Abstract
Integrin αvβ6 is an epithelial cell-specific receptor that is not normally expressed by resting epithelium but its expression is induced during wound healing. The function of αvβ6-integrin in wound repair is not clear. In the present study, we showed that β6-integrin expression was strongly up-regulated in the epidermis in human chronic wounds but not in different forms of skin fibrosis. To test whether increased β6-integrin expression plays a role in abnormal wound healing we developed four homozygous transgenic mouse lines that constitutively expressed human β6-integrin in the epithelium. The mice developed normally and did not show any histological abnormalities in the skin. The rate of experimental skin wound closure was unaltered and the wounds healed without significant scar formation. However, during breeding program 16.1 to 27.0% of transgenic mice developed spontaneous, progressing fibrotic chronic ulcers. None of the wild-type animals developed these lesions. The chronic lesions had areas with severe fibrosis and numerous activated macrophages and fibroblasts expressing transforming growth factor (TGF)-β. The level of TGF-β1 was significantly increased in the lesions as compared with normal skin. The findings suggest that increased αvβ6-integrin in keratinocytes plays an active part in abnormal wound healing possibly through a mechanism involving increased activation of TGF-β.
Normal wound healing requires coordinated function of epithelial, inflammatory, and connective tissue cells. Disruption of this interplay may lead to abnormal or deficient wound healing. The interaction of inflammatory and connective tissue cells in wound healing has been well documented. Interestingly, recent studies have also indicated that epithelium regulates inflammation and connective tissue formation. For example, keratinocytes can modulate dermal fibroblast functions including collagen synthesis1–3 and altered function of epithelial cells may contribute to onset of psoriasis.4,5
Cell adhesion receptors of the integrin family play a major role in the regulation of keratinocyte function and in wound healing. Integrins are heterodimeric adhesion molecules that are composed of an α- and β-subunit. Integrins play a role in initiating intracellular signaling cascades in response to extracellular ligands, participate in growth factor activation, and collaborate with growth factor receptor signaling.6 On wounding, integrin expression by keratinocytes is up-regulated and they also start to express novel integrins, including αvβ6.6–8 The function of αvβ6-integrin in wound healing is not known. In cell culture, αvβ6-integrin facilitates cell adhesion and migration on fibronectin, vitronectin, and tenascin that are components of the early wound provisional matrix.9–13 Early expression of αvβ6-integrin during keratinocyte migration into wound suggests that αvβ6-integrin may regulate this process also in vivo.8,13–15 However, β6-integrin-deficient mice did not show any change in wound closure rate.16,17 Although αvβ6-integrin is expressed by migrating keratinocytes in early wounds, the maximal expression coincides with basement membrane and granulation tissue formation when the migrating edges of the wound epithelium have joined.8,13,18 The temporal localization of αvβ6-integrin in epithelium suggests that αvβ6-integrin may also be involved in epithelial cell differentiation, regeneration of basement membrane, regulation of inflammatory reaction, and formation of granulation tissue. Interestingly, integrin αvβ6 can localize transforming growth factor (TGF)-β1 to cell surface by binding to the latency-associated peptide leading to TGF-β activation presumably by a nearby cell.19 TGF-β1 plays an important role in wound repair by regulating re-epithelialization, suppressing inflammation, and promoting connective tissue regeneration and scar formation.20 In the absence of αvβ6-integrin there seems to be a deficiency of TGF-β1 activation by the epithelium leading to exaggerated inflammation in response to injury or infection. Transgenic mice with targeted deletion of β6-integrin developed excess lung inflammation that was reversed by restoring β6 expression,11 and they were compromised in their ability to mount an inflammatory response to experimental infection in the gut.21 Activated TGF-βs may also regulate other cellular functions critical for wound healing, including integrin expression, cell migration, proliferation, differentiation and, matrix synthesis and degradation, in an autocrine or paracrine manner.22–24
Chronic, nonhealing wounds are characterized by deficient re-epithelialization and excess formation of granulation tissue. This is associated with ongoing inflammation that maintains the chronic state and prevents healing.25 On the other hand, hypertrophic scars and skin fibrosis result from excess healing response and associate with increased TGF-β1 expression and activation. This leads to enhanced extracellular matrix production by fibroblasts and excess matrix accumulation.26,27 We hypothesized that prolonged expression of αvβ6-integrin expressed by keratinocytes may lead to abnormal healing. To this end, we studied the expression of αvβ6-integrin in human aberrant wound healing (chronic wounds and hypertrophic scars) and skin fibrosis. The findings showed that in human chronic skin wounds, but not in hypertrophic scars or in skin fibrosis, expression of αvβ6-integrin was up-regulated in the epithelium. The αvβ6-integrin-expressing keratinocytes were in close proximity with activated fibroblasts in the underlying connective tissue. To study the function of αvβ6-integrin in wound healing, we created transgenic mice harboring human β6-integrin gene under the control of cytokeratin 14 promoter to target constitutive expression of αvβ6-integrin in the epidermal basal cells. The homozygous mice showed normal wound repair speed, and wound healing did not result in scar formation. However, during breeding of the colony, the transgenic animals developed (unlike wild-type mice) spontaneous chronic wounds that were surrounded by progressing fibrosis. These lesions were characterized by numerous activated fibroblasts and macrophages and abundant expression of TGF-β in connective tissue underneath the αvβ6-integrin-expressing epithelial cells. In chronic lesions from transgenic animals, the level of TGF-β1 was significantly increased. These findings suggest that epithelial integrin αvβ6 plays a role in abnormal wound healing possibly through its ability to activate TGF-β and thereby control cell recruitment and matrix deposition.
Materials and Methods
In Situ Hybridization
A 2.6-kb cDNA containing the entire coding region for human β6-integrin was subcloned into pCDNAIneo plasmid.28 The construct was linearized with PvuII so that a 505-bp anti-sense probe corresponding to nucleotides 2139 to 2644 was obtained using sp6 polymerase whereas linearization with SspI and transcription with T7 gave a 721-bp sense probe corresponding to nucleotides 211 to 932. The specificity of the probe was determined by sequencing.28 The production and specificity of the anti-sense human stromelysin-2 probe used as positive control in the experiments has been described.29 The cDNAs were transcribed in vitro using a commercial kit (Promega Corp., Madison, WI) and labeled with 35S-UTP, as previously described.30
Samples of fibrotic and chronic nonhealing wounds and normal skin wounds were obtained from patients of the Department of Dermatology, University of Helsinki, Helsinki, Finland. The procedures were approved by the corresponding ethical committee. The tissue biopsies were fixed in 10% formalin and processed for paraffin embedding. In situ hybridization was performed as described in detail elsewhere.31 After deparaffinization and rehydration, 5-μm sections cut from paraffin blocks were pretreated with proteinase K and washed in 0.1 mol/L of triethanolamine buffer containing 0.25% acetic anhydride. The sections were covered with 25 to 70 μl of hybridization buffer containing 4 × 104 cpm/L of 35S-labeled anti-sense or sense RNA probe and incubated at 50 to 55°C in a humidified chamber for 18 hours. After hybridization, the slides were washed under stringent conditions, including treatment with RNase A to remove unhybridized probe. After 30 to 50 days of autoradiography, the photographic emulsion was developed, and the slides were stained with hematoxylin and eosin (H&E). All samples were processed in at least two experiments. The samples were examined under dark-field illumination and the bright-field view of a microscope. Samples previously positive for stromelysin-2 (chronic human wounds) were used as positive controls. No signal was detected with the sense β6-integrin probe.
Transgenic Mouse Construction
The entire (2.6 kb) coding region of human β6-integrin cDNA28 was inserted into pUC19 plasmid downstream of the cytokeratin 14 promoter to generate pUC19K14-hβ6 plasmid. The 5.0-kb K14-hβ6 expression cassette was then removed from the plasmid by digestion with XhoI and SalI. The expression cassette was injected into fertilized oocytes from FVB/N F0 mice.32 Founders harboring the transgene in their genome were crossbred back to establish lines of wild-type, homozygous, and heterozygous animals. Animals were kept in an established 12 hour:12 hour light:dark cycle in the animal facility.
Southern Blotting
We used Southern blotting to screen for the presence of the transgene in F1 or later generation animals and to determine the copy number of the transgene. Genomic DNA from mouse tail snips was isolated and digested with BamHI, which cuts twice to yield a 2.6-kb band. Digested DNA (4 μg) was electrophoresed on 1% agarose gel, blotted onto nylon, and probed with a human β6-integrin probe consisting of a 2.6-kb NotI fragment of the human β6-integrin insert. Copy numbers were determined as previously described.32
Polymerase Chain Reaction
Newborn mice were euthanized with inhalation of CO2, and their skins were collected and homogenized on liquid nitrogen. Total RNA was isolated using Trizol (Life Technologies, Inc., Gaithersburg, MD) followed by digestion with DNase I and purification with the RNeasy kit (Qiagen, Valencia, CA). A reverse transcriptase-polymerase chain reaction kit (Life Technologies, Inc.) was used to amplify 0.25 μg of total RNA to cDNA using two primers specific for human β6-integrin to yield a 245-bp band: 5′-TCTGGAGTTGGCGAAAGG-3′ (5′ primer) and 5′-TCCACCGGGTAGTCCTCA-3′ (3′ primer). Amplified DNA fragments were analyzed by electrophoresis through 0.8% agarose gel. As a positive control, total RNA isolated from human keratinocytes treated with TGF-β1 was used.12 Negative control was RNA from the skin from homozygous animals that was not reverse-transcribed.
Excisional Wound Healing in Transgenic Mice
All animal studies were conducted in compliance with Canadian Council on Animal Care guidelines and approved by the University of British Columbia Animal Care Committee. Mice were anesthetized with inhalation of halothane (MTC Pharmaceuticals, Cambridge, Ontario, Canada) and the dorsal skin shaved and then depilated (Nair Cantor Horner, Inc., Mississauga, Ontario, Canada). Four full-thickness, 4-mm bunch biopsy wounds were created on the dorsal surface of age- and sex-matched adult (more than 3 months old) wild-type and homozygous mice (β6F1, β6F2, β6F3, and β6F4). The normal skin was collected for histology and immunohistochemistry. At days 1, 3, 4, 6, 10, 12, 14, 28, 30, 44, 58, and 210 after wounding six to eight animals in each group were euthanized by CO2 inhalation, and the wounds including 5 mm of surrounding unwounded skin, as well as samples of normal tail and ear skin were collected.
At the time of wounding and at days 3, 4, 8, and 12 after wounding, standardized images of 8 to 16 wounds in wild-type and β6F1-transgenic animals were recorded using a digital camera for analysis of wound closure rate. The surface areas of the wounds were quantitated by using NIH Image software.
Wound Breaking Strength
The wound strength was measured by using a previously described method with slight modifications.33 Briefly, for each time point, a 20-mm long full-thickness incisional wound was made to the standardized area of the mid-back in 8 to 14, 4-month-old, sex-matched wild-type and hβ6F1 mice, and the wounds were closed using two surgical clips. After 4, 7, and 14 days the mice were sacrificed and the entire dorsal pelt was excised and cut into a standardized transverse strip perpendicular to the original wound. Unwounded skin collected as above served as control. Perpendicular breaking strength of the wounded or unwounded skin was then measured using an Instron 4301 tensometer with a 500-N maximum load cell (Instron Corp., Canton, MA) and analyzed using Series IX (version 8.12) software (Instron).
Histochemistry and Immunohistochemistry
TGF-β1, -2, and -3 were localized with a monoclonal antibody recognizing the active forms of TGF-β1, -β2, and -β3 (R&D Systems, Inc., Minneapolis, MN). Myofibroblasts were localized with a monoclonal antibody against α-smooth muscle actin (1A4; Sigma Chemical Co., St. Louis, MO). To localize macrophages, sections were stained with a monoclonal antibody F4/80 (Serotec Inc., Raleigh, NC). Activated fibroblasts in human paraffin sections were stained with a rat antibody to N-terminus of the type I procollagen (PC-1) molecule (mAb 1912; Chemicon, Temecula, CA). In frozen mouse sections, human β6-integrin was localized with a monoclonal rabbit antibody (B1) against human αvβ6-integrin.11 This antibody recognizes both the human and mouse β6-integrin subunit. Cytokeratin 14 was stained with a monoclonal anti-CK14 antibody (LL002, Serotec, Inc.). To localize αv integrin subunits in mouse tissues, a polyclonal anti-αv antibody was used (Ab 1930, Chemicon).
Immediately after collection, fresh tissue samples were mounted in Histoprep (Fisher Scientific, New Jersey, NJ) and snap-frozen in liquid nitrogen. Frozen sections (6 μm) were cut and fixed with −20°C acetone for 5 minutes and stored at −70°C until used for staining. A set of samples were fixed for 2 hours with 10% formalin and processed for paraffin embedding. After deparaffinization and rehydration, 5-μm sections cut from paraffin blocks were used for staining.
Immunostaining was performed using the avidin-biotin-peroxidase complex or immunofluorescence techniques.13 The sections were incubated in 0.3% hydrogen peroxide in methanol for 30 minutes and washed in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA; Sigma Chemical Co.). Sections were rinsed and incubated with normal blocking serum (Vectastain; Vector Laboratories, Burlingame, CA) for 60 minutes at room temperature and then incubated with the primary antibody in PBS/BSA in a humid chamber at 4°C for 16 hours. To stain macrophages, slides were pretreated with Proteinase K (Sigma) 20 μg/ml in 50 mmol/L of PBS (pH 7.4) for 5 minutes at room temperature before staining. After washing with PBS/BSA, sections were incubated with an appropriate biotinylated secondary antibody for 60 minutes and then reacted with ABC avidin/peroxidase reagent (Vectastain Elite kit, Vector Laboratories). Chromogen was developed using diaminobenzidine (Chemicon) or VIP (Vector Laboratories) as chromogenic substrate and hematoxylin as counterstain. Reactions were monitored until suitable color development was achieved and then washed with distilled water. The sections were mounted using Permount (Fisher Scientific, Pittsburgh, PA). After immunostaining with the anti-αv integrin antibody, sections were incubated with a fluorescent secondary antibody (Alexa 546; Molecular Probes Inc., Eugene, OR) and mounted with cyanoacrylate glue (Krazy Glue). Control sections were stained as above but the incubation with the primary antibody was omitted.
A set of paraffin sections of mouse skin samples were stained with H&E, phosphotungstic acid hematoxylin, Gomori’s methenamine silver, periodic acid-Schiff, or acid fast stains using standard methods.
Analyses of TGF-β
Viscose cellulose sponges (Cellomeda Oy, Turku, Finland) were used as an inductive matrix for connective tissue formation.34,35 Two pieces of sponge (0.5 × 0.5 cm) were implanted into the dorsal skin of each of the control animals and β6F1- to β6F4-transgenic mice (four age- and sex-matched adult animals in each group). Sponges were removed after 21 or 36 days and subjected to analysis for TGF-β. Normal skin from wild-type transgenic animals was used as a control. The amount of biologically active TGF-β in granulation tissue sponges was studied as described previously using the plasminogen activator inhibitor-I/luciferase assay.36 For this purpose, cellulose sponges were homogenized on liquid nitrogen. TGF-β was extracted by eluting the tissue homogenates (250 mg of tissue/ml) with serum-free Dulbecco’s modified Eagle’s medium containing glutamine (2 mmol/L), pyrogen-free BSA (0.2%), aprotinin (1 μg/ml), and antibiotics at 37°C for 20 hours. The tissue debris was then removed by centrifugation. For TGF-β assays, two samples from each eluate were used. One sample was analyzed as such representing the proportion of active TGF-β in tissue. The other sample representing the total amount (active plus latent) of TGF-β present in the tissue was heated at 80°C for 5 minutes to activate the latent form of TGF-β. The granulation tissue eluate samples were added in duplicate to mink lung epithelial cell culture that were stably transfected with TGF-β-inducible PAI-1 promoter fused to luciferase reporter gene37 and incubated overnight in the elution medium at 37°C. Cells were washed and lysed in Promega passive lysis buffer (Promega, Madison, WI). The lysates were diluted with PBS containing BSA (1 mg/ml) and transferred to a 96-well luminometer plate. An equal volume of Bright-Glo luciferase reagent (Promega) was added to the samples, and the luciferase activity was determined using a microtiter plate luminometer (Polarstar/Fluostar Optima; BMG Labtechnologies Inc., Durham, NC). The assay system is specific for TGF-β1, -2, and -3 and the detection limit is ∼5 to 10 pg/ml.36
To compare the levels of TGF-β1 in skin, normal skin samples were collected from six wild-type animals, from eight β6-integrin-overexpressing mice (line β6F1, n = 4; line β6F2, n = 4) and from chronic lesions from six β6-integrin-overexpressing mice (line β6F1, n = 3; line β6F2, n = 3). The samples were immediately frozen in liquid nitrogen and stored at −80°C until used. For the analyses, the samples were homogenized by grinding over liquid nitrogen. The homogenized tissue was weighted, and the soluble TGF-β was eluted by adding a fixed weight-per-volume ratio of Dulbecco’s modified Eagle’s medium containing 1 μg/ml aprotinin, 0.1% pyrogen-free BSA, and antibiotics (50 μg/ml streptomycin sulfate, 100 U/ml penicillin) and incubating at 37°C for overnight with occasional vortexing. Eluents were cleared of tissue debris by centrifugation. TGF-β1 in the clear supernatants was activated by acidification and quantitated with Quantikine TGF-β1 immunoassay using human TGF-β1 as a standard (R&D Systems).
Statistical Analysis
The wound breaking strength of wild-type and transgenic mice was compared using Student’s t-test. One way analysis of variance was used to compare wound surface areas, TGF-β levels, and prevalence of chronic lesions from different groups of animals. The significance of difference between samples from β6-transgenic mice and wild-type mice was analyzed using Bonferroni’s post test. P values <0.05 were considered significant.
Results
Expression of β6-Integrin in Human Acute and Chronic Skin Wounds and in Dermal Fibrosis
To analyze whether expression of β6-integrin associates with fibrosis or chronic wounds, we analyzed expression of β6-integrin in human samples by in situ hybridization. In keloids, hyperthrophic scars, scleroderma, and a case of dermatofibroma, no β6-integrin expression was demonstrated in the epithelium overlying the lesion (Table 1). However, in 85.7% of samples from nonhealing, chronic wounds, β6-integrin was expressed by the epithelial cells (Table 1). These lesions were histologically characterized by a moderately inflamed open ulcer surrounded with hypercellular, fibrotic connective tissue. In both areas, the epithelium was hyperplastic. Most abundant expression of β6-integrin mRNA localized to the basal keratinocytes at the edge of the open wound and overlying fibrotic, hypercellular connective tissue distant from the open wound (Figure 1). In these lesions, β6-expressing keratinocytes were localized next to fibroblasts that showed strong intracellular immunoreactivity for type I procollagen (Figure 1). In acute dermal wounds, β6-integrin was first expressed 3 days after wounding and remained up-regulated for at least 9 days (Table 1). The most abundant expression localized to the basal epithelial cells of the migrating epithelium in early wounds and to the basal and few suprabasal layers in the joined wound epithelium (data not shown).
Table 1.
Expression of β6-Integrin mRNA in Human Skin Fibrosis and Chronic and Acute Wounds
| Lesion | n | β6-negative | β6-positive | Total n | Total β6-negative | Total β6-positive |
|---|---|---|---|---|---|---|
| Fibrosis | 33 | 33 (100%) | 0 (0%) | |||
| Keloid | 17 | 17 | 0 | |||
| Hypertrophic scar | 6 | 4 | 0 (2; hair follicles) | |||
| Scleroderma | 9 | 9 | 0 | |||
| Dermatofibroma | 1 | 1 | 0 | |||
| Chronic wounds | 28 | 4 (14.3%) | 24 (85.7%) | |||
| Infected wound | 1 | 0 | 1 | |||
| Decubitus | 7 | 0 | 7 | |||
| Diabetes | 5 | 0 | 5 | |||
| Rheumatoid | 1 | 1 | 0 | |||
| Decubitus and rheumatoid | 2 | 0 | 2 | |||
| Venous leg ulcer | 7 | 1 | 6 | |||
| Well granulating wound | 5 | 2 | 3 | |||
| Acute wounds | 11 | 3 (27.3%) | 8 (72.7%) | |||
| 1-day-old | 1 | 1 | 0 (1; hair follicles) | |||
| 3-day-old | 3 | 3 | ||||
| 4-day-old | 3 | 2 | 1 | |||
| 6-day-old | 1 | 1 | ||||
| 7-day-old | 1 | 1 | ||||
| 9-day-old | 2 | 2 |
Expression of β6-integrin was studied in paraffin-embedded tissue sections by in situ hybridization.
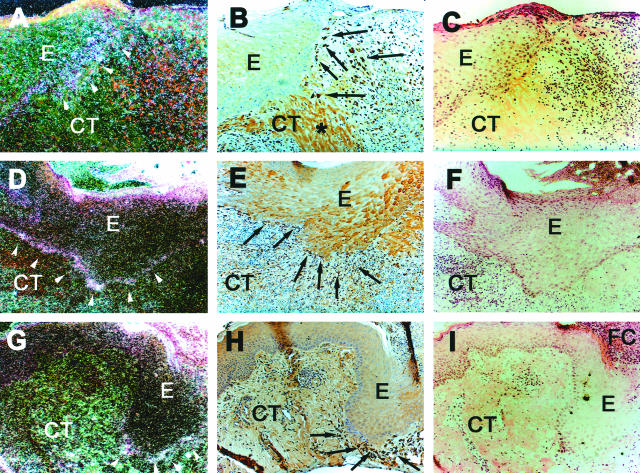
Figure 1.
Expression of β6-integrin mRNA (A, D, G) and immunolocalization of type I procollagen (B, E, H) in chronic human skin wounds. Arrowheads indicate epidermal-dermal junction and arrows indicate fibroblasts that show positive immunoreactivity for type I procollagen. Integrin β6 mRNA is most abundantly localized at the basal epithelial cells. At close proximity to these cells fibroblasts show strong immunoreactivity to type I procollagen. Occasionally, nonspecific intracellular staining in keratinocytes was noted in immunostainings with type I collagen antibody (B, E, and H). A–C: Diabetic ulcer; D–E: decubitus ulcer; G–I: chronic leg ulcer. E, Epithelium; CT, connective tissue; FC, fibrin clot. Asterisk, Collagen fibers. A, D, G: Dark-field image; C, F, I: H&E staining.
Characterization of the Transgenic Mice
To study whether increased expression of β6-integrin leads to abnormal wound healing we developed transgenic mouse lines that constitutively express human β6-integrin (hβ6) in the basal keratinocytes. To this end, fertilized mouse oocytes were microinjected with the K14-hβ6 construct. Screening of progeny by Southern blotting revealed that six of them carried the hβ6 gene. The six founder mice were mated with normal mice to determine stability of the transgene. Four founder mice produced progeny that were heterozygous for the hβ6-integrin. The transgene was inherited autosomally. Mating of heterozygous animals produced homozygous animals that were further bred to produce four mouse lines (β6F1 to β6F4) that were homozygous for hβ6-integrin. The K14-hβ6 transgene in each mouse line was screened by Southern blotting to confirm the homozygous inheritance and determine the gene copy numbers (Figure 2A). Expression of hβ6-integrin in newborn mouse skin was also confirmed by reverse transcriptase-polymerase chain reaction (Figure 2B). The expression of hβ6 was further studied using immunostaining and in situ hybridization (Figure 3). Expression of hβ6 gene and positive immunoreactivity for hβ6-integrin was found in the back skin of all β6F1 to β6F4 animals. The relative expression level was highest in the β6F1 line in which the copy number of the hβ6 gene was also the highest (Figure 2). The expression of cytokeratin 14 and hβ6-integrin co-localized in the epidermis and at the outer root sheath cells of hair follicles (Figure 3). In the epidermis, hβ6 immunostaining localized to the cell membranes of basal and one to two suprabasal cell layers. Immunostaining of αv integrin co-localized with hβ6-integrin at the basal epithelial cells (Figure 3). In addition to various locations of skin, basal cells of tongue epithelium were positive for hβ6 expression (data not shown). Control animals did not express β6-integrin in the epidermis but some expression in the cells of the outer root sheath of hair follicles was noted (Figure 3). The expression of the transgene did not have any apparent effect on the development, weight, behavior, or fertility of the animals. Histologically, the skin in newborn and adult transgenic mice did not show any alterations as compared with wild-type animals (Figure 3).
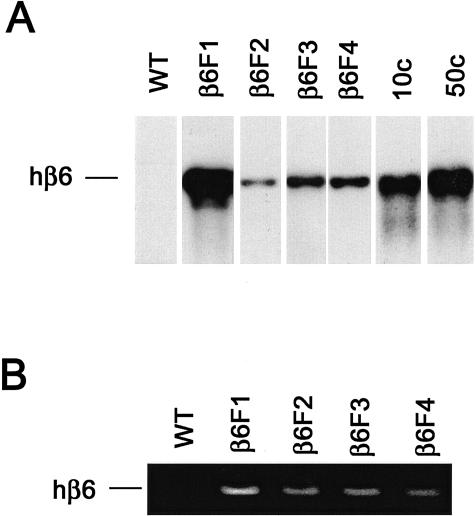
Figure 2.
Southern blotting of mouse genomic DNA for human β6-integrin gene (A) and reverse transcriptase-polymerase chain reaction detection of human β6 mRNA from newborn mouse skin (B). WT, Wild-type mice; β6F1 to β6F4, four homozygous hβ6-integrin-expressing mouse lines. In A, 10c and 50c indicate β6-integrin cDNA standards for 10 and 50 gene copies, respectively.
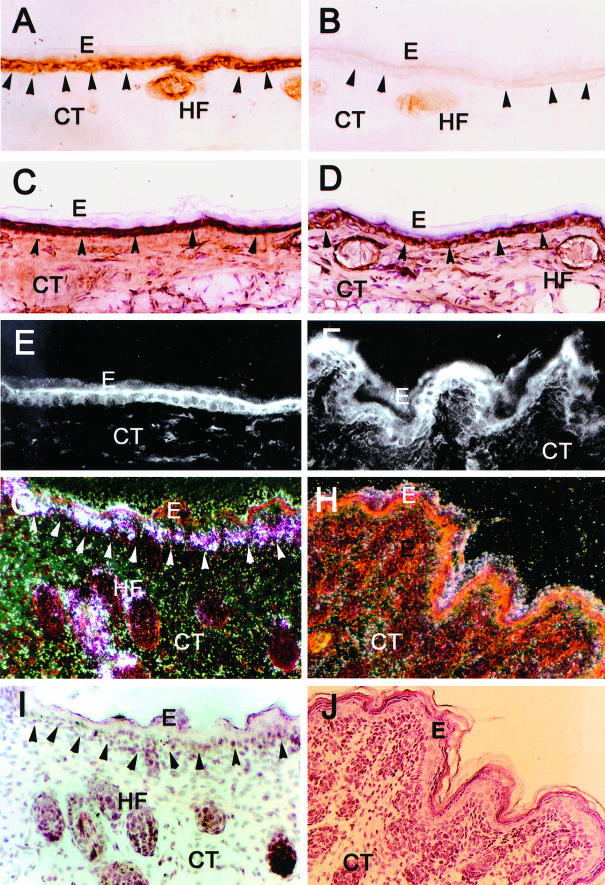
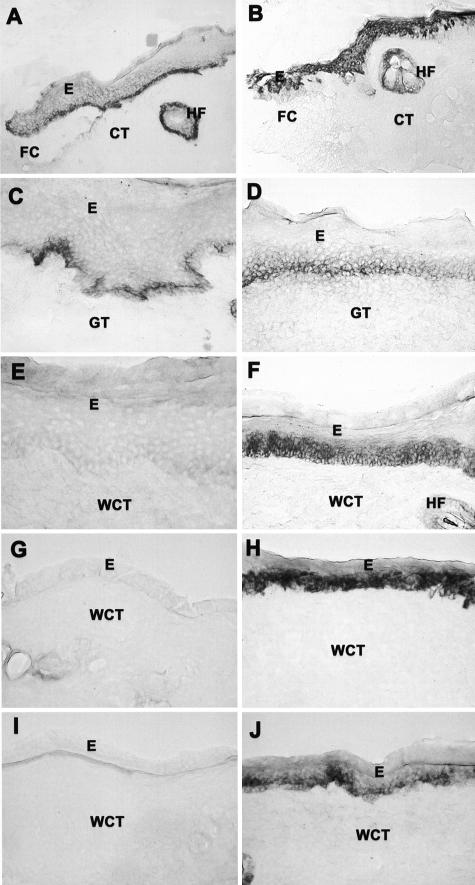
Figure 3.
Localization of human β6-integrin mRNA (G and H) and immunolocalization of β6-integrin (A and B), cytokeratin 14 (C and D), and αv integrin subunit (E and F) in human β6-integrin-transgenic (hβ6F1) (A, C, E, G, I) and wild-type (B, D, F, H, J) mouse skin. I and J: H&E staining of the corresponding samples as in G and H. Arrowheads indicate epidermal-dermal junction. Strong expression of hβ6-integrin mRNA and immunoreactivity for β6-integrin localizes at the basal epithelial cells and hair follicles of transgenic mouse skin. Integrin β6 expression and protein co-localizes with endogenous αv integrin subunit and cytokeratin 14 at the basal epithelial cells. Wild-type animals do not express β6-integrin except for weak immunoreactivity at the outer root sheath cells of hair follicles. E, Epithelium; CT, connective tissue; HF, hair follicle.
Experimental Wound Healing in Transgenic Mice
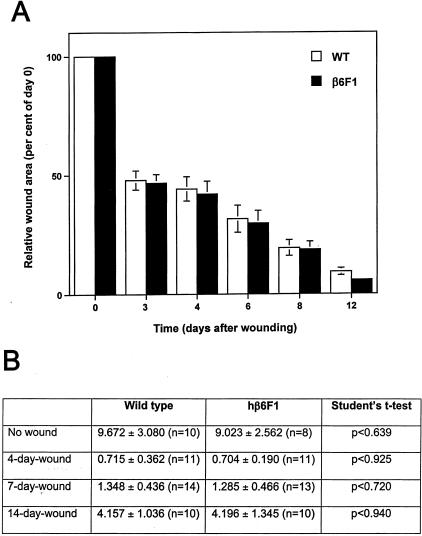
To study whether constitutive expression of β6-integrin in skin affects wound healing, we created excisional wounds to the back skin of the transgenic (β6F1 to β6F4) and normal animals. Wounds in all animals were completely closed at day 12 after wounding and no difference was found in the relative wound areas at different time points (Figure 4A) or in the wound breaking strength between wild-type and human β6-integrin-transgenic mice (Figure 4B). In histological examination, no differences in the re-epithelialization speed, inflammatory reaction, or reorganization of connective tissue was observed (Figure 5). Both wild-type and transgenic animals developed collagen-rich wound connective tissue that was histologically evident by 30 days after wounding (Figure 5). Macroscopically, the skin in both transgenic and wild-type animals healed without hypertrophic scar formation. The results were consistent among different human β6-integrin-transgenic mouse lines.
Figure 4.
Cutaneous wound healing in wild-type and human β6-integrin-transgenic mouse skin. A: Quantitation of wound surface areas at different time points after wounding in wild-type (WT) and in a transgenic (β6F1) mouse line. B: Breaking strength (load in Newtons at breaking point) of incisional wounds and nonwounded skin in wild-type and human β6-integrin-transgenic mice (hβ6F1) at different time points after wounding. There were no differences in wound closure rate (wound surface area at different time points) or in the wound breaking strength between wild-type and human β6-integrin-transgenic mice.
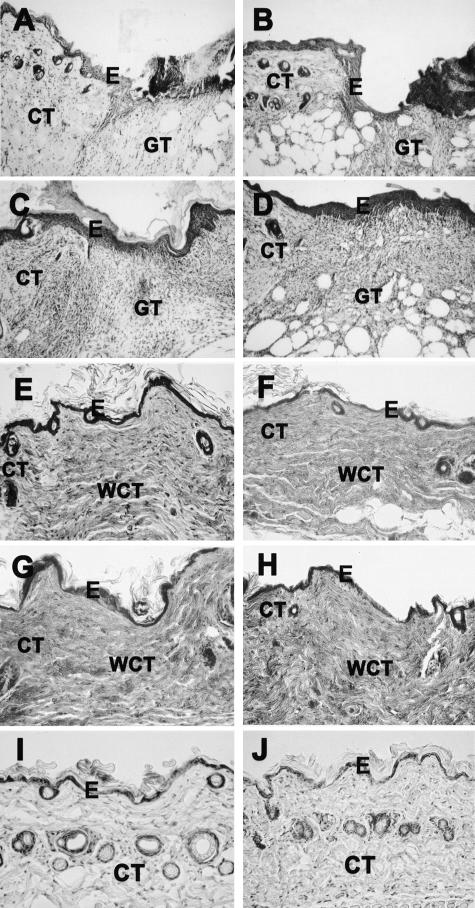
Figure 5.
Histological characterization of cutaneous wound healing in wild-type (A, C, E, G) and human β6-integrin-transgenic mouse (β6F1) skin (B, D, F, H) at 3 (A and B), 7 (C and D), 30 (E and F), and 44 (G and H) days after wounding. I and J: Histological sections of nonwounded skin in wild-type and in the β6-integrin-transgenic mouse, respectively. Histologically, both wild-type and transgenic animals showed similar wound healing response with complete re-epithelialization at day 7 and formation of collagen-rich wound connective tissue by day 30 after wounding. A–D and I and J: H&E staining; E–H: phosphotungstic acid hematoxylin staining. E, Epithelium; CT, connective tissue; GT, granulation tissue; WCT, wound connective tissue.
To study expression of β6-integrin during wound healing, we used a rabbit monoclonal antibody that recognizes both human and mouse β6-integrin subunits. In the wild-type mice, 3 days after wounding the migrating wound epithelium expressed β6-integrin most abundantly at the basal epithelial cells (Figure 6A). There was also a weak positive immunoreaction at the cell membranes of suprabasal cells (Figure 6A). Seven days after wounding when the migratory epithelial fronts had just joined to cover the wound, basal epithelial cells still expressed β6-integrin (Figure 6C). At day 14, the expression of β6-integrin was strongly down-regulated and no expression was seen at the 28- or 44-day-old wound epithelium of the wild-type mice (Figure 6; E, G, and I). In contrast, expression of β6-integrin was strongly increased at all layers of the early migrating epithelium in the transgenic animals 3 days after wounding (Figure 6B). Unlike in the control animals, basal and some suprabasal epithelial cells expressed β6-integrin still strongly from day 14 until day 28 after wounding (Figure 6, F and H). After 44 days the wound epithelium was morphologically identical with unwounded epithelium and expressed abundantly β6-integrin similar to unwounded epithelium of the transgenic animals (Figures 6J and 3A, respectively).
Figure 6.
Immunohistochemical localization of β6-integrin in cutaneous wound healing in wild-type (A, C, E, G, I) and human β6-integrin-transgenic mouse (hβ6F1) skin (B, D, F, H, J). Results show β6-integrin expression at 3 (A and B), 7 (C and D), 14 (E and F), 28 (G and H), and 44 (I and J) days after wounding. E, Epithelium; FC, fibrin clot; CT, connective tissue; GT, granulation tissue; WCT, wound connective tissue.
To quantitate the amount of TGF-β1, -2, and -3 in mouse skin wounds, we placed viscous cellulose sponges subcutaneously into back skin of β6F1- to β6F4-transgenic mice and wild-type animals to induce granulation tissue formation. After 21 and 36 days, the sponges were removed and TGF-β was measured in the sponge extracts using a luciferase assay. No differences between wild-type and transgenic animals were found in the levels of active or latent TGF-β (data not shown).
Development of Chronic Lesions in Transgenic Mice
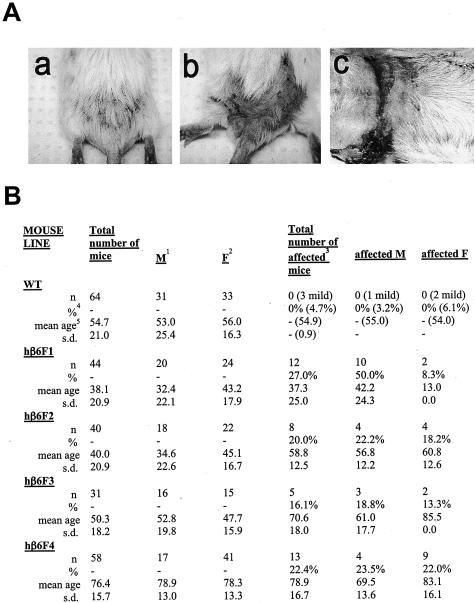
During breeding of the colony of transgenic β6 mice, they started to develop spontaneous chronic lesions (Figure 7). The lesion usually started to develop at ∼10 to 20 weeks of age and was initially characterized macroscopically by hair loss in the lower back area (Figure 7A). Throughout time (usually within 22 to 72 weeks) this affected area of skin slowly developed into a fibrotic lesion that also had areas with open, superficial chronic ulcerations. The lesion was progressive and no regression was observed. The mice did not usually develop multiple lesions but the single lesions could become relatively large in size (Figure 7A). The fibrotic area of skin was very thick and progressed often to the abdominal area where it caused constriction (Figure 7A). The prevalence of the chronic lesions was followed for up to 2 years. The wild-type mice did not develop chronic lesions (Figure 7B). Three wild-type mice showed very mild cases of chronic lesions involving some hair loss and some thickening of the skin, but these lesions did not progress to form constricting fibrosis. In the transgenic animals, 16.1% (β6F3) to 27.0% (β6F1) of the animals developed chronic lesions. The prevalence was the highest in males of β6F1 mice (50.0%, Figure 7B). The difference in the prevalence of chronic lesions was significantly higher (P < 0.0001, Student’s t-test) in human β6-integrin-transgenic mice (38 of 173 hβ6F1, hβ6F2, hβ6F3, and hβ6F4 mice had lesions) as compared with wild-type mice (none of 64 mice had lesions). When the human β6-integrin-transgenic mouse lines were compared individually against the wild-type mice, hβ6F1 (P < 0.01), hβ6F2 (P < 0.05), and hβ6F4 (P < 0.01) mouse lines showed significantly increased prevalence of chronic lesions (analysis of variance). The difference in the prevalence of the lesions between hβ6F3 mouse line and wild-type mice was not statistically significant (P > 0.05, analysis of variance).
Figure 7.
A: Representative clinical appearance of a chronic progressing wound in a human β6-integrin-transgenic (hβ6F1) mouse. a: Early lesion; b: progressed lesion; c: advanced lesion showing progression to the abdominal area. B: Prevalence of skin lesions in the wild-type and human β6-integrin-transgenic mice. 1Males; 2females; 3affected: Mice with progressing scarring and/or chronic wounds in the lower back; 4percent of affected mice of total number of mice or percent of affected males or females of total number of males or females, respectively; 5mean age of the mice in weeks. WT, wild-type mice; hβ6F1, hβ6F2, hβ6F3, hβ6F4 transgenic mouse lines.
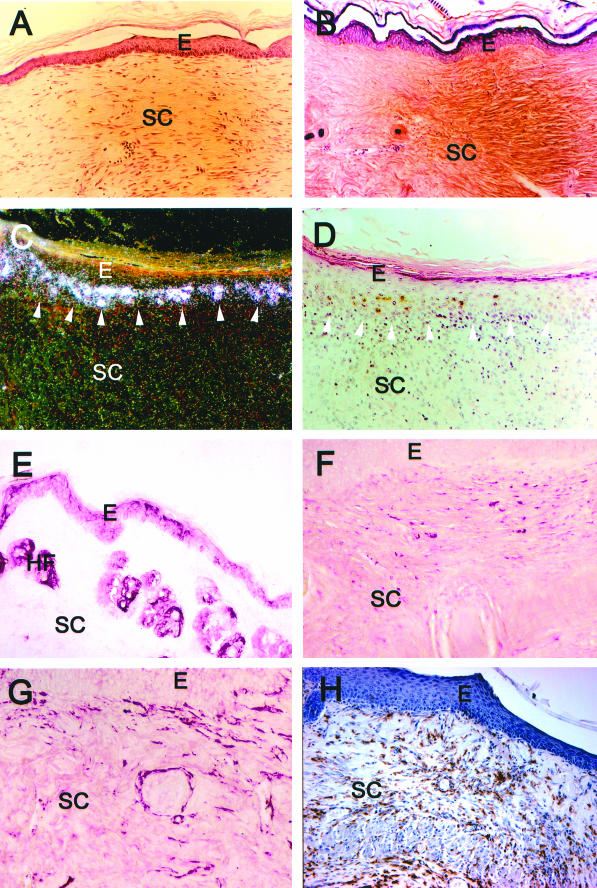
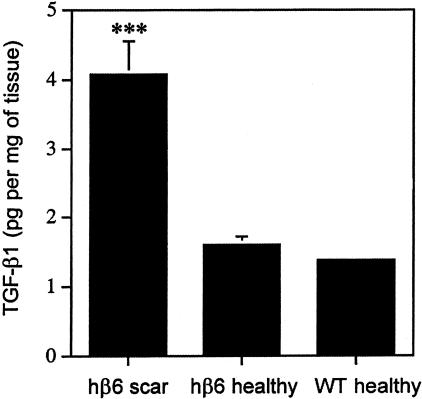
Histologically, almost all of the lesions from the β6F1- to β6F4-transgenic mice showed either a superficial ulceration with focal polymorphonuclear neutrophil infiltrate (Figure 8) or focal hyperkeratosis and granulation tissue indicative of recent and resolving ulceration. Stainings of representative lesions with acid fast did not reveal acid fast bacilli in the lesions (data not shown). Likewise, Gomori’s methenamine silver and periodic acid-Schiff stains were negative for microorganisms and fungi in the dermis (data not shown). The ulcerations were surrounded by hyperplastic epithelium and strongly thickened connective tissue (Figures 8 and 9). The epithelium covering these fibrotic areas and at the edge of open wounds expressed αvβ6-integrin in the basal and suprabasal epithelial cells (Figure 9). Strong localization of β6-integrin mRNA was also demonstrated at the basal epithelial cells. In connective tissue surrounding the ulcerations, all lesions showed a vast expansion of the papillary and reticular dermis with a mixed infiltrate of activated fibroblasts and abundant macrophages (Figure 9). Macrophage infiltration was evident in some cases as deep as the subcutaneous fat. The macrophages in the dermis were diffusely scattered between fibroblasts and were not immediately obvious by H&E staining. The connective tissue next to the ulcerations showed abundant collagen accumulation (Figures 8 and 9) and contained fibroblasts that showed positive immunoreactivity for α-smooth muscle actin (Figure 9). Immunostaining with an antibody against TGF-β1, -β2, and -β3 isoforms localized in the cytoplasms of connective tissue cells in the fibrotic areas with heavy collagen accumulation. We then analyzed levels of total TGF-β1 in the healthy skin in wild-type and β6F1- and β6F2-transgenic animals as compared with the chronic scars from the same β6-integrin-overexpressing mouse lines by using the TGF-β1 immunoassay. The chronic lesions from β6-integrin-overexpressing mice contained significantly higher levels of TGF-β1 than healthy skin from transgenic and wild-type animals (Figure 10). No difference in the level of TGF-β1 was found between healthy skin from wild-type and transgenic animals (Figure 10).
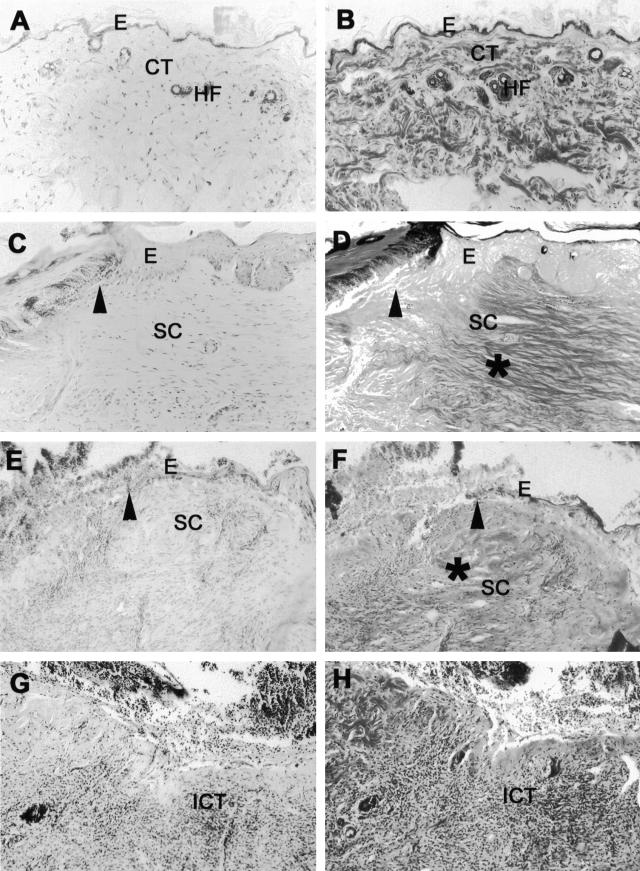
Figure 8.
Histological characterization of superficial ulcer areas in representative chronic, progressing lesions from human β6-integrin-transgenic mice. A, C, E, and G: H&E staining; B, D, F, H: Phosphotungstic acid hematoxylin staining. A and B: Normal healthy skin from human β6-integrin-transgenic mouse (hβ6F1). C–F: Edge of an open ulceration in a chronic, progressing wound from hβ6F1 (C and D) and hβ6F2 (E and F) human β6-integrin-transgenic mice. The connective tissue next to the ulcer is strongly thickened and shows abundant collagen accumulation (asterisk in D and F). The epithelium next to the ulceration can be thickened (C and D) and/or show hyperkeratosis (E and F). G and H: Mid-ulcer area from the chronic, progressing wound from the hβ6F2 mouse. The connective tissue beneath the superficial ulceration is heavily infiltrated with inflammatory cells. Arrowhead, Open ulcer edge; E, epithelium; CT, connective tissue; HF, hair follicle; SC, scar tissue; ICT, inflamed connective tissue.
Figure 9.
Immunohistochemical characterization of representative areas next to the superficial ulcers in chronic, progressing wound samples from a human β6-integrin-transgenic mouse (hβ6F1). A: H&E staining; B: Phosphotungstic acid hematoxylin staining; C: in situ hybridization of human β6-integrin (dark-field image); D: H&E illustration of the same sample as in C; E: immunolocalization of β6-integrin; F: immunolocalization of TGF-β1, -2, and -3; G: immunolocalization of α-smooth muscle actin; H: immunostaining of macrophages. E, Epithelium; SC, scar tissue; HF, hair follicle.
Figure 10.
Level (pg/mg of tissue) of total TGF-β1 (active + latent) in chronic scars from β6-integrin-overexpressing mice (hβ6 scar; β6F1, n = 3 and β6F2, n = 3) and in healthy skin from β6-integrin-overexpressing (hβ6 healthy; β6F1, n = 3 and β6F2, n = 3) and from wild-type mice (WT healthy, n = 8) was analyzed with the TGF-β1 immunoassay. The amount of TGF-β1 in chronic scars from transgenic animals was significantly higher as compared with normal skin from transgenic and control animals (P < 0.001, analysis of variance).
Discussion
The present study showed that induced expression of αvβ6-integrin associated with chronic human skin wounds but not with keloids, hypertrophic scars, scleroderma, or a single case of dermatofibroma. This suggested to us that expression of αvβ6-integrin was either induced secondarily because of chronic state of the wound or αvβ6-integrin could be actually a contributing factor for the development of these lesions. The chronic wounds were characterized by open ulcers that were surrounded by hypercellular, fibrotic connective tissue. In these lesions, expression of β6-integrin was localized at the basal epithelial cells. Underneath the β6-integrin-expressing keratinocytes, strong immunoreactivity for type I procollagen localized at the cytoplasms of fibroblasts suggesting that the cells were actively secreting collagen. Integrin αvβ6 can bind latent TGF-β to the cell membrane and induce its activation.19 TGF-β can potently stimulate recruitment and growth of connective tissue cells and induce collagen deposition by fibroblasts.2 Therefore, it is possible that αvβ6-integrin-expressing keratinocytes actively modulate connective tissue cell functions in chronic wounds through activation of TGF-β and therefore actively contribute to the development of the lesions.
To test whether αvβ6-integrin plays an active role in abnormal wound healing, we developed transgenic mouse lines that constitutively expressed human β6-integrin under the control of cytokeratin 14 promoter. The localization of human β6-integrin expression in the basal and some suprabasal epidermal cell layers and in outer root sheath cells of the hair follicles was consistent with previous reports of cytokeratin 14 promoter expression.38 The hβ6 integrin was localized at the cell membranes of keratinocytes and co-localized with endogenous αv integrin. The formation of a heterodimer from αv- and β6-integrin subunits is the prerequisite for the assembly of a functional, integral cell membrane αvβ6-integrin receptor.39 The transgenic animals did not show any developmental abnormalities and histologically the skin in newborn and adult animals was unaltered as compared with wild-type mice. Additionally, the speed of normal wound healing was unchanged as compared with wild-type mice. In human cutaneous and mucosal wounds, αvβ6-integrin is up-regulated during early keratinocyte migration and remains up-regulated at least until day 14 when re-epithelialization is complete.8,13–15 As in human wounds, β6-integrin expression was induced by basal epithelial cells of the migrating epithelium at day 3 but strongly down-regulated after 14 days of healing. Transgenic mice showed, however, increased expression of β6-integrin in all cell layers of migrating epithelium at day 3 and the expression remained high throughout the healing period. Thus, although the endogenous β6-integrin was down-regulated in wound epithelium within 14 days, the transgene expression remained high at the wound epithelium during later phases of wound healing. It seems obvious, therefore, that the different expression levels of αvβ6-integrin do not modulate normal wound healing because β6 −/−16 or β6-overexpressing (our study) animals do not show abnormal wound healing response. It is possible that αvβ6-integrin plays a role only when wound healing is compromised by infection, immunosuppression, or other conditions as suggested by our data from chronic human wounds.
Interestingly, when the mouse colonies were bred, 16.1 to 27.0% of the transgenic mice developed spontaneous chronic wounds. Throughout the time these lesions developed into progressing, fibrotic scars with areas of nonhealing open ulcerations. In an advanced stage, the fibrosis caused constriction of the scar tissue that often progressed to the abdominal region at the time when the experiment was terminated. We noted that when transgenic or control mice were caged together, they occasionally injured each other to the skin in the lower back during territorial fighting. Therefore, wounding as a result of territorial fighting may have contributed to the development of the chronic wounds in the β6-integrin-overexpressing mice. All of the chronic lesions appeared to be associated with ulcerative trauma and, therefore, it is possible that superficial infection contributed to the lesions. However, no microorganisms or fungi were present in the histological sections from the lesions.
In mice, bleomycin treatment leads to lung fibrosis because of increased activation of TGF-β.19 The αvβ6-integrin plays an active role in this process because in mice lacking β6-integrin, bleomycin failed to induce lung fibrosis.19 In culture, the β6-integrin −/− cells could not activate latent TGF-β1,19 and the β6-integrin-deficient mice did not show the increased expression of TGF-β1-regulated genes as was detected in the wild-type animals.40 Therefore, it is possible that constitutive expression of αvβ6-integrin by keratinocytes provides a mechanism that promotes TGF-β1 activation also in the skin that in turn could lead to altered wound healing response. Increased expression of TGF-β1 has been shown to associate with reduced rate of wound re-epithelialization and increased collagen deposition.40 In our study, closure speed of experimental wounds was unaltered in the transgenic animals, and the wounds did not heal by excessive scar formation. Additionally, the levels of active and latent TGF-β1, -2, and -3 in subcutaneously implanted cellulose sponges from 21 and 36 days after wounding were similar in the β6-integrin-overexpressing and wild-type mice. Therefore, activation of TGF-β by αvβ6-integrin may not play as important role in normal skin wound healing as in bleomycin-induced lung fibrosis. However, in a compromised healing situation, as in the case of development of chronic lesions in this study, activation of TGF-β by αvβ6-integrin may play a role. The chronic lesions in this study were composed abundantly of activated macrophages and fibroblasts. Many of the cells underneath the αvβ6-expressing epithelium showed positive intracellular staining with an antibody recognizing TGF-β1, -2, and -3. Additionally, the level of TGF-β1 in the lesions of transgenic animals was significantly higher than in healthy skin. There were also many myofibroblasts present in a collagen-rich extracellular matrix. It is possible that in chronic lesions, keratinocytes constitutively expressing αvβ6-integrin could activate TGF-β leading to induction of fibroblast differentiation into myofibroblasts. These activated fibroblasts could then produce more TGF-β that would stimulate collagen deposition. Additionally, one possibility is that in the chronic lesions, increased TGF-β at the dermoepidermal junction causes a sufficient increase in MCP1 secretion by dermal fibroblasts41 leading to situation in which induced macrophage influx fails to reach a normal equilibrium. However, we cannot rule out the possibility that increased TGF-β in the lesions is a result of chronic inflammation and wound environment and not directly linked to increased activation of TGF-β by αvβ6-integrin-expressing keratinocytes.
Because the transgenic mice, unlike wild-type mice, developed chronic progressing scars and ulcerations, we suggest that it is possible that the expression of αvβ6-integrin by the epithelium contributes to the development of the chronic lesions. The idea that epithelial cells could play a significant role in the development of lesions in the skin has been proposed previously. For example, suprabasal integrin expression resulted in development of psoriasis-like changes in transgenic mice,4 and excess production of latent TGF-β1 by keratinocytes enhances collagen production by connective tissue cells.42 Based on the previous findings with β6-integrin knockout animals16 and on our study, we hypothesize that epidermal αvβ6-integrin is not critical for normal wound repair but it may play a role when wound healing is compromised for example in conditions involving chronic inflammation or immunosuppression.
Acknowledgments
We thank Tuula Oinonen and Cristian Sperantia for expert assistance.
Footnotes
Address reprint requests to Dr. Lari Häkkinen, Faculty of Dentistry, Department of Oral Biological and Medical Sciences, Laboratory of Periodontal Biology, University of British Columbia, 2199 Wesbrook Mall, Vancouver, BC, Canada V6T 1Z3. E-mail: lhakkine@interchange.ubc.ca.
Supported by the Canadian Institutes for Health Research (grant MOP-12589).
The present address of M. L. is the A. I. Virtanen Institute, University of Kuopio, Kuopio, Finland; the present address for U.S-K. is the Department of Dermatology, Karolinska Institute, Soder Hospital, Stockholm, Sweden; and the present address for H.R. is Department of Biosciences and Institute of Biotechnology, Neuroscience Center, University of Helsinki, Finland.
References
- Garner WL. Epidermal regulation of dermal fibroblast activity. Plast Reconstr Surg. 1998;102:135–139. doi: 10.1097/00006534-199807000-00021. [DOI] [PubMed] [Google Scholar]
- Bauer BS, Tredget EE, Marcoux Y, Scott PG, Ghahary A. Latent and active transforming growth factor beta1 released from genetically modified keratinocytes modulates extracellular matrix expression by dermal fibroblasts in a coculture system. J Invest Dermatol. 2002;119:456–463. doi: 10.1046/j.1523-1747.2002.01837.x. [DOI] [PubMed] [Google Scholar]
- Nowinski D, Hoijer P, Engstrand T, Rubin K, Gerdin B, Ivarsson M. Keratinocytes inhibit expression of connective tissue growth factor in fibroblasts in vitro by an interleukin-1alpha-dependent mechanism. J Invest Dermatol. 2002;119:449–455. doi: 10.1046/j.1523-1747.2002.01841.x. [DOI] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001;108:527–536. doi: 10.1172/JCI12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L, Uitto V-J, Larjava H. Cell biology of gingival wound healing. Periodontology. 2000;24:127–152. [PubMed] [Google Scholar]
- Larjava H, Koivisto L, Häkkinen L. Keratinocyte interactions with fibronectin during wound healing. Heino J, Kähäri V-M, editors. Georgetown: Landes Biosciences, Eurecah. com; Cell Invasion. 2002:pp 42–64. [Google Scholar]
- Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. [PubMed] [Google Scholar]
- Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins alpha9beta1, alphavbeta3, and alphavbeta6 on cell proliferative responses to tenascin. Roles of the beta subunit extracellular and cytoplasmic domains. J Biol Chem. 1996;71:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol. 1998;19:636–642. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- Koivisto L, Larjava K, Hakkinen L, Uitto VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun. 1999;7:245–257. doi: 10.3109/15419069909010806. [DOI] [PubMed] [Google Scholar]
- Häkkinen L, Hildenbrand HC, Berndt A, Kosmehl H, Larjava H. Immunolocalization of tenascin-C, α9 integrin subunit and αvβ6 integrin during wound healing in human oral mucosa. J Histochem Cytochem. 2000;48:985–998. doi: 10.1177/002215540004800712. [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Abelda SM, Kramer RH, Pytela R. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Clark RA, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MW. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol. 1996;135:46–51. [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark RA, Uitto VJ, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, Miller HRP. Enteric expression of the integrin vβ6 is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol. 2002;161:771–779. doi: 10.1016/s0002-9440(10)64236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol. 1995;129:853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Westermarck J, Leppa S, Hakkinen L, Koivisto L, Lopez-Otin C, Peltonen J, Heino J, Kahari VM. Collagenase 3 (matrix metalloproteinase 13) gene expression by HaCaT keratinocytes is enhanced by tumor necrosis factor alpha and transforming growth factor beta. Cell Growth Differ. 1997;8:243–250. [PubMed] [Google Scholar]
- Kulkarni AB, Thyagarajan T, Letterio JJ. Function of cytokines within the TGF-beta superfamily as determined from transgenic and gene knockout studies in mice. Curr Mol Med. 2002;2:303–327. doi: 10.2174/1566524024605699. [DOI] [PubMed] [Google Scholar]
- Pierce GF. Inflammation in nonhealing diabetic wounds: the space-time continuum does matter. Am J Pathol. 2001;159:399–403. doi: 10.1016/S0002-9440(10)61709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29:5–17. doi: 10.1016/s1357-2725(96)00115-x. [DOI] [PubMed] [Google Scholar]
- Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92:2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989;135:1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, Albers KM, Garlick JA, Harrington R, Taichman LB. Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc Natl Acad Sci USA. 1993;90:10270–10274. doi: 10.1073/pnas.90.21.10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DW, Beljan JR. Tensile strength testing methodology for partially healed rat skin wounds: engineering considerations. J Assoc Adv Med Instrum. 1971;5:191–197. [PubMed] [Google Scholar]
- Niinikoski J, Rajamaki A, Kulonen E. Healing of open wounds: effects of oxygen, disturbed blood supply and hyperemia by infrared irradiation. Acta Chir Scand. 1971;137:399–401. [PubMed] [Google Scholar]
- Jalkanen M, Haapanen T, Lyytikainen AM, Larjava H. Wound fluids mediate granulation tissue growth phases. Cell Biol Int Rep. 1983;7:745–753. doi: 10.1016/0309-1651(83)90202-3. [DOI] [PubMed] [Google Scholar]
- Yang L, Qiu CX, Ludlow A, Ferguson MW, Brunner G. Active transforming growth factor-beta in wound repair: determination using a new assay. Am J Pathol. 1999;154:105–111. doi: 10.1016/s0002-9440(10)65256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci USA. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen P, Heino J. The selective regulation of alpha Vbeta 1 integrin expression is based on the hierarchical formation of alpha V-containing heterodimers. J Biol Chem. 2002;277:24835–24841. doi: 10.1074/jbc.M203149200. [DOI] [PubMed] [Google Scholar]
- Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000;97:1778–1783. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J, Unemori E, Hunt TK, Amento E. Monocyte chemotactic protein-1 (MCP-1) mRNA is down-regulated in human dermal fibroblasts by dexamethasone: differential regulation by TGF-beta. Growth Factors. 1995;12:151–157. doi: 10.3109/08977199509028961. [DOI] [PubMed] [Google Scholar]
- Chan T, Ghahary A, Demare J, Yang L, Iwashina T, Scott P, Tredget EE. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-beta1 transgenic mouse. Wound Repair Regen. 2002;10:177–187. doi: 10.1046/j.1524-475x.2002.11101.x. [DOI] [PubMed] [Google Scholar]