Abstract
Mycoplasma hominis is a commensal of the genitourinary tract. It mostly causes infections to associated structures of this system; however, occasionally it is a pathogen in nongenitourinary tract infections. Since, M. hominis strains require special growth conditions and cannot be Gram stained, they may be missed or delay diagnosis. This report describes a deep wound infection caused by M. hominis after neuromuscular scoliosis surgery; M. hominis was recovered by real-time polymerase chain reaction (PCR). An awareness of the role of M. hominis as an extragenital pathogen in musculoskeletal infections, especially in neuromuscular scoliosis, being a high-risk group for postoperative wound infection, it is necessary to identify this pathogen. Real-time PCR for postoperative deep wound infection, in patients with a history of genitourinary infections, decreases the delay in diagnosis and treatment. In these cases rapid real-time PCR on deep cultures should be considered.
Keywords: Mycoplasma hominis, Wound infection, Neuromuscular scoliosis, PCR
Introduction
Deep wound infections complicating spinal surgery are sources of major morbidity in neuromuscular scoliotic patients. Enterobacter, Enterococcus, Escherichia coli, Proteus and Staphylococci account for the majority of postoperative spinal wound infections in these patients [28]. Increasingly, previously uncommon pathogens are being identified in surgical infections. These organisms may be difficult to identify and/or resistant to most of the broad-spectrum antibiotics used for preoperative prophylaxis and for the treatment of postoperative wound infections. Among these pathogens, Mycoplasma hominis has been recognized as a cause of postoperative wound infections [25, 27, 31]. M. hominis is a commensal bacterium in humans and is distinguished phenotypically from other bacteria by the minute size and lack of a cell wall. As a result, M. hominis cannot be Gram stained and is resistant to penicillin and other antibiotics that interfere with the cell wall metabolism. In addition, it is a fastidious slow-growing organism, which may not be readily identified by using routine culture protocols. Early diagnosis, however, is of utmost importance for adequate institution of appropriate antimicrobial therapy.
To our knowledge, only two cases of postoperative spinal wound infections due to M. hominis have previously been reported [18]. We describe a case report of a M. hominis postoperative deep wound infection in a patient with a progressive myelomeningocele scoliosis who underwent posterior scoliosis surgery with bone allograft. Real-time polymerase chain reaction (PCR) provided a fast and secure diagnosis, which prevented further complications.
Case presentation
An 11-year-old girl with a complete paraplegia at level L2–L3 caused by a myelomeningocele was admitted to our hospital for surgical correction of a progressive right convex scoliosis. Preoperative physical examination revealed a flexible right convex thoracic scoliosis with a left convex thoracolumbar curve. The unsupported sitting anteroposterior radiograph showed a right convex thoracic scoliosis of 55°and a left convex thoracolumbar curve of 42°with no pelvic obliquity. Her medical history revealed a shunted hydrocephalus with an Arnold Chiari type II malformation, chronic urinary tract infections, and an auto-augmentation of the bladder.
The patient was treated with single stage scoliosis correction involving posterior instrumentation (Xia™ spinal system, Stryker Spine, Cestas, France) from T3 to L5. The spondylodesis was completed by applying allograft bone chips (Netherlands Bone Bank Foundation, Leiden, The Netherlands) over the laminae in the thoracolumbar region. In addition, Collagraft® (Neucoll, Campbell, CA, USA), a synthetic bone graft substitute composed of collagen and a composite mineral (hydroxyapatite and tricalcium phosphate), was applied posterolateral in the lumbosacral region.
Prophylactic antibiotics, cefazolin (cefalosporin, Kefzol®) 1,000 mg IV, were administered at the induction of anaesthesia, and as a second and third dose 8 and 16 h postoperatively, respectively [6]. Because of a chronic urinary tract infection including a positive culture with E. coli, cefradin (cefalosporin, Velosef®) 500 mg orally was continued for 9 days at 8-h intervals.
The postoperative course was uneventful. At day 8 the patient was discharged. At discharge the wound produced negligible clear fluid at the distal end without any signs of infection. However, at day 18 postoperatively, the patient developed fever (39°C) and was readmitted to our centre. On physical examination, an enlarged distal wound dehiscence was seen with increased fluid production. Infection parameters showed an increased C-reactive protein (CRP) (120 mg/l; normal <10 mg/l) and white blood cell (WBC) count (10.9 cells×109/l; normal 4–10 cells×109/l). A surgical intervention was performed including a thorough debridement of necrotic tissue and removal of the bone grafts. The instrumentation was left in place. According to the established guidelines, cultures were taken of various deep areas by fluid aspiration and from the applied bone graft [15, 28, 32]. After extensively irrigating with pulsatile lavage, the wound was closed leaving gentamicin collagen fleeces (Septocoll®, Biomed, Darmstadt, Germany) over the instrumentation. Adjuvant therapy with gentamicin (aminoglycoside, Garamycinl®) 210 mg IV and flucloxacillin (isoxazolylpenicillin, Floxapen®) 2,000 mg IV were initiated immediately after obtaining appropriate intraoperative cultures, and was continued postoperatively at 6-h intervals.
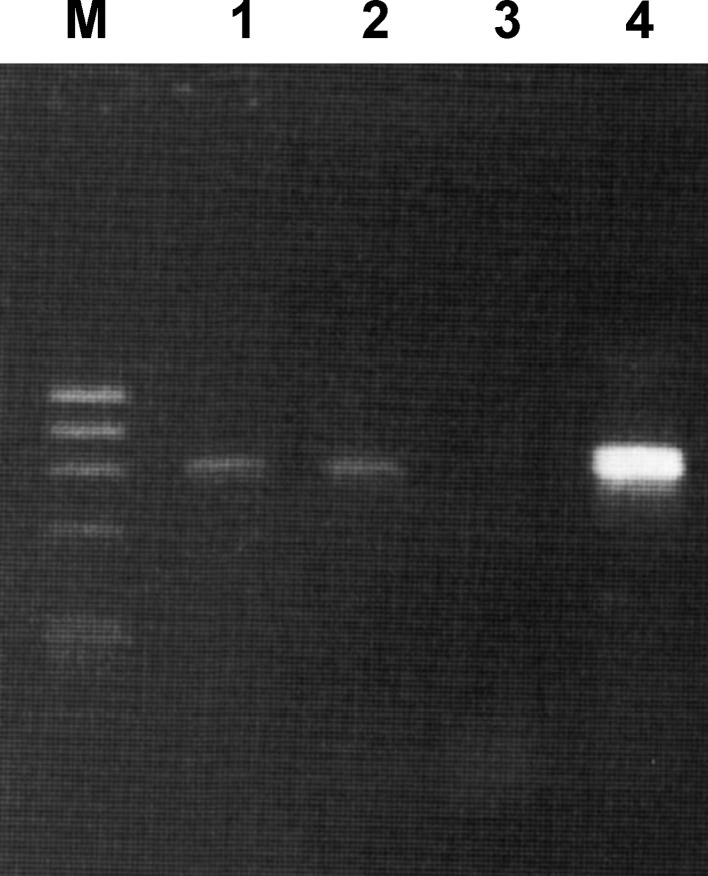
Intraoperative cultures from both the fluid aspiration and the bone graft yielded Mycoplasma after 4 days incubation. Real-time PCR on the peri-operative biopsy material was only positive for Mycoplasma and the species was identified by 16S DNA amplification as M. hominis (Fig. 1). Separately a Collagraft® sample of the same batch was tested for Mycoplasma, however, the culture as well as real-time PCR remained negative. Gentamicin and flucloxacillin were stopped, and doxycycline (tetracycline, Vibra-S®) 100 mg was administered orally at 12-h intervals and continued for 3 weeks. The patient was discharged 5 days after surgery.
Fig. 1.
Agarose gel analysis of duplo PCR amplification of Mycoplasma DNA. M Molecular weight marker, Lanes 1 and 2 positive PCR amplification of clinical sample, lane 3 negative control, lane 4 positive control. Subsequent sequencing results revealed M. hominis
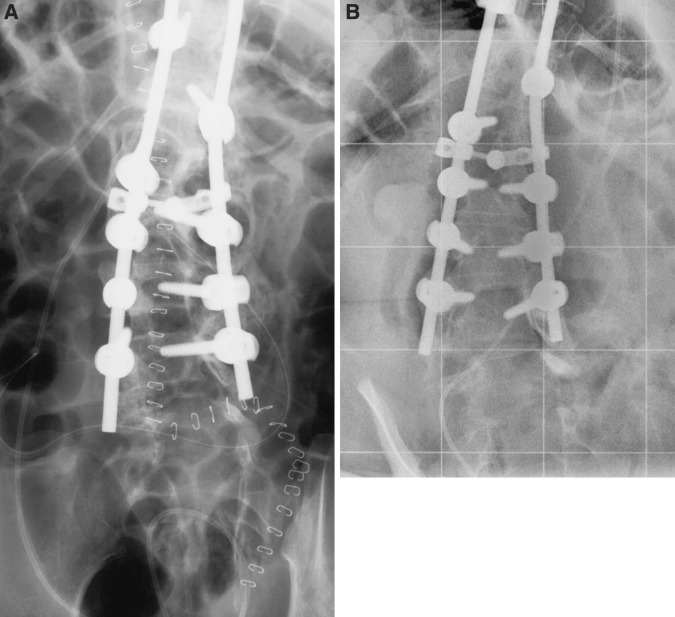
Wound healing and temperature were monitored at regular intervals at the outpatient clinic. After 3 months, the infection parameters were normalized and the wound was healed (Fig. 2). Four years after surgery there were no signs of infection. Radiographs at final follow-up demonstrated no instrumentation failure and no loss of correction of the scoliotic deformity (Fig. 3).
Fig. 2.
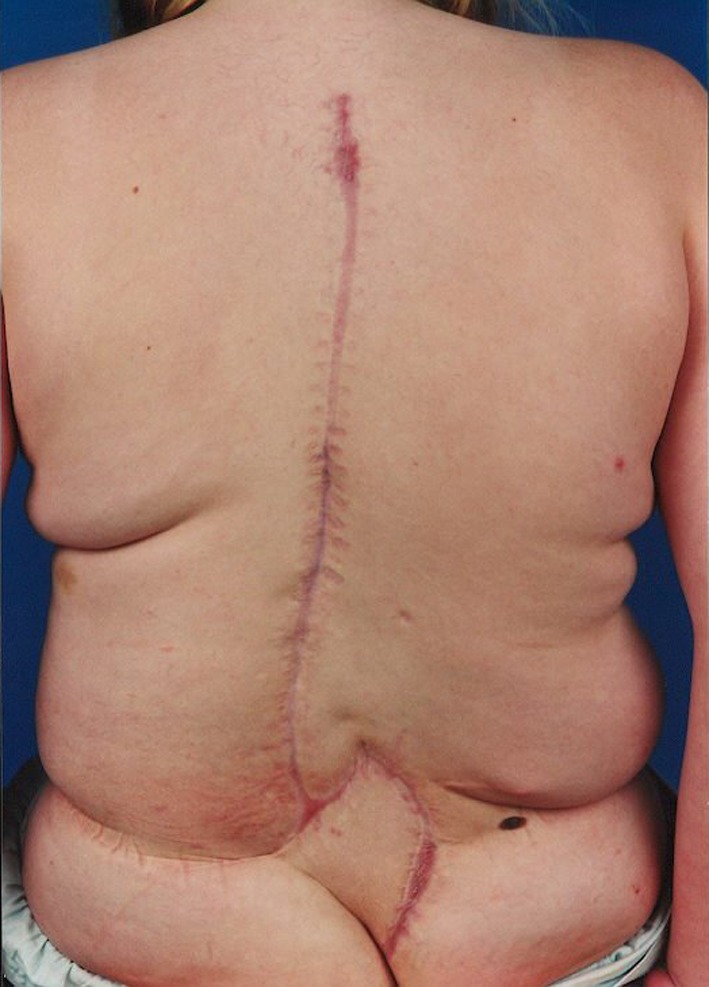
Clinical appearance 3 months after surgical intervention for deep wound infection, located at the distal end of the wound
Fig. 3.
AP radiographs of the lumbar spine at the site of the deep wound infection immediately after surgical intervention and debridement of necrotic tissue including removal of the bone grafts (a) and at 4 years of follow-up (b)
Discussion
Mycoplasma hominis is a commensal bacterium in humans. The organism is commonly associated with infections of the genitourinary tract, particularly in females. Rates of colonization in the urogenital tract range from 21 to 54% among women and from 4 to 13% among men [20]. Reports on M. hominis infections outside the genitourinary tract are scarce. Most of them are case reports describing extragenital infections, such as septic arthritis [17, 21], septicemia [11, 12], prosthetic valve endocarditis [3, 10], postoperative wound infections [25, 31], peritonitis [13], neonatal encephalitis [30], meningitis [7, 9], brain abscess [33], thrombophlebitis [27], and mediastinitis [19]. Extragenital infections are mainly related to patients with immunosuppression [3, 7, 9, 17, 19, 21, 25, 27, 30, 33]. Osteomyelitis caused by M. hominis is predominantly reported in combination with hypogammaglobulinemia [5, 14, 16, 24]. In an extensive overview of nongenitourinary M. hominis infections, Madoff and Hooper [18] described only two cases of postoperative deep wound infections caused by M. hominis after orthopedic surgery. Both cases concerned with deep wound infections after scoliosis surgery, one of them with a history of pyelonefritis.
Postoperative deep wound infections after scoliosis surgery are more common in neuromuscular patients than in patients with idiopathic scoliosis [28]. Risk factors associated with increased postoperative wound infection rates include a generalized decline in the immune status of neuromuscular patients, poor personal hygiene, and soiling of the wound. Sponseller et al. [28] reported 25 patients (12%) with a deep wound infection out of a series of 210 surgically treated patients with neuromuscular scoliosis in a 10-year retrospective study. From these 25 patients, 16 had a scoliosis related to myelomeningocele. Two risk factors were found to be significant: the degree of cognitive impairment and use of bone allograft. Furthermore, 52% of the infections were polymicrobial, which could point to contamination during or after surgery. However, the authors did not report any case of M. hominis wound infections in their series.
Antibiotics are used routinely to prevent postoperative wound infection in patients undergoing spinal implant procedures. Currently, first or second-generation cephalosporin (e.g. cefazolin) are recommended as prophylaxis [6]. Prophylactic cefazolin was also routinely given in our case. Since, cephalosporins act on the cell wall of organisms in a manner similar to the penicillins, postoperative wound infection by M. hominis was not prevented. Antimicrobial treatment for M. hominis include protein-synthesis inhibitors such as tetracycline (e.g. doxycycline) [26] and doxycycline was administered accordingly. The postoperative deep wound infection resolved favorably after surgical debridement and appropriate antimicrobial treatment with doxycycline.
The source of M. hominis deep wound infection in our case is unclear. M. hominis is commonly associated with infections of the genitourinary tract [20] and patients with meningomyelocele are known to have a high incidence of urinary tract infections [8]. In addition, it has been shown that children with myelomeningocele have a high incidence of urological complications after surgical treatment of scoliosis [4]. Possibly, in our case the M. hominis could have been present in the urinary tract infection and spread hematogenous or by contamination, either at the time of surgery or secondarily through the wound. Other possible sources are the materials that were used during surgery. Obviously, the instrumentation and the applied Collagraft®, were sterilized. In addition, cultures and PCR on the same batch of the Collagraft® were negative. Bone allograft, used to induce and facilitate spinal fusion, could also have been the source of the infection. The fact that the bone allograft was positive for M. hominis, proved that it was infected before retrieval, however, not that it was the source of the infection itself. Bone allograft has not been reported as a carrier of M. hominis in the literature, however, it must be noted that the standard screening at the Netherlands Bone Bank Foundation does not include screening on M. hominis. Unfortunately, no other specimen of the donor could be retrieved for testing. As a result, haematogenous spread or contamination from a colonized urogenital tract or contamination by the bone allograft as source of the infection could not be excluded.
In the presented case, cultures taken during the reintervention proved to be positive for Mycoplasma. However, the diagnosis of a Mycoplasma infection was delayed due to the fastidious nature of mycoplasmas. To identify the Mycoplasma species, which in our case was cultured, 16S DNA amplification was used. Because isolation of M. hominis is difficult, time consuming, and not routinely done, a rapid specimen processing is required. Real-time PCR on deep cultures could provide a rapid alternative with a higher sensitivity and specificity than culture for the detection of M. hominis [1, 2].
In conclusion, M. hominis infections should be considered in postoperative deep wound infection after neuromuscular spinal surgery, especially in patients with genitourinary tract comorbidity. Since, M. hominis is not covered by routine prophylactic and therapeutic antibiotics, rapid real-time PCR is advised in these patients to initiate appropriate antibiotic treatment.
References
- 1.Abele-Horn M, Wolff C, Dressel P, Zimmermann A, Vahlensieck W, Pfaff F, et al. Polymerase chain reaction versus culture for detection of Ureaplasma urealyticum and Mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur J Clin Microbiol Infect Dis. 1996;15:595–598. doi: 10.1007/BF01709369. [DOI] [PubMed] [Google Scholar]
- 2.Baczynska A, Svenstrup HF, Fedder J, Birkelund S, Christiansen G. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 2004;4:35. doi: 10.1186/1471-2180-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasco M, Torres L, Marco ML, Moles B, Villuendas MC, Garcia Moya JB. Prosthetic valve endocarditis caused by Mycoplasma hominis. Eur J Clin Microbiol Infect Dis. 2000;19:638–640. doi: 10.1007/s100960000333. [DOI] [PubMed] [Google Scholar]
- 4.Boemers TM, Soorani-Lunsing IJ, Jong TP, Pruijs HE. Urological problems after surgical treatment of scoliosis in children with myelomeningocele. J Urol. 1996;155:1066–1069. doi: 10.1016/S0022-5347(01)66393-6. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla HF, Chenoweth CE, Tully JG, Blythe LK, Robertson JA, Ognenovski VM, et al. Mycoplasma felis septic arthritis in a patient with hypogammaglobulinemia. Clin Infect Dis. 1997;24:222–225. doi: 10.1093/clinids/24.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Brown EM, Pople IK, Louvois J, Hedges A, Bayston R, Eisenstein SM, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29:938–945. doi: 10.1097/00007632-200404150-00023. [DOI] [PubMed] [Google Scholar]
- 7.Cano-Sanchez A, Cano-Gracia H, Fernandez-Pardo J, Artero-Galan JM, Gonzalez-Sicilia L. Aseptic meningitis caused by Mycoplasma pneumoniae in a 19-year-old woman. Eur J Clin Microbiol Infect Dis. 1999;18:228–229. doi: 10.1007/s100960050266. [DOI] [PubMed] [Google Scholar]
- 8.Cass AS, Luxenberg M, Johnson CF, Gleich P. Incidence of urinary tract complications with myelomeningocele. Urology. 1985;25:374–378. doi: 10.1016/0090-4295(85)90492-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M, Kubak B. Mycoplasma hominis meningitis complicating head trauma: case report and review. Clin Infect Dis. 1997;24:272–273. doi: 10.1093/clinids/24.2.272. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JI, Sloss LJ, Kundsin R, Golightly L. Prosthetic valve endocarditis caused by Mycoplasma hominis. Am J Med. 1989;86:819–821. doi: 10.1016/0002-9343(89)90479-8. [DOI] [PubMed] [Google Scholar]
- 11.Dan M, Robertson J. Mycoplasma hominis septicemia after heart surgery. Am J Med. 1988;84:976–977. doi: 10.1016/0002-9343(88)90085-X. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez Guerrero ML, Manuel RJ, Soriano F. Mycoplasma hominis bacteraemia not associated with genital infections. J Infect. 1999;39:91–94. doi: 10.1016/S0163-4453(99)90109-3. [DOI] [PubMed] [Google Scholar]
- 13.Haller M, Forst H, Ruckdeschel G, Denecke H, Peter K. Peritonitis due to Mycoplasma hominis and Ureaplasma urealyticum in a liver transplant recipient. Eur J Clin Microbiol Infect Dis. 1991;10:172. doi: 10.1007/BF01964452. [DOI] [PubMed] [Google Scholar]
- 14.Jorup-Ronstrom C, Ahl T, Hammarstrom L, Smith CI, Rylander M, Hallander H. Septic osteomyelitis and polyarthritis with ureaplasma in hypogammaglobulinemia. Infection. 1989;17:301–303. doi: 10.1007/BF01650712. [DOI] [PubMed] [Google Scholar]
- 15.Kwasny O, Bockhorn G, Vecsei V. The use of gentamicin collagen floss in the treatment of infections in trauma surgery. Orthopedics. 1994;17:421–425. doi: 10.3928/0147-7447-19940501-07. [DOI] [PubMed] [Google Scholar]
- 16.La Scola B, Michel G, Raoult D. Use of amplification and sequencing of the 16S rRNA gene to diagnose Mycoplasma pneumoniae osteomyelitis in a patient with hypogammaglobulinemia. Clin Infect Dis. 1997;24:1161–1163. doi: 10.1086/513631. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, Kanj SS, Corey GR, Lins RE, Spinner RJ, Mallon WJ, et al. Mycoplasma hominis septic arthritis: two case reports and review. Clin Infect Dis. 1994;19:1067–1070. doi: 10.1093/clinids/19.6.1067. [DOI] [PubMed] [Google Scholar]
- 18.Madoff S, Hooper DC. Nongenitourinary infections caused by Mycoplasma hominis in adults. Rev Infect Dis. 1988;10:602–613. doi: 10.1093/clinids/10.3.602. [DOI] [PubMed] [Google Scholar]
- 19.Martinez OV, Chan J, Cleary T, Cassell GH. Mycoplasma hominis septic thrombophlebitis in a patient with multiple trauma: a case report and literature review. Diagn Microbiol Infect Dis. 1989;12:193–196. doi: 10.1016/0732-8893(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 20.McCormack WM. Epidemiology of Mycoplasma hominis. Sex Transm Dis. 1983;10:261–262. [PubMed] [Google Scholar]
- 21.McDonald MI, Moore JO, Harrelson JM, Browning CP, Gallis HA. Septic arthritis due to Mycoplasma hominis. Arthritis Rheum. 1983;26:1044–1047. doi: 10.1002/art.1780260817. [DOI] [PubMed] [Google Scholar]
- 22.McMahon DK, Dummer JS, Pasculle AW, Cassell G. Extragenital Mycoplasma hominis infections in adults. Am J Med. 1990;89:275–281. doi: 10.1016/0002-9343(90)90338-E. [DOI] [PubMed] [Google Scholar]
- 23.Meyer RD, Clough W. Extragenital Mycoplasma hominis infections in adults: emphasis on immunosuppression. Clin Infect Dis. 1993;17(Suppl 1):243–249. doi: 10.1093/clinids/17.supplement_1.s243. [DOI] [PubMed] [Google Scholar]
- 24.Mohiuddin AA, Corren J, Harbeck RJ, Teague JL, Volz M, Gelfand EW. Ureaplasma urealyticum chronic osteomyelitis in a patient with hypogammaglobulinemia. J Allergy Clin Immunol. 1991;87:104–107. doi: 10.1016/0091-6749(91)90219-E. [DOI] [PubMed] [Google Scholar]
- 25.Mossad SB, Rehm SJ, Tomford JW, Isada CM, Taylor PC, Rutherford I, et al. Sternotomy infection with Mycoplasma hominis: a cause of “culture negative” wound infection. J Cardiovasc Surg (Torino) 1996;37:505–509. [PubMed] [Google Scholar]
- 26.Myhre EB, Mardh PA. Treatment of extragenital infections caused by Mycoplasma hominis. Sex Transm Dis. 1983;10:382–385. [PubMed] [Google Scholar]
- 27.Sielaff TD, Everett JE, Shumway SJ, Wahoff DC, Bolman RM, III, Dunn DL. Mycoplasma hominis infections occurring in cardiovascular surgical patients. Ann Thorac Surg. 1996;61:99–103. doi: 10.1016/0003-4975(95)00826-8. [DOI] [PubMed] [Google Scholar]
- 28.Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine. 2000;25:2461–2466. doi: 10.1097/00007632-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Stemberger A, Grimm H, Bader F, Rahn HD, Ascherl R (1997) Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur J Surg Suppl 17–26 [PubMed]
- 30.Waites KB, Rudd PT, Crouse DT, Canupp KC, Nelson KG, Ramsey C, et al. Chronic Ureaplasma urealyticum and Mycoplasma hominis infections of centralervous system in preterm infants. Lancet. 1988;331:17–21. doi: 10.1016/S0140-6736(88)91002-1. [DOI] [PubMed] [Google Scholar]
- 31.Wilson ME, Dietze C. Mycoplasma hominis surgical wound infection: a case report and discussion. Surgery. 1988;103:257–260. [PubMed] [Google Scholar]
- 32.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop. 2004;427:86–93. doi: 10.1097/01.blo.0000143571.18892.8d. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Olson DA, Tully JG, Watson HL, Cassell GH, Gustafson DR, et al. Isolation of Mycoplasma hominis from a brain abscess. J Clin Microbiol. 1997;35:992–994. doi: 10.1128/jcm.35.4.992-994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]