Abstract
Epithelioid sarcoma is a rare and highly malignant soft tissue tumor that is commonly found in the extremities and rarely in the trunk area. This malignant tumor often mimics granuloma or nodular fasciitis, which causes a delay in establishing the diagnosis. This type of cancer has a high recurrence rate. Surgical treatment requires wide radical resection. The objective of this case report is to highlight the unique location of a rare neoplasm and to illustrate the relentless course of epithelioid sarcoma despite initial radical resection. A 14-year-old boy was admitted to our facility with a soft tissue mass on the right lower thoracic spine. The large tumor mass had deeply penetrated into the muscles, infiltrated the neuroforamen of T9–T10 level, and compressed the dural sac. Immunohistological study of the biopsy was highly consistent with an epithelioid sarcoma. Wide excision of the mass, laminectomy and spine fusion with instrumentation was performed. The patient received chemotherapy and irradiation. The first recurrence of the neoplasm was seen as a contralateral metastasis 21 months after the resection. On the last follow-up, 3 years postoperatively, the patient was in a good general condition. However, further progression of the sarcoma had to be recognized. Our case encompasses multiple features that represent negative prognostic factors. Initial wide excision of the neoplasm and adjuvant therapy including chemotherapy and irradiation seem to slow down the relentless course of epithelioid sarcoma in the trunk.
Keywords: Epithelioid sarcoma, Thoracic spine, Survival
Introduction
Epithelioid sarcoma is a rare type of a fibrohistiocytic tumor, which is not extensively documented in the literature. It is common at the extremities, and rare at the trunk area. The incidence of epithelioid sarcoma in the trunk is 4% with a range from 0 to 44% [2, 12, 14]. It affects mainly adolescent males [1–3, 11, 13, 14, 17]. To our knowledge there are only two cases reported of localization in the spine (lumbosacral junction and sacrum) [7, 14], and there are no reports on epithelioid sarcoma at the thoracic spinal level. Laskowski [9] has first described the unique histological appearance in 1961. It resembles a chronic inflammatory process, necrotizing granuloma, squamous cell carcinoma or other fibrohistiocytic tumors. Electron microscopic appearance suggests origin from synovial structures [6, 10]. There is 85% local recurrence rate and 30–50% likelihood for metastases predominately lymphatic or in the lungs [2, 4, 11]. The 5-year survival rate varies from 65 to 100% [1, 12, 13]. Factors adversely affecting the survival rate are [2, 12]:
Size of the tumor;
Infiltration of the tumor into muscular, neural or vascular structures;
Primary location in the trunk;
Pulmonary metastasis.
Wide surgical resection is indicated, since epithelioid sarcoma frequently follows a relentless course with multiple recurrences [4, 17]. Chemotherapy and irradiation are recommended to decrease recurrence rate [2, 8].
Case report
A 14-year-old overweight boy presented at an orthopaedist’s private practice with a painless, bulging mass on his back that was incidentally discovered by his grandmother. He was 183 cm tall and weighted 107 kg. Besides obesity no other medical problems were reported. There was no family history of a soft tissue mass. On the physical examination the tumor had a soft consistence and could not be shifted from the underlying tissue. Neurological examination did not reveal any deficit. Magnetic resonance imaging (MRI) with gadolinium of his thoracolumbar spine revealed a right paravertebral cystic mass. The soft tissue tumor extended from T7 to T11 level and measured 9.5×3×3 cm. The mass infiltrated via the neuroforamen at T9–T10 levels compressing one quarter of the spinal cord (Fig. 1). There was no osseous involvement. Open biopsy of the mass showed necrobiotic granuloma including epithelioid and fusiform cells. Immunohistochemical studies revealed a co-expression of vimentin and cytokeratin, with pathological diagnosis of epithelioid sarcoma grade III. During the tumor staging a craniolateral metastasis was depicted on the PET scan (Fig. 2). Consecutive MRI confirmed a soft tissue mass of 1 cm diameter, located about 10 cm distant from the main mass. Wide mass resection, including the locoregional metastasis, led to a sacrifice of large parts of the paravertebral thoracic muscles. In addition a hemilaminectomy of T9–T10 vertebra with resection of the adjacent pedicles and careful dissection from the dural sac was performed. There was no sparing of right ninth nerve root. The tumor resection was intralesional. Posterior transpedicular instrumentation from T6 to L1 was carried out. After surgery no new motor or sensory deficits were noted. Chemotherapy was initiated on the 6th postoperative week and irradiation on 10th postoperative week. The patient received nine courses of chemotherapy within 25 weeks (Table 1) and a total dose of 3-Gy irradiation over 4 weeks. He developed a wound dehiscence 2 weeks after initiation of the irradiation therapy. Under daily dressing no infection was observed. The wound was finally closed with a pedicle musculocutaneous flap using the Latissimus dorsi muscle 4 weeks after the completing the course of irradiation. Routine follow-up consisted of a physical examination, plain radiograms of the thoracic spine plus MRI Scans every 3 month (Fig. 3).
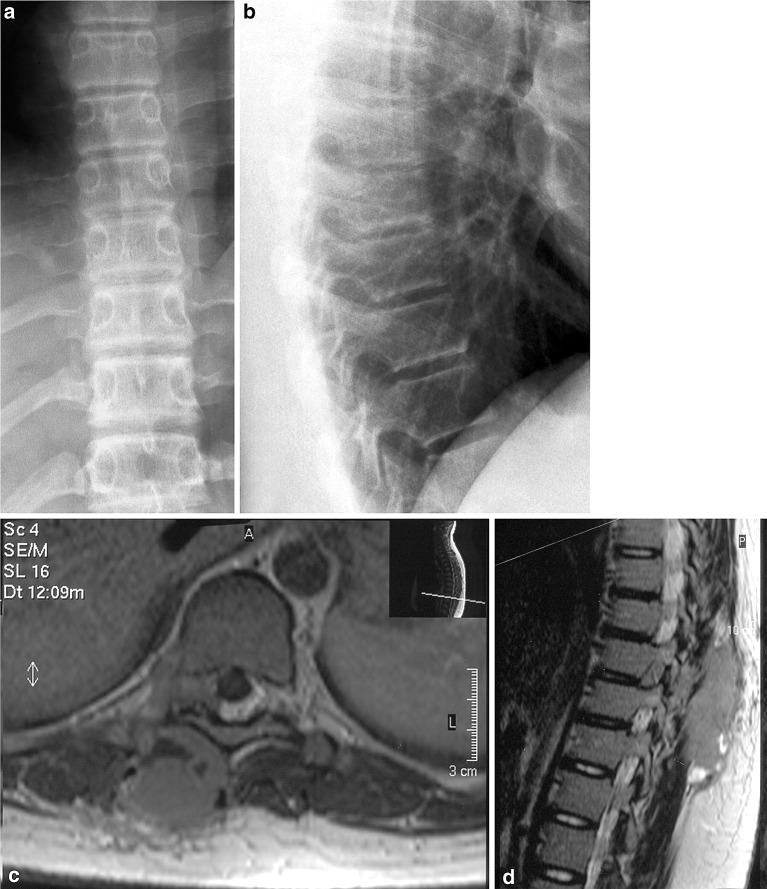
Fig. 1.
Preoperative antero-posterior (a) and lateral (b) plain X-rays of the middle thoracic spine. On the ap view no irregular formation of the pedicles can be depicted—the lateral view of the thoracic spine show apophyseal endplate changes as a feature of Scheuermans disease. No signs of osteolytic lesion can be detected. Preoperative transverse (c) and sagittal (d) MRI scan (T2 sequences: TR=3,070, TE=120) demonstrating the tumor mass in the region of the paravertebral muscles, reaching and compressing the dural sac via the right neuroforamen Th9/10
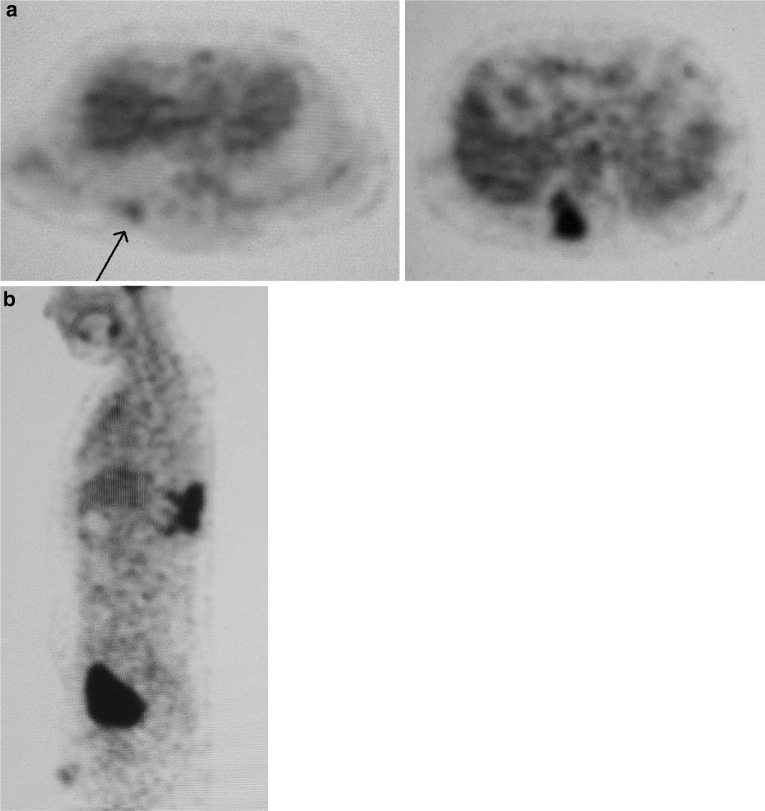
Fig. 2.
On the PET scan a craniolateral metastasis was detected (a) and the paravertebral tumor mass was confirmed (b). No further satellite lesions were found
Table 1.
Agents and dosage of the quadruple chemotherapy
| Agent (trade name) | Total dose (mg) |
|---|---|
| Ifosfamid (Holoxan®) | 100,440 |
| Vincristin (Onkocristin®) | 20 |
| Actinomycin D (Lyovac®) | 12 |
| Doxorubicin (Ribodoxo®) | 494 |
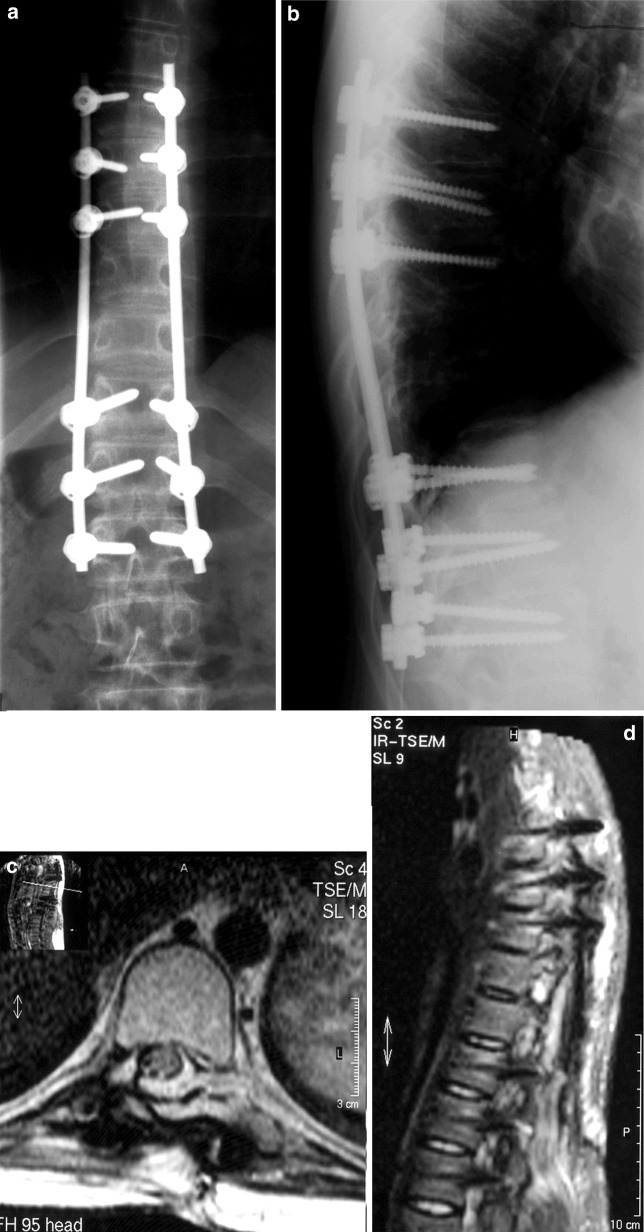
Fig. 3.
Postoperative antero-posterior (a) and lateral (b) plain radiograph of the thoracic spine at final follow-up. Postoperative transverse (c) and sagittal (d) MRI scan (T2 sequences: TR=3,682, TE=120) at 1.5 year showing no sign of recurrence
A metastasis at the fifth intercostal space of the chest contralateral to the initial tumor location was observed at 21 months follow-up. At this stage the patient denied further intervention. Two years postoperatively pulmonary metastases were detected on CT scans in addition to a local recurrence on the right thoracic spine. There was no significant progression of the tumor growth at the last follow-up 37 month after surgical treatment. The young male was in good general condition fully participating in social life.
Discussion
Epithelioid sarcoma is a rare soft tissue neoplasm with a high local recurrence rate. Epithelioid sarcoma presents histologic characteristics similar to inflammatory processes and other benign soft tissue tumors, which frequently delays the diagnosis and adequate treatment. Typical for this soft tissue sarcoma is an immunohistochemical co-expression of cytokeratin and/or epithelial membrane antigen (EMA) and vimentin, and in half of the cases an immunoreactivity for CD34, an occasional reactivity for smooth muscle actin (SMA), desmin, neuron specific enolase and S100 protein [2, 5]. In our specimen a strong positive immunoreaction with antibodies to cytokeratin, vimentin and EMA was registered (Fig. 4). Histomorphology and immunohistochemical pattern are typical and separates this tumor entity from other sarcomas. To differentiate from osteogenic sarcoma, which may also co-express cytokeratin and vimentin, and may demonstrate a immunoreactivity with SMA and CD99, epithelioid sarcoma, however, does not produce tumor osteoid, which is the key finding in osteogenic sarcoma.
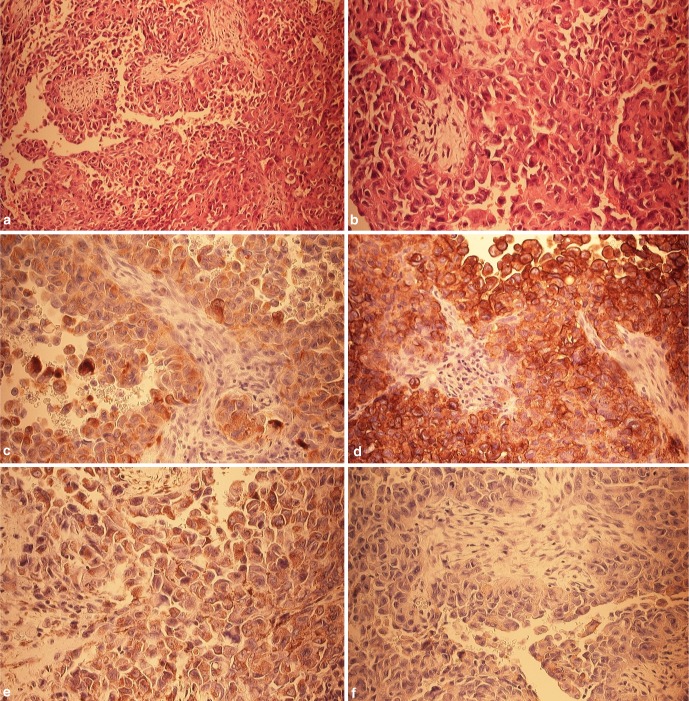
Fig. 4.
a Histomorphologic aspect of epithelioid sarcoma with solid and nodular growth pattern, H&E (10×). b The tumor cells are roundish, admixed with spindle shaped cells and are featuring an eosinophilic cytoplasm with vesicular, irregular nuclei (20×). c Immunohistochemical investigation with antibodies against cytokeratin shows a strong positive immunoreaction (20×). d The immunoreaction against epithelial membrane antigen (EMA) demonstrates a comparable picture (20×). e The immunoreactivity against smooth muscle actin (SMA) features a weak staining (20×). f The immunoreaction against CD 99 is negative (20×)
Wide local resection is the recommended treatment of epithelioid sarcoma [1, 8, 11, 14]. In the spine however, total eradication of the sarcoma by an enbloc excision is compromised by the presence of neural structures [16]. Thus the concept of intralesional resection was followed both for the main tumor mass and the metastasis. Nerve sheaths may serve as a pathway for the spread of the sarcoma [1, 2, 8, 11, 17]. Enzinger [4] reported six cases with a mass infiltration along nerve sheaths or vascular structures. In our case there was involvement of the right ninth nerve root. The mass expansion into the right T9–T10 neuroforamen with compression of the dural sac necessitated a wide hemilaminectomy with resection of the facet joints and the adjacent pedicles. The wide paraspinal muscle resection required six levels segmental instrumentation and fusion to avoid scoliotic deformity due to muscular imbalance [15]. Pulmonary metastasis and local recurrence of the sarcoma were first diagnosed 24 month after the resection. In comparison, a survival period of 22 month after the initial surgery was described in a case report of epithelioid sarcoma in the lumbosacral spine [14]. Moreover, on first diagnosis, our case embodied many factors that promote recurrence of this neoplasm: large size, localization in the trunk and infiltration in both muscle and neural structures. Recurrence as the typical feature of epithelioid sarcoma cannot be prevented even in extremities, where a radical surgical regimen can be pursued [8]. In conclusion, epithelioid sarcoma of the spine is an extremely rare entity, which differs from other sarcoma subtypes in propensity for local recurrence and spread along the lymphatic pathways. Initial radical resection followed by adjuvant chemotherapy and irradiation can retard the relentless course of this malignancy.
References
- 1.Bos GD, Pritchard DJ, Reiman HM, et al. Epithelioid sarcoma. An analysis of fifty-one cases. J Bone Joint Surg Am. 1988;70:862–870. [PubMed] [Google Scholar]
- 2.Chase DR, Enzinger FM. Epithelioid sarcoma. Diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241–263. doi: 10.1097/00000478-198504000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger FM. Epithelioid sarcoma. A sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029–1041. doi: 10.1002/1097-0142(197011)26:5<1029::AID-CNCR2820260510>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger FM, Weiss SW. Epithelioid sarcoma. In: Enzinger FM, Weiss SW, editors. Soft tissue tumours. St Louis: Mosby; 1995. pp. 787–795. [Google Scholar]
- 5.Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 6.Gabbiani G, Fu YS, Kaye GI, et al. Epithelioid sarcoma. A light and electron microscopic study suggesting a synovial origin. Cancer. 1972;30:486–499. doi: 10.1002/1097-0142(197208)30:2<486::AID-CNCR2820300229>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Ilaslan H, Sundaram M, Unni KK, Shives TC. Primary vertebral osteosarcoma: imaging findings. Radiology. 2004;230:697–702. doi: 10.1148/radiol.2303030226. [DOI] [PubMed] [Google Scholar]
- 8.Kawai A, Hasizume H, Sugihara S, et al. Treatment of bone and soft tissue sarcomas of the hand and wrist. Int Orthop. 2002;26:26–30. doi: 10.1007/s002640100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laskowski J. Aponeurotic sarcoma. Pol Med J. 1971;10:12–17. [PubMed] [Google Scholar]
- 10.Patchefsky AS, Soriano R, Kostianovsky M. Epithelioid sarcoma: ultrastructural similarity to nodular synovitis. Cancer. 1977;39:143–152. doi: 10.1002/1097-0142(197701)39:1<143::AID-CNCR2820390125>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Prat J, Woodruff JM, Marcove RC. Epithelioid sarcoma: an analysis of 22 cases indicating the prognostic significance of vascular invasion and regional lymph node metastasis. Cancer. 1978;41:1472–1487. doi: 10.1002/1097-0142(197804)41:4<1472::AID-CNCR2820410436>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Ross HM, Lewis JJ, Woodruff JM, Brennan MF. Epithelioid sarcoma: clinical behavior and prognostic factors of survival. Ann Surg Oncol. 1997;4:491–495. doi: 10.1007/BF02303673. [DOI] [PubMed] [Google Scholar]
- 13.Soule EH, Enriquez P. Atypical fibrous histiocytoma, malignant fibrous histiocytoma, malignant histiocytoma, and epithelioid sarcoma. A comparative study of 65 tumors. Cancer. 1972;30:128–143. doi: 10.1002/1097-0142(197207)30:1<128::AID-CNCR2820300119>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Steib JP, Pierchon F, Farcy JP, et al. Epithelioid sarcoma of the spine: a case report. Spine. 1996;21:634–638. doi: 10.1097/00007632-199603010-00019. [DOI] [PubMed] [Google Scholar]
- 15.Walton JM, Bass J, Sambey E, Rubin SZ. Use of human dura in pediatric chest wall reconstruction after tumor resection. J Pediatr Surg. 1994;29:1189–1191. doi: 10.1016/0022-3468(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein JN (1994) Spine neoplasms. In: Weinstein SL (ed) The pediatric spine. Principles and practice, vol I. Raven Press. New York, pp 887–917
- 17.Wevers AC, Kroon BB, Albus-Lutter CE, Gortzak E. Epithelioid sarcoma. Eur J Surg Oncol. 1989;15:345–349. [PubMed] [Google Scholar]