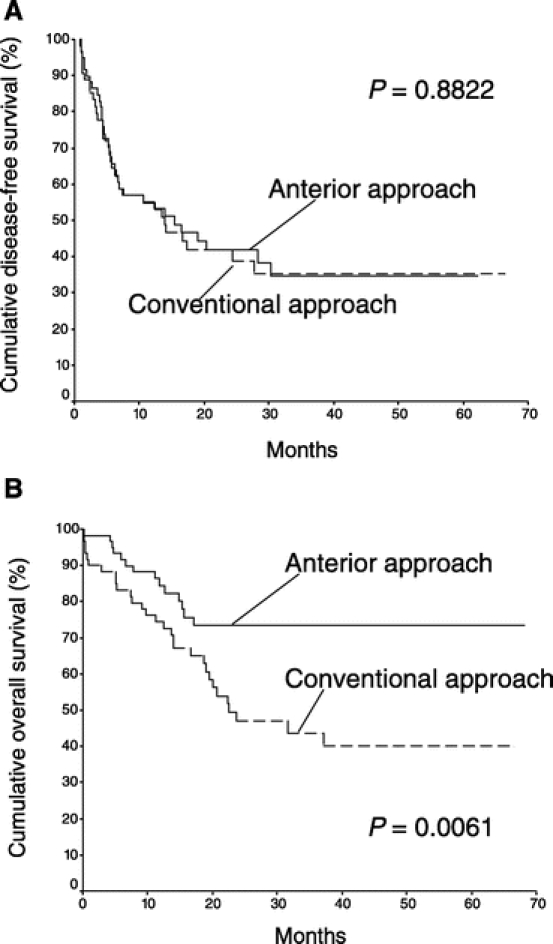
FIGURE 2. Cumulative disease-free survival (A) and overall survival (B) of patients who underwent major right hepatectomy using anterior approach (60 patients) and conventional approach (60 patients).
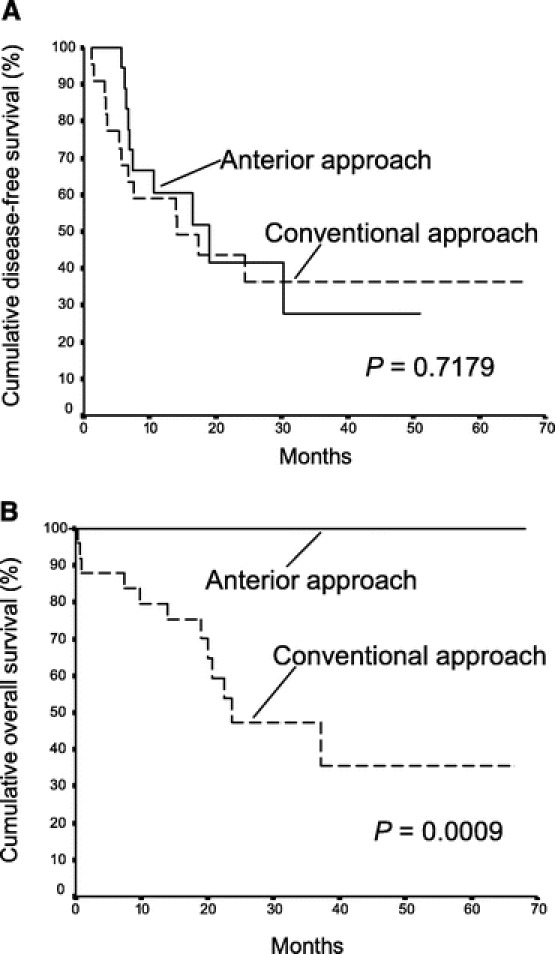
FIGURE 3. Cumulative disease-free survival (A) and overall survival (B) of patients with stage II disease in the anterior approach group (18 patients) and the conventional approach group (25 patients).
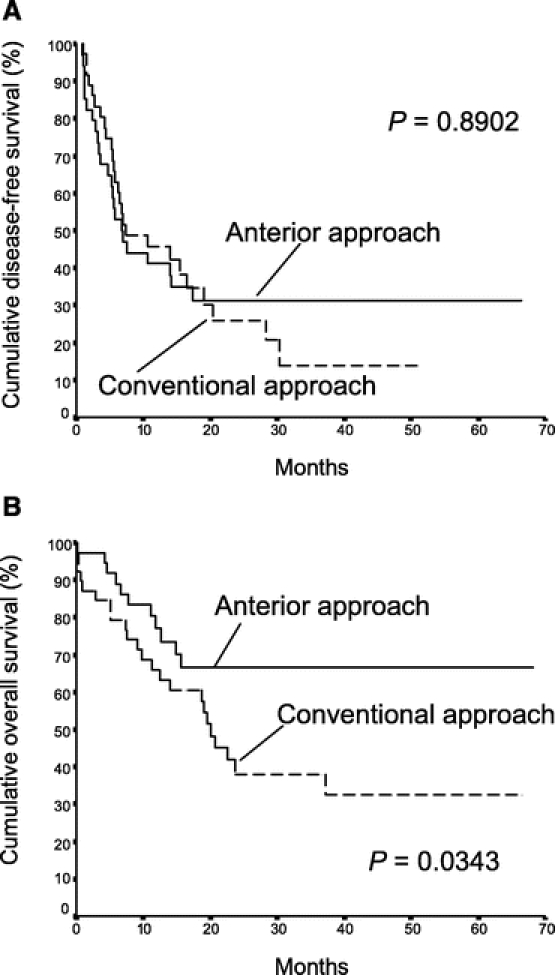
FIGURE 4. Cumulative disease-free survival (A) and overall survival (B) of patients with lymphovascular permeation of tumor in the anterior approach group (37 patients) and the conventional approach group (39 patients).
Statistical analysis was performed to identify independent factors that were associated with overall survival of the entire patient population in this study. Twelve factors were examined, including patient factors (age, gender, preoperative indocyanine green clearance, and serum total bilirubin), tumor factors (preoperative serum alpha-fetoprotein, size of the tumor, lymphovascular permeation, and tumor staging), and operative factors (operative blood loss, intraoperative transfusion, resection margin involved by the tumor, and anterior approach hepatic resection). On multivariate analysis, tumor staging (HR, 1.608; 95% confidence interval, 1.177–2.198, P = 0.003), anterior approach hepatectomy (HR, 0.416; 95% confidence interval, 0.22–0.787, P = 0.007), and resection margin involved by the tumor (HR, 4.419; 95% confidence interval, 1.488–13.127, P = 0.007) were independent factors affecting overall survival.
Quantitative Assessment of Cell-Free Circulating Albumin-mRNA
Comparing the two liver resection techniques, patients in the AA group had significantly lower levels of albumin-mRNA both before parenchymal transection [ΔCT (mobilization): median, 0.57; interquartile range, 1.65 vs. median, 1.52; interquartile range, 3.47; P = 0.026] and at the end of surgery after delivery of the tumor [ΔCT (liver surgery): median, 1.54; interquartile range, 3.05 vs. median, 2.73; interquartile range, 4.57; P = 0.037] compared with patients in the CA group (Fig. 5).
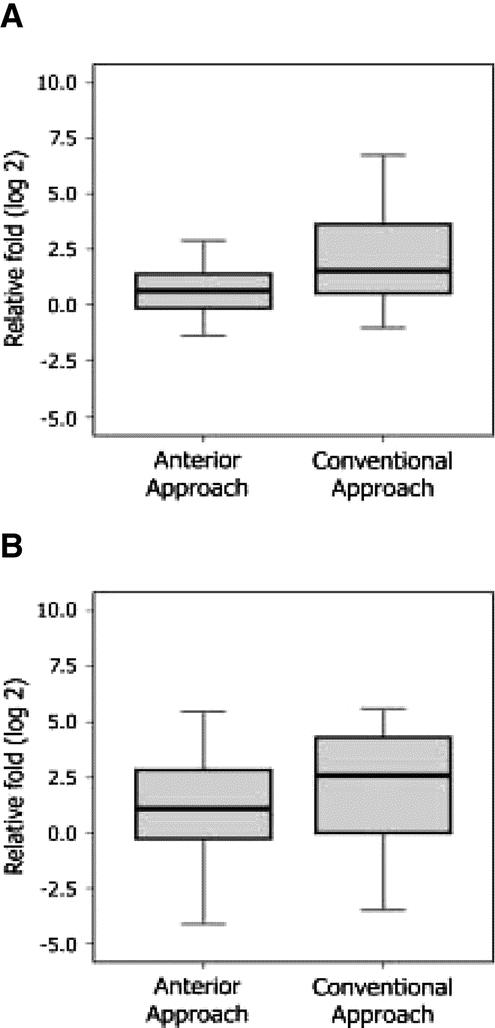
FIGURE 5. Cell-free circulating albumin-mRNA level was significantly lower in the anterior approach group compared with the conventional approach group, (A) before parenchymal transection (P = 0.034) and (B) at the end of surgery after delivery of the tumor (P = 0.040).
DISCUSSION
Major right hepatectomy for large HCC is associated with significant operative morbidity and mortality and remains a major surgical challenge, especially when underlying liver cirrhosis is present.13,14 HCC is well known to be a soft, friable, and highly vascular tumor. The potential disadvantages of mobilization of the right liver together with the large tumor using the conventional approach have been well recognized. These included excessive bleeding caused by avulsion of the hepatic vein and caval branches, prolonged ischemia of the liver remnant from rotation of the hepatoduodenal ligament, iatrogenic tumor rupture, and spillage of cancer cells into the systemic circulation. The anterior approach technique was first described by Ozawa as one of the “nonconventional approaches” to advanced liver cancer to avoid prolonged rotation and displacement of the hepatic lobes, leading to impairment of the afferent and efferent circulation.15 Using this “no touch technique,” the right liver together with the tumor are completely separated from the IVC before mobilization. It has the theoretical advantage of avoiding squeezing the tumor cells into the circulation during mobilization of the tumor when the venous outflow is still intact.
In our previous report, 54 patients with HCC of ≥5 cm in diameter situated at the right liver undergoing hepatectomy using the anterior approach technique were retrospectively evaluated.6 When compared with the 106 patients with similar clinical parameters who underwent hepatectomy using the conventional approach during the same study period, patients in the anterior approach group had significantly less intraoperative blood loss and blood transfusion requirement, a lower hospital mortality rate, lower incidence of pulmonary metastases, and better median disease-free survival and median overall cumulative survival. Thereafter, several reports have supported the use of the anterior approach technique as the preferred approach in patients with large HCC in the right liver.16–21 A French group reported the experience of anterior approach hepatectomy for resection of massive tumors in 14 patients, and reported a favorable operative outcome without any patient suffering from postoperative liver failure.16 Nevertheless, the theoretical advantages of the anterior approach technique over the conventional approach technique have not been documented prospectively.
The present prospective randomized controlled study shows that the anterior approach results in better operative and survival outcomes compared with the conventional approach in patients with large HCC. With the improvement of the surgical technique and careful use of the ultrasonic dissector for parenchymal transection, the overall operative blood loss in the present study was much less than that reported previously,6 and 82.5% of the patients did not require blood transfusion. As a result, there was no significant difference in the overall operative blood loss between the two groups. However, more patients in the CA group had massive blood loss and required more blood transfusion than the AA group (Table 4). This observation verified the postulation that difficult mobilization of the right liver with a huge HCC was risky and associated with an increased operative blood loss. Excessive intraoperative bleeding has been reported to have a detrimental effect on the postoperative liver function and result in increased perioperative mortality.22 Perioperative transfusion has also been suggested to associate with early recurrence of HCC after hepatic resection, leading to short disease-free and overall survival.23–25
With better selection of patients, improved perioperative care and surgical technique, hospital mortality after hepatic resection for HCC was only infrequently encountered.7,26 The current series represented a hospital mortality rate of 5.8% in 120 patients who underwent major right hepatic resection for HCC, among whom 87.5% had underlying chronic hepatitis or cirrhosis. In our previous retrospective study reported in 2000, the anterior approach was shown to associate with a lower hospital mortality rate compared with the conventional approach. With improvement in perioperative care and operative technique over the years, the present study was obviously under power to show any significant difference in hospital mortality between the two groups (1.7% in the AA group and 10% in the CA group, P = 0.114). However, it was interesting to observe that all 6 mortalities in the CA group were related to liver failure or multiorgan failure secondary to liver failure, whereas none of the patients in the AA group suffered from these conditions. The observation was consistent with the suggestion by Ozawa in his initial proposal that the anterior approach could contribute to better preservation of postoperative liver function by avoiding prolonged rotation and displacement of the hepatic lobes causing impairment of the afferent and efferent circulation of the liver remnant.15
The median disease-free survival of the AA group was not statistically different from that of the CA group. However, the overall cumulative survival of the AA group was significantly better than that of the CA group. Significantly better survival outcome of the AA group was observed in patients with stage II disease or with lymphovascular permeation. This observation was associated with more widespread metastases in the CA group, a phenomenon probably related to more tumor manipulation in the conventional approach.
Hematogenous dissemination of malignant tumor cells has been reported during surgical resection of biliary-pancreatic cancer,27,28 colorectal cancer,29 and prostatic cancer.30 It was considered to be related to manipulation of the tumors during surgery, and the “no-touch isolation technique” has been reported to reduce intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer.31 In patients with HCC, venous permeation or vascular invasion of the tumor is a frequent phenomenon. This phenomenon may be responsible for the high incidence of hematogenous spread before resection, but compression of the tumor during mobilization may enhance the spread of tumor cells into the systemic circulation32 or the intrahepatic portal venous system.33 The advantage of minimizing tumor cell dissemination using the anterior approach technique is substantiated by the observation that significantly lower levels of albumin-mRNA were detected in various stages of surgery in the AA group compared with the CA group. In this study, quantitative assay of cell-free plasma albumin-mRNA was chosen because the previous assays for circulating cancer cell markers based on isolation of nucleated cells in the blood were prone to have errors during processing.34 It has recently been shown that assays for nucleic acid in the blood are as efficacious as cell-based assays in the detection of micrometastases.35 Moreover, being different from alpha-fetoprotein, albumin was expressed by nearly all HCCs.36
The present prospective randomized study is the first report, to the best knowledge of the authors, to show that modification of the surgical technique is associated with improved operative and survival outcomes of patients undergoing cancer surgery. The current study exerts its potential impacts not only on patients who undergo hepatectomy for HCC, but also on those who undergo surgery for other malignancies.37 Whether the advantages of this “no touch technique” for patients with HCC can be extrapolated to patients with other malignancies should be subjected to further evaluations.
Despite its advantages over the conventional approach, the anterior approach can potentially be dangerous. Torrential bleeding can occur at the deeper plane of parenchymal transection from the right hepatic vein or middle hepatic vein. Without prior mobilization of the right liver and the tumor, and control of the right hepatic vein, bleeding can be substantial and difficult to control. When it occurs, Pringle maneuver or total vascular occlusion should be used to identify and control the site of bleeding rather than converting to the conventional approach. Nevertheless, with adequate accumulation of experience in liver resection and refinement of surgical technique, accurate parenchymal transection can be accomplished with the help of an ultrasonic dissector with little blood loss, even without inflow vascular control. Massive bleeding during liver transection of anterior approach hepatectomy seldom occurs in our recent experience.
To minimize the risk of massive venous bleeding and to facilitate hepatic parenchymal transection, modification of the anterior approach technique was advocated by Belghiti et al.18 They proposed the technique of “hanging maneuver,” which consisted of a blind passage of a long vascular clamp along the midline of the anterior surface of the IVC, on the left side of the inferior right hepatic vein, and cranially up to the space between the right and the middle hepatic veins. The liver is lifted up with a tape during parenchymal transection. With such modification of the anterior approach technique by the hanging maneuver, the risk of massive venous bleeding is minimized. However, there is a potential risk of bleeding from the caudate hepatic veins induced by the blind passage of an instrument anterior to the IVC. Bleeding from these branches can be substantial and difficult to stop, especially in patients with liver cirrhosis and portal hypertension. Without the use of the hanging maneuver, the median blood loss of patients in the AA group was 800 mL in the present study and blood transfusion was only required in 4 (7%) patients. It appears that the hanging modification may not be necessary in most circumstances.
CONCLUSION
The anterior approach is the preferred technique for major right hepatectomy for large HCC because it results in improved operative and survival outcomes of the patients. It should be recommended as the standard technique for curative resection of large HCC in the right liver.
Footnotes
Supported by the Earmarked Research Grant of the Research Grants Council of Hong Kong.
Reprints: Sheung Tat Fan, MD, PhD, Department of Surgery, University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China. E-mail: stfan@hku.hk.
REFERENCES
- 1.Blumgart LH, Jarnagin WR, Fong Y. Liver resection for benign diseases and for liver and biliary tumors. In: Blumgart LH, ed. Surgery of the Liver and the Biliary Tract, 3rd ed. London: Saunders, 2000:1639–1713. [Google Scholar]
- 2.Fortner JG, Kim DK, Maclean BJ, et al. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Bell RH, Beart RW, et al. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet. 1975;141:429–437. [PMC free article] [PubMed] [Google Scholar]
- 4.Makuuchi M, Yamamoto J, Takayama T, et al. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology. 1991;38:176–179. [PubMed] [Google Scholar]
- 5.Lai EC, Fan ST, Lo CM, et al. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314–317. [DOI] [PubMed] [Google Scholar]
- 6.Liu CL, Fan ST, Lo CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 9.Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. [DOI] [PubMed] [Google Scholar]
- 10.Couinaud C. Etudes anatomiques et chirurgicales. Paris: Mason, 1957. [Google Scholar]
- 11.American Joint Committee on Cancer Staging. Liver (including intrahepatic bile duct). In: American Joint Committee on Cancer Staging Manual, 6th ed. New York: Springer, 2002:131–138. [Google Scholar]
- 12.Strasberg SM, Belghiti J, Clavien PA, et al. The Brisbane 2000 terminology of liver anatomy and resections. HBP. 2000;2:333–339. [Google Scholar]
- 13.Capussotti L, Polastri R. Operative risks of major hepatic resections. Hepatogastroenterology. 1998;45:184–190. [PubMed] [Google Scholar]
- 14.Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa K. Hepatic function and liver resection. J Gastroenterol Hepatol. 1990;5:296–309. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay D, Marin-Hargreaves G, Castaing D, et al. The anterior approach: the right way for right massive hepatectomy. J Am Coll Surg. 2001;192:412–417. [DOI] [PubMed] [Google Scholar]
- 17.Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563–585. [DOI] [PubMed] [Google Scholar]
- 18.Belghiti J, Guevara OA, Noun R, et al. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–111. [DOI] [PubMed] [Google Scholar]
- 19.Belghiti J, Ogata S. Assessment of hepatic reserve for the indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:1–3. [DOI] [PubMed] [Google Scholar]
- 20.Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin North Am. 2003;12:51–63. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Unno M, Katayose Y, et al. Hepatic resection through an anterior approach employing a modified liver hanging maneuver in patients with a massive liver tumor severely oppressing the inferior vena cava. Hepatogastroenterology. 2004;51:1459–1463. [PubMed] [Google Scholar]
- 22.Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma: clinical features and long-term prognosis. Ann Surg. 1987;205:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumata T, Ikeda Y, Hayashi H, et al. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866–1871. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 25.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono F, Takao S, Natsugoe S, et al. Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am J Surg. 1999;177:475–479. [DOI] [PubMed] [Google Scholar]
- 28.Nomoto S, Nakao A, Kasai Y, et al. Detection of ras gene mutations in perioperative peripheral blood with pancreatic adenocarcinoma. Jpn J Cancer Res. 1996;87:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4:343–348. [PubMed] [Google Scholar]
- 30.Oefelein MG, Kaul K, Herz B, et al. Molecular detection of prostate epithelial cells from the surgical field and peripheral circulation during radical prostatectomy. J Urol. 1996;155:238–242. [PubMed] [Google Scholar]
- 31.Hayashi N, Egami H, Kai M, et al. No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery. 1999;125:369–374. [PubMed] [Google Scholar]
- 32.Louha M, Nicolet J, Zylberberg H, et al. Liver resection and needle liver biopsy cause hematogenous dissemination of liver cells. Hepatology. 1999;29:879–882. [DOI] [PubMed] [Google Scholar]
- 33.Matsumata T, Kanematsu T, Takenaka K, et al. Lack of intrahepatic recurrence of hepatocellular carcinoma by temporary portal venous embolization with starch microspheres. Surgery. 1989;105:188–191. [PubMed] [Google Scholar]
- 34.Paterlini-Brechot P, Vona G, Brechot C. Circulating tumorous cells in patients with hepatocellular carcinoma: clinical impact and future directions. Semin Cancer Biol. 2000;10:241–249. [DOI] [PubMed] [Google Scholar]
- 35.Kopreski MS, Gocke CD. Cellular- versus extracellular-based assays: comparing utility in DNA and RNA molecular marker assessment. Ann NY Acad Sci. 2000;906:124–128. [DOI] [PubMed] [Google Scholar]
- 36.Krishna M, Lloyd RV, Batts KP. Detection of albumin messenger RNA in hepatic and extrahepatic neoplasms: a marker of hepatocellular differentiation. Am J Surg Pathol. 1997;21:147–152. [DOI] [PubMed] [Google Scholar]
- 37.Liu CL, Fan ST. Anterior approach for right hepatectomy for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:292–294. [DOI] [PubMed] [Google Scholar]


