Abstract
Ipsilateral motor evoked potentials (MEPs) in spinal cord surgery intraoperative monitoring is not well studied. We show that ipsilateral MEPs have significantly larger amplitudes and were elicited with lower stimulation intensities than contralateral MEPs. The possible underlying mechanisms are discussed based on current knowledge of corticospinal pathways. Ipsilateral MEPs may provide additional information on the integrity of descending motor tracts during spinal surgery monitoring.
Keywords: Motor evoked potential, Ipsilateral, Contralateral, Corticospinal, Intraoperative monitoring, Scoliosis
Introduction
Intraoperative monitoring (IOM) of the motor pathways is a routine procedure for ensuring integrity of corticospinal tracts during scoliosis surgery. In combination with somatosensory evoked potentials, motor evoked potential (MEP) monitoring is widely utilized in operations with significant risks of spinal cord damage [2, 14].
MEPs are most effectively obtained with multi-pulse cortical electrical stimulation during IOM [3]. However, anesthetic agents, which cause suppression of cortical and spinal motor neuron excitability, affect them [4, 5]. While most IOM protocols involving MEPs utilize total intravenous anesthesia (TIVA), we have previously reported success with desflurane as a halogenated inhalational anesthetic agent [15].
In IOM, MEPs are elicited mostly with contralateral cortical electrical stimulation. Ipsilateral MEP responses have not been adequately studied in this context. Our paper describes observations of ipsilateral and contralateral MEPs with bilateral recordings, in conjunction with TIVA or desflurane during IOM of scoliosis surgery.
Methods
We studied nine patients (mean age 16.2 years; range 14–17 years; 1 male) over a 6-month period in a prospective manner. The local ethical committee has approved the study protocol. All patients did not have medical conditions contraindicating transcranial electrical stimulation. Apart from scoliosis, they were healthy and had normal neurological examinations.
Multi-pulse transcranial electrical stimulation was performed using two constant-current stimulators connected in parallel configuration from a Dantec Keypoint EMG machine (Dantec, Skovlunde, Denmark). A train of five square wave stimuli 0.5 ms in duration was delivered at 4 ms (250 Hz) interstimulus intervals. Stimulating electrodes consisted of 9 mm gold-plated disc electrodes at C3C4 (International 10–20 system) affixed with collodion. Stimulation output was increased from 50 mA in steps of 5 mA until a reproducible MEP was elicited. The intensity was then increased and fixed at 10% above this threshold intensity to obtain a supramaximal MEP response. Each stimulator was capable of delivering a maximum output of 100 mA (200 mA in total). MEP recordings were obtained with 13 mm disposable subdermal needles (Technomed Europe, Beek, Netherlands) in the tibialis anterior (TA) bilaterally. Filter settings were set at 10 Hz and 2 kHz. Input impedance of stimulating and recording electrodes were maintained below 5 kΩ.
For induction of anesthesia, sodium thiopentone at 4 mg/kg and fentanyl at 2 mcg/kg was administered; 0.8 mg/kg of intravenous atracurium was used to facilitate endotracheal intubation. No further doses of neuromuscular blocking agents were used subsequently. In the desflurane group, anesthesia was maintained using 60% nitrous oxide in oxygen. Desflurane was introduced through a calibrated vaporizer up to an end-tidal concentration of 2.1–4.3 %, with a mean concentration of 3.4% (approximately 0.5 maximum alveolar concentration). This was measured using an Ohmeda respiratory gas monitor 5250 (BOC Group, Louisville, USA). Closed circuit mechanical ventilation was adjusted to maintain end-tidal carbon dioxide levels between 32 and 35 mmHg.
In the TIVA group, anesthesia was maintained using the regime of 10 mg/kg of propofol for the first 10 min, 8 mg/kg for the next 10 min and 5 mg/kg for the subsequent length of operation; 50% air in oxygen was administered. In both groups, morphine was titrated as required for pain relief.
Monitoring included electrocardiography, pulse oximetry, capnography and direct radial artery pressures. All patients were kept nornothermic with a warming blanket. Normotensive anesthesia was maintained throughout the operation.
After approximately 45 min post-induction, a train of four-twitch assessment was performed using a nerve stimulator (Fischer Paykel NS242, UK). Cortical stimulation was commenced only when the amplitude of the fourth was visibly similar to the first. An interval of 3–5 min was allowed between two trains of cortical stimulation. This alternated with monitoring of somatosensory evoked potentials from posterior tibial nerve stimulation.
We measured two parameters: MEP amplitude, onset latency and initial stimulation intensity. Peak to peak amplitudes (between two largest peaks opposite in polarity) and onset latency was utilized for all MEP responses recorded bilaterally. Hence, ipsilateral MEPs refer to MEPs recorded from the TA on the same side as cortical stimulation. Within each patient, ten consecutive supramaximal MEPs obtained before insertion of pedicle screws used as a baseline were averaged to obtain the first two parameters. The initial stimulation intensity was defined as the minimal intensity required to obtain five consistent visible MEP responses at a vertical gain of 20 μV per division.
During insertion of pedicle screws and instrumentation, a 50% reduction of MEP amplitude or 10% prolongation of latency was brought to the surgeon’s attention.
Statistical analyses using Student’s t-tests were obtained with Microsoft SPSS for Windows Version 10.1. Statistical significance was considered at P < 0.05.
Results
There were no complaints of headache, seizures or skin burns postoperatively; all patients had normal neurological examination.
MEPs were successfully obtained from all patients with TA recordings bilaterally. There were four patients in the TIVA group and five in the desflurane group. Mean ages for desflurane (16.2) and TIVA (15.7) groups were not significantly different (P = 0.6).
None of the patients had MEP amplitude or latency changes exceeding our set limits so as to require immediate surgical attention during and after pedicel screw insertion and spinal instrumentation.
The ipsilateral MEP amplitudes (standard deviation) were significantly larger than contralateral MEP amplitudes [68.9 (27.1) vs. 52.5 (15.7) μV, P < 0.01, paired t-test]. The initial stimulation intensity to obtain ipsilateral MEPs was significantly lower than for contralateral MEPs [66.9 (12.3) vs. 74.4 (10.1) mA, P < 0.05, paired t-test]. However, there were no significant latency differences for ipsilateral and contralateral MEPs [32.0 (2.1) vs. 31.5 (2.0) ms, P = 0.3, paired t-test).
There was no significant difference between use of TIVA or desflurane anesthesia for MEP amplitudes obtained, with ipsilateral (P = 0.06, unpaired t-test) and contralateral (P = 0.09, unpaired t-test) stimulation. Additionally, there was also no significant difference between right and left sided MEP amplitudes, either with ipsilateral (P = 0.9, paired t-test) or contralateral (P = 0.7, paired t-test) stimulation.
We consecutively studied an additional 17 subjects monitored for scoliosis surgery using an identical protocol (1 men, mean age 16.1 years, range 14–22). All had the TIVA anesthetic regimen. With right cortex stimulation, mean initial stimulation intensity to obtain ipsilateral MEPs [39.7 (9.9) mA] was significantly lower than to obtain contralateral MEPs [47.1 (11.3) mA, paired t-test, P < 0.0005]. With left cortex stimulation, similar findings were obtained [40.6 (10.7) vs. 50.3 (11.8) mA, paired t-test, P < 0.0005]. With right cortex stimulation, mean ipsilateral MEP amplitudes [107.1 (35.7) μV] were significantly larger than mean contralateral MEP amplitudes [90 (37.1) μV, paired t-test, P = 0.01]. With left cortex stimulation, similar findings were again observed [112.1 (37) vs. 82.3 (30.9) μV, paired t-test, P = 0.0004]. With right cortex stimulation, mean ipsilateral MEP latencies [30.2 (2.4) ms] were not significantly different from mean contralateral MEP latencies [30 (2.3) ms, paired t-test, P = 0.3). With left cortex stimulation, similar findings were again observed [30 (2.1) vs. 30.4 (2.3) ms, paired t-test, P = 0.2].
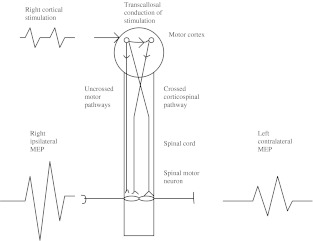
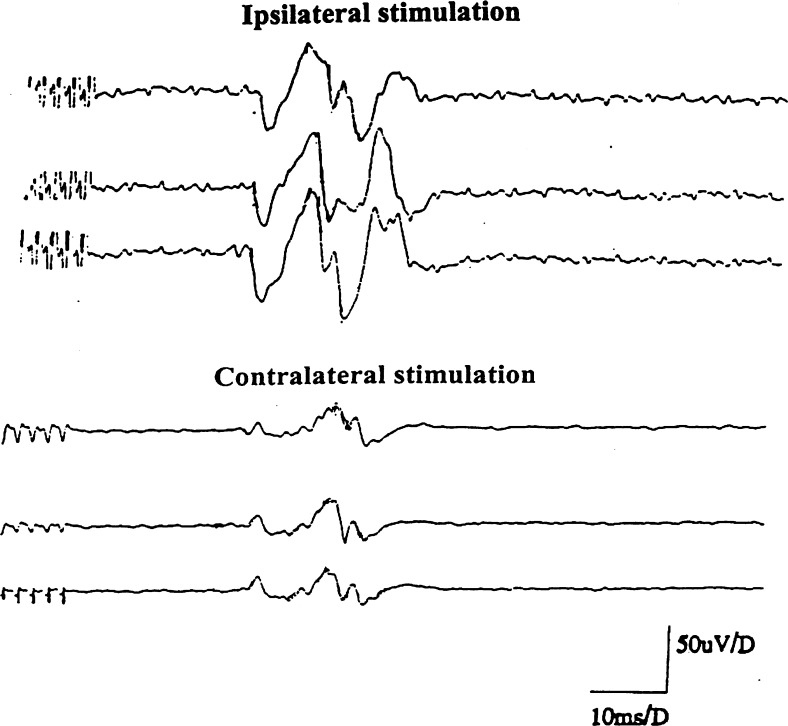
Examples of MEPs obtained with both ipsilateral and contralateral stimulation are shown in schematically in Fig. 1 and 2.
Fig. 1.
Schematic diagram showing right cortical stimulation, resulting in ipsilaterally and transcallosally conducted corticospinal impulses activating the spinal cord anterior horn cell. The right ipsilateral MEP recording is from the TA. Summation of ipsilaterally conducted and transcallosally generated descending impulses may thus result in larger ipsilateral MEPs from right cortical stimulation
Fig. 2.
Actual consecutive MEPs obtained from a patient, showing larger amplitude responses with ipsilateral stimulation. Both recordings were made from the TA at 70% stimulation intensity
Discussion
The present study showed that ipsilateral MEPs have significantly larger amplitudes and were elicited with significantly lower stimulation intensities than contralateral MEPs. However, onset MEP latencies were not significantly different.
The origin of ipsilateral MEPs in humans is not well understood. In animal studies, cat corticospinal neurons have been shown to evoke ipsilateral actions via ipsilaterally descending reticulospinal tracts, as well as via contralaterally descending reticulospinal neurons, both by synapsing spinal interneurons [9]. Tracer studies in rhesus monkeys have quantified ipsilateral corticospinal fibers as approximately 9–12% of the total descending corticospinal projections [14]. Thus, current evidence points to contralateral corticospinal fibers as the predominant pathway for spinal motorneuron activation.
The presence of ipsilateral MEPs has mostly been described in pathological conditions. Patients with congenital mirror movements [11], schizencephaly [12], and Kallmann’s syndrome [13] show ipsilateral MEPs, which likely result from abnormal structure and function of ipsilateral corticospinal fibers. However, functional reorganization and unmasking of ipsilateral corticospinal pathways may contribute to the generation of ipsilateral MEPs after adult stroke [6] and congenital hemiparesis [18]. One study involving 50 normal children suggested that presence of ipsilateral MEPs might be a normal state of ontogeny. Their disappearance after 10 years old is likely due to increasing transcallosal inhibitory influences [16]. In our study, all patients were above 10 years of age, and did not have clinical features to suggest presence of underlying conditions mentioned above. Another study comparing healthy adults with stroke patients has suggested ipsilateral MEPs may be conducted via corticoreticulospinal or corticopropriospinal pathways in normal subjects [1].
What are the possible underlying mechanisms, which explain our findings? Firstly, it is possible that ipsilateral MEPs may be solely due to transcallosal stimulation of the contralateral motor cortex. Additionally, the effects of anesthesia on corticospinal excitability may facilitate this, hence resulting in significantly lower initial stimulation intensity to obtain ipsilateral MEPs. While evidence to suggest this is scarce, rat brain studies have demonstrated widespread action of anesthesia at multiple binding sites [8]. Magnetic resonance brain imaging has also demonstrated increased callosal T2 changes with anesthesia, suggesting structural alterations at a molecular level [19]. It is also possible that longstanding scoliosis has led to spinal cord plasticity changes. Motor pathway reorganization and spinal cord plasticity have been well documented in response to cord injury [7] in an activity-dependent manner [10]. Thus, structural and postural changes of longstanding scoliosis may have resulted in reorganization of cortical or subcortical motor pathways, including ipsilateral corticoreticular fibres leading to our observations [17]. However, lack of lateralization of MEP amplitudes with ipsilateral or contralateral stimulation was not supportive of this hypothesis.
Additionally, lack of significant ipsilateral and contralateral latency differences suggest bilateral motor cortex stimulation has resulted in ipsilateral MEPs, which may have comprised early ipsilaterally conducted components and late transcallosally stimulated components (Fig. 1). This might also explain the larger amplitudes of ipsilateral MEPs obtained than MEPs derived from contralateral motor cortex stimulation. Further studies clarifying the predominant mechanisms responsible would be interesting.
Are ipsilateral MEP responses useful and relevant in clinical settings? Ipsilateral MEPs are readily elicited, as shown in this study. While the relative contributions of ipsilaterally and transcallosal conducted MEPs remain uncertain, bilateral MEP recordings during spinal surgery IOM may provide additional information regarding the integrity of descending motor tracts. Together with the electrophysiological findings presented here, future studies clarifying these aspects would be justified. It thus may be feasible to routinely monitor MEPs bilaterally in future IOM protocols for spinal surgery.
References
- 1.Alagona G, Delvaux V, Gerard P, Pasqua V, Pennisis G, Delwaide PJ, Nicoletti F, Maertens Noordhout A. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute stroke patients. Stroke. 2001;32:1304–1309. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Lichtor JL, Lane BS, Coalson DW, Korttila KT. Awakening, clinical recovery, and psychomotor effects after desflurane and profofol anesthesia. Anesth Analg. 1996;83:721–725. doi: 10.1097/00000539-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Burke D, Hicks RG. Surgical monitoring of motor pathways. J Clin Neurophysiol. 1998;15:194–205. doi: 10.1097/00004691-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Byas-Smith M, Frolich MA, Votaw JR, Faber TL, Hoffman JM. Cerebral blood flow during propofol induced sedation. Mol Imaging Biol. 2002;4:139–146. doi: 10.1016/S1536-1632(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 5.Calancie B, Klose KJ, Baier S. Isoflurane-induced attenuation of motor evoked potentials caused by electrical motor cortex stimulation during surgery. J Neurosurg. 1991;74:897–904. doi: 10.3171/jns.1991.74.6.0897. [DOI] [PubMed] [Google Scholar]
- 6.Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol. 2000;11:1990–1996. doi: 10.1016/S1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 7.Cohen LG, Roth BJ, Wassermann EM, Topka H, Fuhr P, Schultz J, Hallett M. Magnetic stimulation of the human cerebral cortex, an indicator of reorganization in motor pathways in certain pathological conditions. J Clin Neurophysiol. 1991;8:56–65. doi: 10.1097/00004691-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Eckenhoff MF, Eckenhoff RG. Quantitative autoradiograohy of halothane binding in rat brain. J Pharmacol Exp Ther. 1998;285:371–376. [PubMed] [Google Scholar]
- 9.Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motorneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SM, Mitchell GS. Activity-dependent plasticity of descending synaptic inputs to spinal motorneurons in an in vitro turtle brainstem-spinal cord preparation. J Neurosci. 2000;20:3487–3495. doi: 10.1523/JNEUROSCI.20-09-03487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. J Neurol Neurosurg Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YH, Jang SH, Han BS, Kwon YH, You SH, Byun WM, Park JW, Yoo WK. Ipsilateral motor pathway confirmed by diffusion tensor tractography in a patient with schizencehaly. Neuroreport. 2004;15:1899–1902. doi: 10.1097/00001756-200408260-00013. [DOI] [PubMed] [Google Scholar]
- 13.Krams M, Quinton R, Ashburner J, Friston KJ, Frackowiak RS, Rouloux PM, Passingham RE. Kallmann’s syndrome: mirror movements associated with bilateral corticospinal tract hypertrophy. Neurology. 1999;10:816–822. doi: 10.1212/wnl.52.4.816. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- 15.Lo YL, Dan YF, Tan YE, Nurjannah S, Tan SB, Tan CT, Raman S. Intraoperative monitoring in scoliosis surgery with multi-pulse cortical stimuli and desflurane anesthesia. Spinal Cord. 2004;42:342–345. doi: 10.1038/sj.sc.3101605. [DOI] [PubMed] [Google Scholar]
- 16.Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- 17.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nature Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 18.Staudt M, Krageloh-Mann I, Grodd W. Ipsilateral corticospinal pathways in congenital hemiparesis on routine magnetic resonance imaging. Pediatr Neurol. 2005;32:37–39. doi: 10.1016/j.pediatrneurol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Whitfield A, Douglas RH. Effect of general anethesia on the magnetic resonance imaging signal from the brain. Br J Anes. 1989;62:694–696. doi: 10.1093/bja/62.6.694. [DOI] [PubMed] [Google Scholar]