Abstract
Objective:
To propose a cholangiographic classification for intraductal growth type intrahepatic cholangiocarcinoma (IG-ICC) and its precursor, collectively termed intraductal papillary mucinous neoplasm of the bile ducts (IPMN-B).
Summary Background Data:
For the extensive clinicopathologic variations of IPMN-B, a detailed characterization of cholangiography for IPMN-B is beneficial for determining the optimal therapeutic strategy.
Methods:
A total of 124 patients with cholangiography-available and pathologically proven IPMN-B were retrospectively studied. Numbers of IPMN-B type 1, type 2, type 3, and type 4 were 33, 17, 15, and 59, respectively. A cholangiographic classification was proposed based on the presence of hepatolithiasis, mucobilia, neoplasia localization, and concomitant malignancies. The demographics, histologic grading, management, and survival were also analyzed.
Results:
All 33 IPMN-B type 1 and 12 of 17 IPMN-B type 2 displayed cholangiographic pattern IA demonstrating hepatolithiasis-related biliary stricture. The remaining 5 IPMN-B type 2 displayed cholangiographic pattern IB or IC, which demonstrated mucobilia without discernible neoplasia. Seven of 15 IPMN-B type 3 and 52 of the 59 IPMN-B type 4 displayed cholangiographic pattern IIA or IIB, which demonstrated overt intraductal neoplasia. Seven IPMN-B type 3 or 4 displayed cholangiographic pattern IIIA or IIIB, which demonstrated IPMN-B and concomitant malignancies. For those presenting with cholangiographic pattern IA, IC, IIA, IIB, and IIIA, straightforward hepatectomies for the diseased lobes were performed. For those with pattern IB, surgical resections were performed only when there was emergence of mucin-producing neoplasia. For those with IIIB, the concomitant malignancies were considered inoperable. No disease-related death occurred in IPMN-B type 1and 2. The mean survival rates of IPMN-B type 3 and type 4 were 55.5 ± 17.1 months and 36.9 ± 6.3 months, respectively.
Conclusion:
The presented cholangiographic classification facilitates the management for IPMN-B. Significant survival discrepancy at the various stages warrants a more aggressive surgical strategy.
A cholangiographic classification was proposed to guide the management of intraductal papillary mucinous neoplasm of the bile ducts.
Macroscopic types of intrahepatic cholangiocarcinoma (ICC) are mainly classified into the mass-forming type, periductal-infiltrating type, and intraductal growth type (IG-ICC).1,2 Most ICCs are of the former 2 types (non-IG-ICC), which invade liver parenchyma early in their growth; in contrast, the IG-ICC is less common but associated with more favorable outcome. Biliary papillomatosis, IG-ICC, and mucin-producing ICC, intraductal papillary neoplasm of the bile duct (IPMN-B) describes essentially the same disease process.3–5 This category of IPMN-B histologically resembles intraductal papillary mucinous neoplasm of the pancreas.6 We recently categorized IPMN-B from the viewpoint of its histologic evolution, both of IG-ICC and its precursors.7 Variable gastrointestinal metaplasia phenotypes, such as colon-like metaplasia, goblet cell, Paneth cell, foveolar metaplasia, and enriched mucin production, are characteristically found in IPMN-B.7 Although most non-IG-ICC have a limited resectability and poor prognosis, IPMN-B is considered of relatively low-grade malignancy and merits consideration for an aggressive surgery.1,2,8–10 Early and precise diagnosis of this specific disease entity is therefore important to maximize patient survival. Several image studies of IPMN-B using computed tomography have been reported.11–13 The aim of this study was to characterize the cholangiographic patterns of IPMN-B, based on which the optimal management could be designed.
PATIENTS AND METHODS
Definition and Pathologic Description
IPMN-B is characterized by innumerable frondlike papillary infoldings consisting of columnar epithelial cells surrounding slender fibrovascular stalks. Type 1 is lined by biliary epithelium of low-grade dysplasia; type 2, lined by those of high-grade dysplasia; type 3, lined by in situ and microinvasive adenocarcinoma; and type 4, lined by papillary lesions with stromal invasion of adenocarcinoma. Accordingly, IPMN-B type 3 and type 4 correspond to traditional IG-ICC with varying degrees of stromal invasion.
Patient Selection
From 1977 to 2005, a total of 124 patients with cholangiography-available and pathologically proven IPMN-B managed in the Department of Surgery, Chang Gung Memorial Hospital were retrospectively studied. All 124 IPMN-B had their lesion predominately confined at the intrahepatic bile duct. Patient population of IPMN-B type 1, type 2, type 3, and type 4 were 33, 17, 15, and 59, respectively. All patients except 3, who had concomitant hepatocellular carcinoma (n = 2) and gallbladder cancer (n = 1) at the contralateral lobe with respect to the existing IPMN-B, underwent intended curable hepatectomy. The mean age of patients with IPMN-B type 1, 2, 3, and 4 were 60 ± 11, 58 ± 13, 58 ± 8, and 60 ± 9, respectively. The female prevalence in IPMN-B type 1, 2, 3, and 4 was 67% (22 of 33), 76% (13 of 17), 67% (10 of 15), and 66% (39 of 59), respectively. At the same time period, the total number of non-IG-ICC who had undergone surgery in our institute during 1977 to 2005 was 386. Of them, 108 (28%) had undergone an intended curable resection and enrolled into this study for survival comparison with that of IPMN-B.
Preoperatively, all patients had undergone complete cell count, serologic biochemistry, serum tumor markers (CEA, CA19-9), ultrasonography, computed tomography, endoscopic retrograde cholangiography (ERC), percutaneous transhepatic cholangiography (PTC), and magnetic resonance image (MRI), and magnetic resonance cholangiography (MRC), in various combinations. All patients were thereafter followed up with a median time period of 13 months (mean, 21.2 ± 22.4 months; range, 1.4–142.5 months).
Patterns of Cholangiography
Before 2002, direct cholangiography (ERC and/or PTC) was routinely used to examine the bile duct lesions for those with IPMN-B in the current series. MRC was not available until 1996 in our institute, and since 2002 it was our consensus to have MRC as the first-line diagnostic tool for those with suspected biliary neoplasia, based on which direct cholangiography was added for therapeutic purpose such as biliary decompression, bile duct biopsy, bile cytology study, and choledocholithotripsy. The proposed cholangiographic classification for IPMN-B was based on direct cholangiography before 2002 and MRC was added thereafter, with respect to the presence of hepatolithiasis, mucobilia, neoplasia localization, and concomitant malignancies. The subjects were categorized into the following cholangiographic patterns: type 1A, hepatolithiasis with biliary stricture; type 1B, fusiform biliary tree with amorphous floating filling defects in absence of discernible neoplasia; type 1C, disproportional biliary dilatation in absence of discernible neoplasia; type IIA, intrahepatic polypoid or cystic neoplasia; type IIB, intrahepatic polypoid or cystic neoplasia extending to the extrahepatic bile duct; type IIIA, type 1 or type 2 with operable concomitant malignancy; type IIIB, type 1 or type 2 with inoperable concomitant malignancy.
Statistics
All continuous data were presented as mean ± standard deviation. Comparison of numerical variables was analyzed using Student t test, whereas comparison of categorical variables was performed using Pearson χ2 method. Kaplan-Meier curves and log-rank test were performed to compare survival discrepancy between groups. Statistical significance was set at P < 0.05.
RESULTS
Clinical Manifestations
The cardinal symptomatology of 124 patients with IPMN-B included right hypochondralgia (87%), fever and chills (58%), and jaundice (29%). Sixteen (13%) of 124 patients presenting with incidental findings such as positive ultrasonography (Fig. 1), ERC, and/or elevated serum tumor markers (CEA and/or CA19-9) were asymptomatic. Elevated serum CEA was detected in 0% (0 of 33) of IPMN-B type 1 and 2 cases tested compared with its presence in 25% (16 of 63) of IPMN-B type 3 and 4 cases tested (P < 0.01). Elevated serum CA19.9 was detected in 35% (11 of 31) of IPMN-B type 1and 2 cases tested compared with 61% (27 of 44) of IPMN-B type 3 and 4 cases tested (P > 0.05). Thirty-nine (31%) patients with IPMN-B had previous one or multiple biliary surgeries, mainly for hepatolithiasis disease. Three patients with long-term T-tube equipment for surveillance of recurrent hepatolithiasis were positive for mucobilia (mucin in bile) during regular percutaneous fibrocholedochoscopic examination, which turned out to be IPMN-B type 3 (n = 1) and type 4 (n = 2).
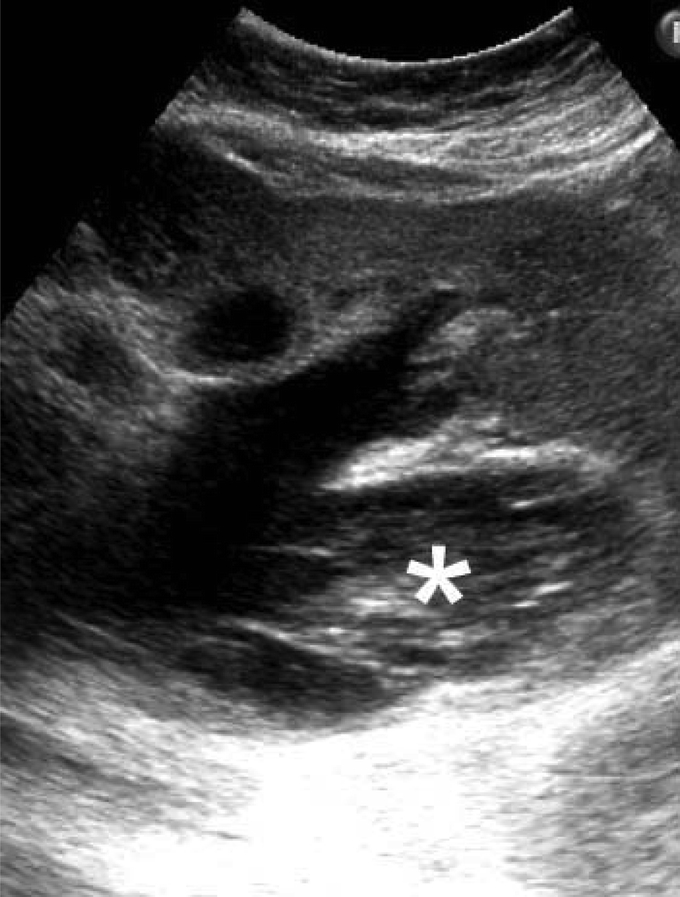
FIGURE 1. Ultrasonography showed a layer-sign suggesting presence of mucobilia (*).
Cholangiography Patterns Stratified by Histologic Classification
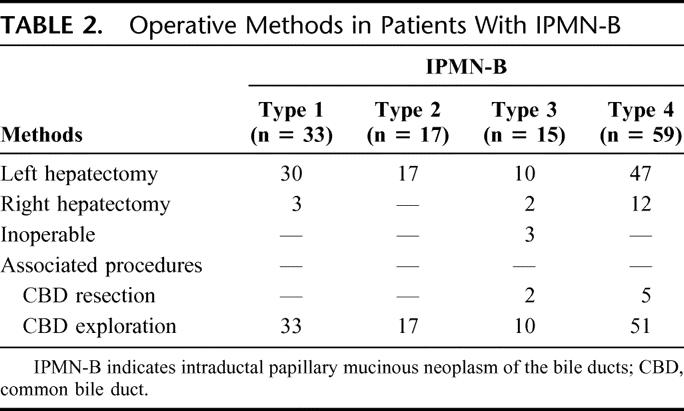
The cholangiographic patterns stratified by histologic classification in 124 IPMN-B cases were tabulated (Table 1). All 33 IPMN-B type 1 and 12 of 17 IPMN-B type 2 displayed cholangiographic pattern IA (Fig. 2) and underwent hepatectomy for the purpose of removal of hepatolithiasis without diagnosis of IPMN-B preoperatively, whereas the remaining 5 IPMN-B type 2 displayed cholangiographic pattern IB (n = 2) (Fig. 3) and IC (n = 3) (Fig. 4), which suggested the presence of mucobilia preoperatively. Among 15 IPMN-B type 3, there were 5 cholangiographic type IIA (Fig. 5) and 2 cholangiographic type IIB, which led to either left or right hepatectomy without and with extrahepatic bile duct resection, respectively. One case of IPMN-B type 3 displayed cholangiographic pattern IB; the patient underwent regular percutaneous fibrocholedochoscopic examination through T-tube tract for removal of recurrent hepatolithiasis. In this case, mucobilia was present for 2 years before definitive operation for IPMN-B. Another IPMN-B type3 displayed cholangiographic pattern IIIA, and there was concomitant intraductal papillary mucinous tumor of the pancreas. Cholangiographic pattern IIIB was detected in the remaining 3 IPMN-B type 3, from which coexisting hepatocellular carcinoma (n = 2) and gallbladder cancer (n = 1) was detected at the contralateral lobe with respect to the existing IPMN-B. For the former two, the hepatocellular carcinomas were treated using transarterial chemoembolization, while their IPMN-B's were managed expectantly. Of the 59 cases with IPMN-B type 4, cholangiographic pattern IIA and IIB were detected in 48 and 4, which led to left or right hepatectomy, without and with extrahepatic bile duct resection, respectively. Cholangiographic pattern IB was detected in 3 cases, repeated cholangiographic and cholangioscopic examinations, and intraductal biopsies before their definite surgeries was warranted. Finally, cholangiographic pattern IIIA was detected in the remaining 3 IPMN-B type 4, from which concomitant common bile duct carcinoma, salivary cystic carcinoma, and cervical cancer were found. For these 3 patients, their IPMN-B and concomitant malignancies could be extirpated simultaneously or consecutively. In all, the operative procedures of 124 patients with IPMN-B were tabulated (Table 2). Three IPMN-B type 3 and 4 IPMN-B type 4 cases had a positive malignant section margin. One IPMN-B type 3 and 3 type 4 cases had a dysplasia change at section margin. Hospital mortality rates in IPMN-B type 1, 2, 3, and 4 were 0 (0%), 1 (5.8%), 1 (6.6%), and 4 (6.7%), respectively (P > 0.05).
TABLE 1. Cholangiographic Types Stratified by Histologic Grading in Patients With IPMN-B
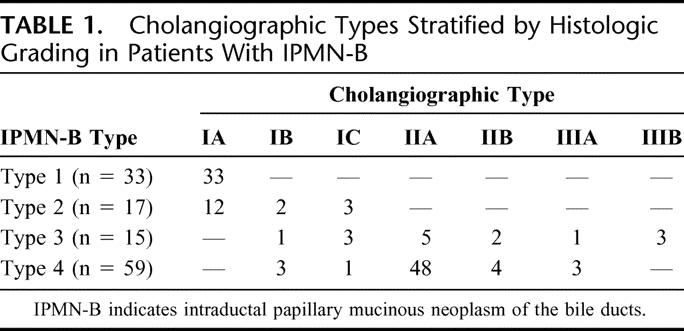
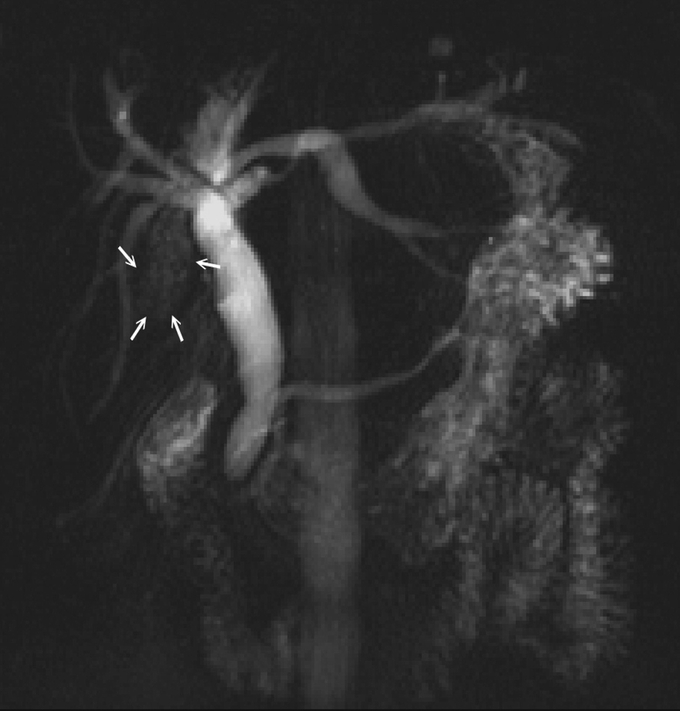
FIGURE 2. Magnetic resonance cholangiography (MRC) showed hepatolithiasis-related biliary stricture at the right posterior inferior duct (arrows).
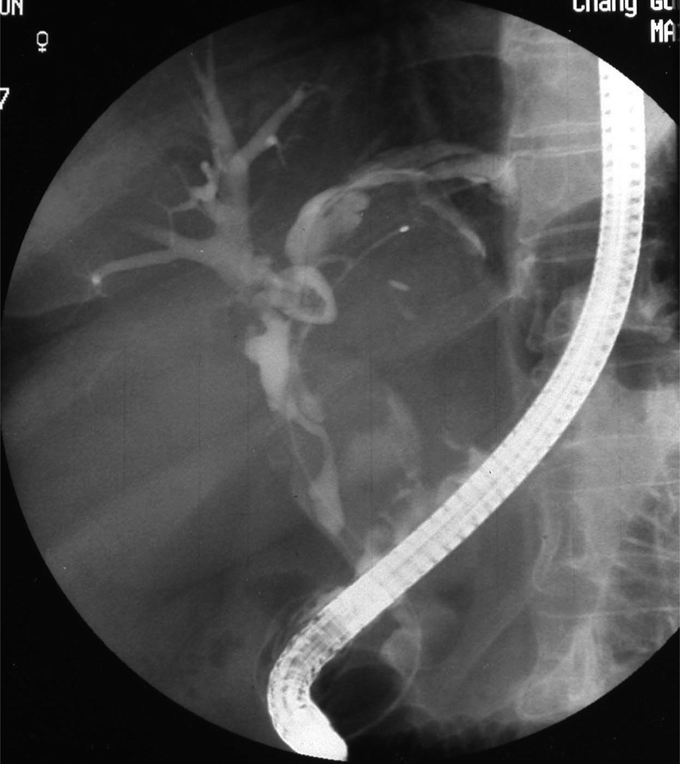
FIGURE 3. Endoscopic retrograde cholangiography showed amorphous filling defect, suggesting the presence of mucobilia.
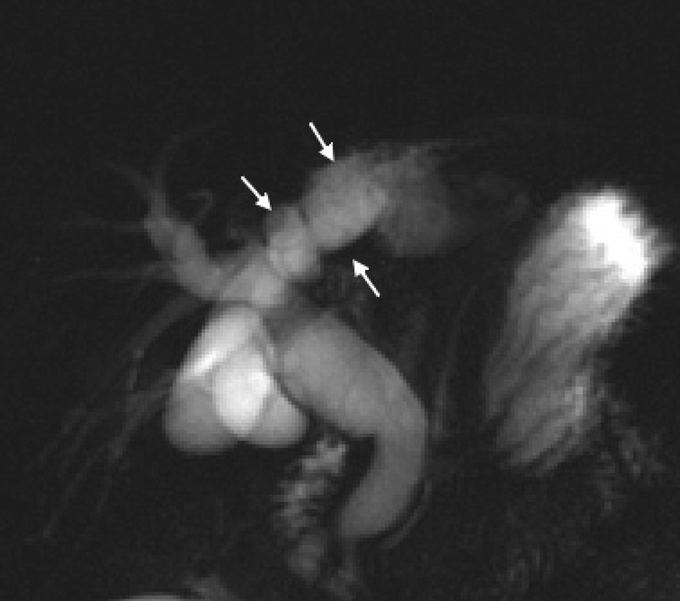
FIGURE 4. MRC showed a disproportional dilatation of intrahepatic duct without a discernible neoplasia (arrows).
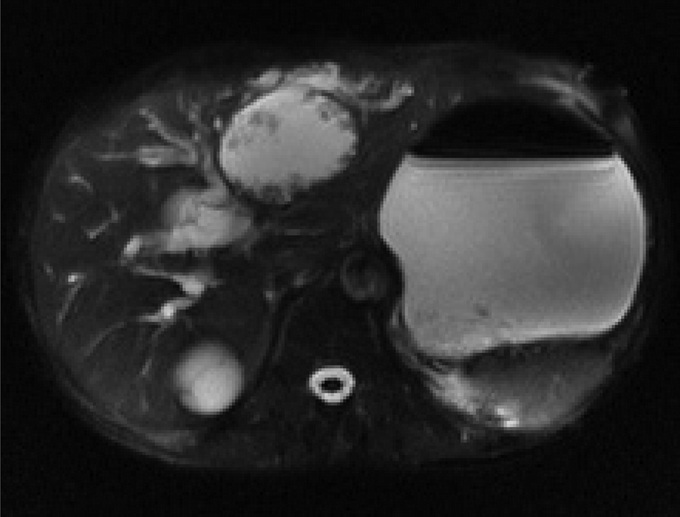
FIGURE 5. Magnetic resonance image (MRI)-T2 showed a cystic lesion with obvious mural nodules at the left hepatic duct.
TABLE 2. Operative Methods in Patients With IPMN-B
Analysis of Long-term Survival
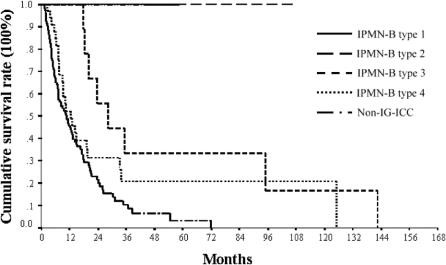
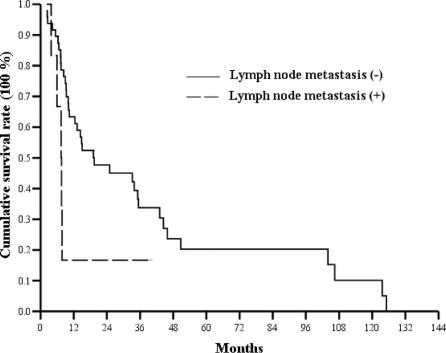
Long-term survival of patients with IPMN-B and non-IG-ICC was analyzed (Fig. 6). At this moment of survival analysis, no disease-related death had occurred in those with IPMN-B type 1and 2. One-, three-, and five-year survival rates were 100%, 33%, and 17% in IPMN-B type 3, 56%, 32%, and 14% in IPMN-B type 4, and 45%, 9%, and 3% in non-IG-ICC. The mean survival of IPMN-B type 3 and type 4 was 55.5 ± 17.1 months (95% CI, 22–89) and 36.9 ± 6.3 months (95% CI, 24.7–49.2), respectively (log-rank test, P = 0.12). There was no lymph node metastasis in IPMN-B type 1, 2, and 3, whereas there were 7 cases with lymph node metastasis at the hepatoduodenal ligament in IPMN-B type 4. Survival analysis stratified by lymph node metastasis in IPMN-B type 4 approached a marginal statistical significance (log-rank test, P = 0.047) (Fig. 7). The mean survival of IPMN-B type 4 with and without lymph node metastasis was 12.1 ± 5.1 months (95% CI, 2.0–22.0) and 39.0 ± 6.7 months (95% CI, 25.9–52.1), respectively. Finally, all 3 IPMN-B type 3 and 4 IPMN-B type 4 with positive malignant section margin died of the disease within 2 years following the operation.
FIGURE 6. Long-term survival analysis of patients with IPMN-B type 1, 2, 3, and 4 and nonintraductal growth type intrahepatic cholangiocarcinoma (non-IG-ICC).
FIGURE 7. Long-term survival of patients with IPMN-B type 4 stratified by lymph node metastasis status.
DISCUSSION
IPMN-B represents a disease spectrum from benign to invasively malignant following the course of chronic inflammation, dysplasia, carcinoma in situ to invasive carcinoma. According to our present and previous reports,14–16 hepatolithiasis played an important role in initiating cholangiocarcinogenesis. This is reflected by the fact that association of hepatolithiasis in IPMN-B type 1, 2, 3, and 4 was as high as 100%, 70%, 66%, and 64%, respectively. Hepatolithiasis not only obscured a precise diagnosis of underlying IPMN-B but also exacerbated the clinical course.14 Signs such as fever, chills, and jaundice were more pronounced in IPMN-B with hepatolithiasis than those without.14 As intraductal neoplasia intermingled with tenacious mucin, hepatolithiasis is particularly liable to occlude both the extrahepatic bile duct and segmental ducts, causing suppurative cholangitis and jaundice.
In the present study, the proposed classification of cholangiographic patterns for a cohort of IPMN-B gives rise to a rational algorithm for optimal management. Cholangiographic type IA represented the hepatolithiasis-related biliary stricture and poststenotic dilatation, which impossibly suggested the presence of underlying IPMN-B. Nevertheless, accumulated surgical experience of our institute during the past 3 decades indicated that cholangiocarcinoma might have been derived from hepatolithiasis with an incidence around 5% to 10%.14–16 Therefore, with the presence of long-standing hepatolithiasis, lobar atrophy, and biliary stricture, hepatectomy is selectively performed for the diseased lobe to prevent refractory retained and ongoing recurrent hepatolithiasis increasing the potential risk of cholangiocarcinoma.
Mucobilia is the most characteristic for the diagnosis of IPMN-B,3,7 which could be ascertained by “layers sign” detected by ultrasonography, giant or patulous papilla detected by duodenoscopy, and specific cholangiographic patterns evidenced on cholangiography. MRCP, however, failed to diagnose the presence of mucobilia consistently in our series. Although both of cholangiographic type IB and IC indicated the presence of mucobilia, the 2 disease patterns were managed quite differently. Cholangiographic type IC represented a disproportional dilated intrahepatic duct without a discernible neoplasia, for which we applied an aggressive surgical attitude to resect the presumed diseased lobe even though a gross neoplasia could not be recognized by the image studies. Based on our data, among the 7 cases with cholangiographic pattern IC, 6 did not have an obvious neoplasia on examination of their surgical specimen, whereas the pathologic examination yielded the final diagnosis of either IPMN-B type 2 or type 3 microscopically. The remaining 1 case was found to have an IPMN-B type 4. The management for those with cholangiographic type IB remained challenging. Mucobilia was a herald sign for the underlying IPMN-B; nevertheless, precise and early localization of the slow-growing mucin-producing neoplasia sometimes is extremely difficult.13 For such cases, modalities including sequential computed tomography, cholangiography, and cholangioscopy examination should be used before definite surgical action can be applied. Management decision-making for those with cholangiographic type IIA and IIB involved the necessity of extrahepatic bile duct resection or not. For those with cholangiographic type IIA, the surgical decision-making was more straightforward, either right or left hepatectomy based on the localization of the neoplasia. For those with cholangiographic type IIB, surgical procedures included lobectomy, resection of extrahepatic bile duct, followed by intrahepatic biliary reconstruction, and the surgical morbidities were considerably increased.3 Additionally, caudate lobectomy was sometimes warranted to achieve a curative eradication for those with cholangiographic type IIIB.
The concomitant malignancy in addition to IPMN-B was another important issue. For those with cholangiographic type IIIA, the concomitant malignancies were considered to be operable and resectable with respect to their existing IPMN-B, such as common bile duct cancer, intraductal papillary mucinous tumor of the pancreas, cervical cancer, and salivary cancer in our series. On the other hand, for those with cholangiographic type IIIB, the concomitant malignancies such as hepatocellular carcinoma and gallbladder cancer at the contralateral lobe with respect to the existing IPMN-B were considered inoperable. Although the association is unclear, the prevalence of 5.6% (7 of 124 IPMN-B) was higher compared with that of age-matched general population. This issue merits further investigation.
The survival of IPMN-B in the present study was significantly better than that of non-IG-ICC (Fig. 6) as in agreement with others' reports.1–3,8–10,14,15 The mean survival of IPMN-B type 3 and type 4 were 55.5 ± 17.1 months and 36.9 ± 6.3 months, respectively. Nevertheless, there was no statistical difference in survival between IPMN-B type 3 and type 4. Several explanations are considered. First, all IPMN-B type 3 patients with malignant and dysplasia section margin did not undergo rehepatectomy due to limited liver reservoir, patient refusal, or by surgeons' decision. Second, multicentric dysplastic biliary epithelium might reside in the remnant liver, and the sensitivity of current diagnostic tool is too low for detection yet. Third, the surgical volume of IPMN-B type 3 was too small to reach a statistical significance. Additionally, the clearance of section margin and hilar lymph node metastasis affected the prognosis of patients with IPMN-B considerably as seen in Figure 7. Relatively rare lymph node metastasis, higher resectability rate, and promising long-term survival of IPMN-B, compared with those of non-IG-ICC, justified a more aggressive surgical attitude toward IPMN-B cases.
CONCLUSION
A classification of cholangiographic patterns for IPMN-B was proposed based on the presence of hepatolithiasis, mucobilia, extension to the extrahepatic bile duct, and the concomitant malignancies. Those with IPMN-B type 1 and type 2 predominately displayed cholangiographic type IA, IB, and IC, which demonstrated either hepatolithiasis-related biliary stricture or mucobilia without recognition of gross neoplasia. Most of IPMN-B type 3 and type 4 displayed cholangiographic type IIA and IIB, which demonstrated overt intrahepatic intraductal neoplasia and led surgical strategy straightforward. Concomitant malignancy was an interesting issue, which however complicates management decision-making. Based on the cholangiographic spectrum, the optimal management strategy could be tailored accordingly. Significant survival discrepancy at the various stages of IPMN-B warrants a more aggressive surgical strategy.
Footnotes
Reprints: Miin-Fu Chen, MD, or Ta-Sen Yeh, MD, PhD, Department of Surgery, Chang Gung Memorial Hospital, 5 Fu-Hsing Street, Taoyuan, Taiwan. E-mail: surglab@adm.cgmh.org.tw or chenmf@adm.cgmh.org.tw.
REFERENCES
- 1.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(suppl VI):vi1–vi9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. [DOI] [PubMed] [Google Scholar]
- 3.Chen MF, Jan YY, Chen TC. Clinical studies of mucin-producing cholangiocellular carcinoma: a study of 22 histopathology-proven cases. Ann Surg. 1998;227:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh KS, Roth HR, Koh YT, et al. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology. 2000;31:12–17. [DOI] [PubMed] [Google Scholar]
- 5.Shibahara H, Tamada S, Goto M, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:327–338. [DOI] [PubMed] [Google Scholar]
- 6.Yeh TS, Ho YP, Chiu CT, et al. Aberrant expression of cdx2 homeobox gene in intraductal papillary-mucinous neoplasm of the pancreas but not in pancreatic ductal adenocarcinoma. Pancreas. 2005;30:233–238. [DOI] [PubMed] [Google Scholar]
- 7.Chen TC, Nakanuma Y, Zen Y, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651–658. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki A, Aramaki M, Kawano Y, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. Br J Surg. 1998;85:1206–1209. [DOI] [PubMed] [Google Scholar]
- 9.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tajima Y, Kuroki T, Fukuda K, et al. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004;91:99–104. [DOI] [PubMed] [Google Scholar]
- 11.Yoon KH, Ha HK, Roh BS, et al. Malignant papillary neoplasms of the intrahepatic bile ducts: CT and histologic features. AJR Am J Radiol. 2000;175:1135–1139. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Han JK, Kim YH, et al. CT features of intraductal intrahepatic cholangiocarcinoma. AJR Am J Radiol. 2000;175:721–725. [DOI] [PubMed] [Google Scholar]
- 13.Lim JH, Yoon KH, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;24:53–67. [DOI] [PubMed] [Google Scholar]
- 14.Chen MF, Jan YY, Hwang TL, et al. Impact of concomitant hepatolithiasis on patients with peripheral cholangiocarcinoma. Dig Dis Sci. 2000;45:312–316. [DOI] [PubMed] [Google Scholar]
- 15.Chen MF, Jan YY, Wang CS, et al. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461–2465. [DOI] [PubMed] [Google Scholar]
- 16.Jan YY, Chen MF, Wang CS, et al. Surgical treatment of hepatolithiasis: long-term results. Surgery. 1996;120:509–514. [DOI] [PubMed] [Google Scholar]