Abstract
The p38 mitogen-activated protein (MAP) kinase signal transduction pathway regulates the production of interleukin-1 and tumor necrosis factor-α. p38 kinase inhibitors are effective in animal models of arthritis and are currently being developed in rheumatoid arthritis (RA). However, little is known about the upstream kinases that control the activation of p38 in RA synovium. In vitro studies previously identified the MAP kinase kinases (MAPKKs) MKK3 and MKK6 as the primary regulators of p38 phosphorylation and activation. To investigate a potential role for MKK3 and MKK6 in RA, we evaluated their expression and regulation in RA synovium and cultured fibroblast-like synoviocytes (FLS). Immunohistochemistry demonstrated that MKK3 and MKK6 are expressed in RA and osteoarthritis (OA) synovium. Digital image analysis showed no significant differences between OA and RA with regard to expression or distribution. However, phosphorylated MKK3/6 expression was significantly higher in RA synovium and was localized to the sublining mononuclear cells and the intimal lining. Actin-normalized Western blot analysis of synovial tissue lysates confirmed the increased expression of phosphorylated MKK3/6 in RA. Western blot analysis demonstrated constitutive expression of MKK3 and MKK6 in RA and OA FLS. Phospho-MKK3 levels were low in medium-treated FLS, but were rapidly increased by interleukin-1 and tumor necrosis factor-α, although phospho-MKK6 levels only modestly increased. p38 co-immunoprecipitated with MKK3 and MKK6 from cytokine-stimulated FLS and the complex phosphorylated activating transcription factor-2 in an in vitro kinase assay. These data are the first documentation of MKK3 and MKK6 activation in human inflammatory disease. By forming a complex with p38 in synovial tissue and FLS, these kinases can potentially be targeted to regulate the production of proinflammatory cytokine production in inflamed synovium.
Mitogen-activated protein (MAP) kinases are a family of serine/threonine kinases that mediate signal transduction and orchestrate an appropriate cellular response to environmental stress. In mammalian cells, three principle MAP kinase pathways have been identified, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38.1 Multiple MAP kinase pathways can be simultaneously activated and the relative balance is determined by the parallel upstream kinase cascades known as MAP kinase kinases (MAPKKs) and MAP kinase kinase kinases (MAP3Ks).2
The p38 MAP kinase is of particular interest in inflammatory diseases such as rheumatoid arthritis (RA) because it regulates the production of pathogenic cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α.3,4 p38 is expressed and activated in RA synovium5 and blockade using selective inhibitors decreases inflammation and bone destruction animal models of arthritis.6 However, little is known about the upstream kinases that can activate this pathway in joint tissues. Of the MAPKKs, MKK3 and MKK6 are thought to be especially important regulators of p38 and represent potential therapeutic targets to modulate cytokine production.7 MKK6 and MKK3 have significant homology at the amino acid level, with 82% amino acid identity.8,9 However, there is significantly less nucleotide sequence homology at the DNA level, especially at the C- and N-terminal regions. MKK6 and MKK3 also differ in tissue and cell expression.10,11 Further diversity is provided by numerous tissue-specific splice variants for MKK6.12,13
Both MKK3 and MKK6 are activated upon phosphorylation of serine and threonine residues within subdomain VIII by upstream MAPKK kinases (MAP3Ks).14 MKK3 selectively phosphorylates p38α, γ, and δ whereas MKK6 activates all four p38 isoforms (α, β, γ, and δ).15 This suggests that substrate selectivity might contribute to the distinct functional profiles of MKK activation. Additional specificity results from selective activation of different MKKs. For instance, MKK6 is the major activator of p38 in cells exposed to osmotic stress16 and MKK3 is required for full activation of p38 MAPK in murine embryonic fibroblasts.17
To study the relative contribution of MKK3 and MKK6 in RA, we investigated their expression and function in RA synovial tissue and cultured fibroblast-like synoviocytes (FLS). The data indicate that both MKK3 and MKK6 are activated in RA synovium. However, MKK3 phosphorylation is greater than MKK6 activation in cultured FLS stimulated by IL-1 or TNF-α. Both can form stable signaling complexes with p38 that can phosphorylate downstream substrates. This is the first demonstration of MKK3 and MKK6 activation in human inflammatory disease and suggests that MKK3 or MKK6 are potential therapeutic targets for RA.
Materials and Methods
Cells and Synovial Tissue
FLS were isolated from RA and osteoarthritis (OA) synovial tissues obtained at joint replacement as previously described.18 The diagnosis of RA conformed to the 1987 revised American College of Rheumatology (ACR) criteria.19 Briefly, the tissues were minced and incubated with 1 mg/ml of collagenase in serum-free Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Grand Island, NY) for 2 hours at 37°C, filtered through a nylon mesh, extensively washed, and cultured in DMEM supplemented with 10% fetal calf serum (FCS) (endotoxin content <0.006 ng/ml; Life Technologies), penicillin, streptomycin, gentamicin, and l-glutamine in a humidified 5% CO2 atmosphere. After overnight culture, nonadherent cells were removed and adherent cells were cultivated in DMEM plus 10% FCS. At confluence, cells were trypsinized, split at a 1:3 ratio, and recultured in medium. Synoviocytes were used from passages three through eight in which they comprised a homogeneous population of FLSs (<1% CD11b, <1% phagocytic, and <1% FcgRII receptor-positive).18 The synovial tissue was snap-frozen and processed for Western blot and immunohistochemistry.
Antibodies and Reagents
Affinity-purified rabbit polyclonal MKK3 antibodies, goat polyclonal MKK6 antibodies, and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal phospho-MKK3/6 and phospho-p38 MAPK antibodies and GST-activating transcription factor (ATF)-2 were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Antibodies specific for macrophages (anti-CD68, clone EMBII) and for T cells (anti-CD3, clone UCHTI) were purchased from DAKO (Glostrup, Denmark). Antibody for synovial fibroblasts (anti-CD55, Ab-1) was purchased from Oncogene Research Products (Boston, MA). CD55, which is a decay-activating factor, can be expressed by other cells but has been used routinely to distinguish FLS from macrophages in synovium.20 IL-1β and TNF-α was purchased from R&D systems (Minneapolis, MN). The p38 inhibitor, SB203580 was purchased from Promega (Madison, WI) and the JNK inhibitor, SP600125, was provided by Celgene, Inc. (San Diego, CA).
Western Blot Analysis
FLS were cultured in DMEM with 10% FCS in 100-mm dishes. At 80% confluency, they were synchronized in DMEM with 0.1% FCS for 48 hours. FLS were then stimulated with IL-1 (2 ng/ml) or TNF-α (10 ng/ml) for up to 24 hours. Cells were then washed twice with phosphate-buffered saline (PBS) and lysed using RIPA buffer [50 mmol/L HEPES, 150 mmol/L NaCl, 1% Triton X-100, 10% glycerol, 1 mmol/L MgCl2, 1.5 mmol/L ethylenediaminetetraacetic acid (pH 8.0), 20 mmol/L β-glycerophosphate, 50 mmol/L NaF, 1 mmol/L Na3VO4, 10 μg/ml aprotinin, 1 μmol/L pepstatin A, and 1 mmol/L phenylmethyl sulfonyl fluoride]. For Western blot studies of synovium, the frozen synovial tissue was pulverized and lysed in the same manner. The protein concentrations in the extracts were determined using the DC Protein assay kit (Bio-Rad, Hercules, CA). Whole-cell lysates (50 μg) or tissue lysates (200 μg) were fractionated on Tris-glycine-buffered 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Perkin-Elmer Life Sciences, Inc., Boston, MA). The membranes were blocked with 5% nonfat milk powder for 1 hour at room temperature, followed by incubation with antibody to MKK3, MKK6, phospho-MKK3/6, phospho-MAPK p38, or actin at 4°C overnight. After washing with TBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Immunoreactive protein was detected with chemiluminescence and autoradiography (Eastman Kodak Co., Rochester, NY).
Immunoprecipitation and Kinase Assays
To measure the activities of MKK3 and MKK6, FLS were serum-starved (0.1% FCS) for 48 hours and then treated with either low-serum medium or IL-1 for 15 minutes. The cells were then washed three times with ice-cold PBS and lysed in immunoprecipitation buffer (1% Triton X-100, 50 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L sodium vanadate, 10 μmol/L leupeptin, and 1.5 μmol/L pepstatin). Lysates were clarified by centrifugation at 15,000 × g for 10 minutes. Protein concentration in the supernatant was determined using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc.). The supernatant was precleared with appropriate sera and with protein A- or G-Sepharose (Oncogene Research Products) for 1 hour. Clarified lysates of 500 μg of total protein were incubated with 4 μg of anti-MKK6 or anti-MKK3 antibody for 4 hours, followed by additional incubation with protein A- or G-Sepharose overnight. The immunoprecipitates were washed three times with immunoprecipitation buffer, once with kinase buffer (50 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 0.2 mmol/L dithiothreitol, 1 mmol/L sodium vanadate, 10 μmol/L leupeptin, and 1.5 μmol/L pepstatin), and resuspended in 25 μl of kinase buffer containing 5 μCi of [γ-32P] ATP, 100 μmol/L ATP, and 4 μg of glutathione S-transferase-ATF-2 (Cell Signaling Technology, Inc., Beverly, MA) and incubated at 37°C for 30 minutes. In some experiments, kinase reactions included p38 (SB203580, 3 μmol/L) or JNK (SP600125, 10 μmol/L) inhibitors. Reactions were stopped by addition of SDS sample buffer (100 mmol/L Tris, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.25% bromophenol blue). After electrophoresis, the gel was subjected to autoradiography. The density of target bands was analyzed using National Institutes of Health Image (version 1.61; National Institutes of Health, Bethesda, MD).
For Western blot analysis, the same protocol was used for immunoprecipitation except that the pellets were washed four times with washing buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L ethylenediaminetetraacetic acid, 1% Triton X-100, 10% glycerol), incubated in 2× nonreducing Laemmli sample buffer, and heated for 5 minutes at 95°C. The samples were processed for SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Immunohistochemistry
Immunohistochemistry was performed as previously described.21 Cryosections (5 μm) of synovial tissue from RA and OA patients were fixed with acetone or 4% formalin for 10 minutes and then incubated with anti-MKK3, anti-MKK6 or anti-phospho-MKK3/6 antibody overnight at 4°C. Isotype-matched antibodies served as control. Endogenous peroxidase was depleted with 0.1% H2O2 and 0.1% NaN3. The sections were then stained with biotinylated secondary antibody anti-rabbit or anti-goat IgG and Vectastain ABC (Vector, Burlingame, CA) and developed using diaminobenzidine (Vector). The immunostained samples were counterstained with hematoxylin. Antibodies for synovial fibroblasts, macrophages, and T cells were used to characterize the cells expressing phospho-MKK3/6 in double-labeling staining. Alkaline phosphatase-labeled horse anti-mouse IgG (Vector) was applied as second antibody. Color was developed using Blue (Vector) as substrate.
After immunohistochemical staining, quantification of positively stained cells was evaluated on six high-powered fields from each section by computer-assisted image analysis. The images were acquired using a Nikon Eclipse E800 microscope (Nikon Instruments, Inc., Melville, NY) and equipped with a MicroFire digital camera (Olympus, Melville, NY). Digital image acquisition was performed by MicroFire software. Quantitative analysis was performed using ImagePro Plus programs (Media Cybernetics, Inc., Silver Spring, MD). Specific areas of interest were selected, including the total tissue section, the intimal lining, or the sublining. The percentage of region covered by diaminobenzidine was quantified (percent positive area) and the mean optical density of the positive region determined. The latter parameter determines average intensity of the selected color in the region of interest. Relative protein expression was determined by multiplying the percent positive area by the mean optical density.
Statistical Analysis
Statistics were performed with paired Student’s t-test. A comparison was considered statistically significant if P was <0.05.
Results
Expression and Activation of MKK3 and MKK6 in Synovial Tissues
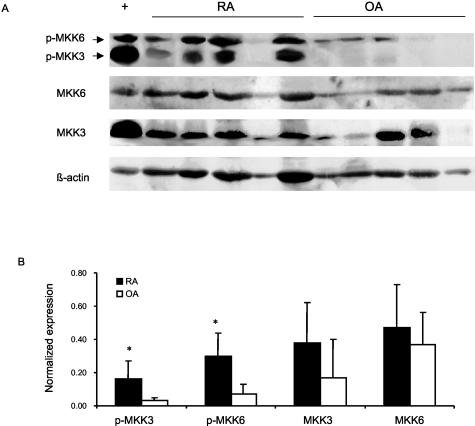
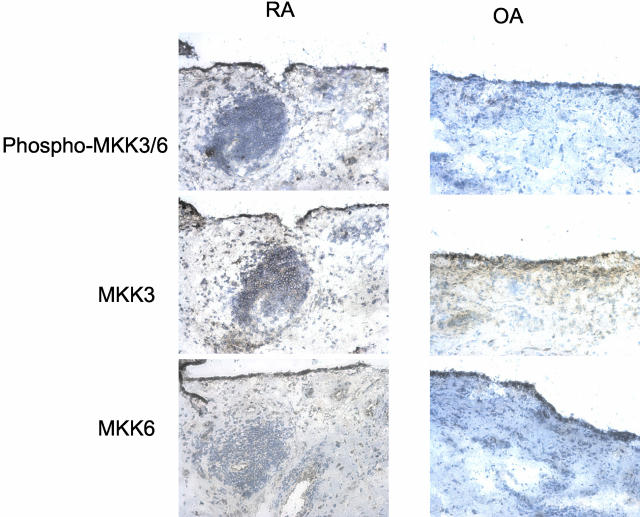
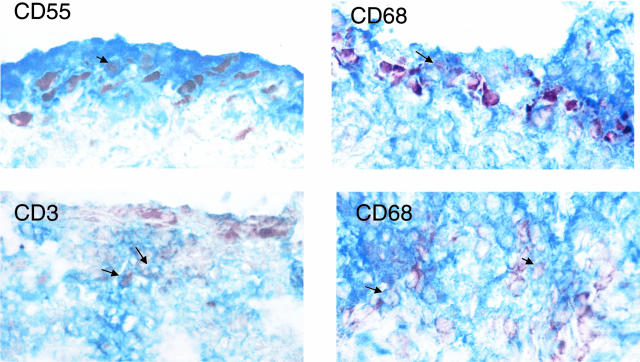
To determine whether key upstream activators of p38 are expressed in RA, Western blot studies were initially performed on RA and OA synovial tissue lysates using specific anti-MKK3 and -MKK6 antibodies. The results for synovial extracts from five RA patients and five OA patients are shown in Figure 1A and demonstrate similar levels of expression for both kinases after actin normalization. However, expression of phosphorylated MKK3 and MKK6 was significantly higher in RA than OA synovium (P < 0.05). Although no antibodies are available that distinguish between phospho-MKK3 and phospho-MKK6, differences in molecular weight allowed us to evaluate the two kinases separately (molecular weight for MKK3 = 43 kd and MKK6 = 45 kd). Immunohistochemistry studies were then performed to localize MKK3 and MKK6 expression and evaluate relative levels in RA and OA tissues (n = 6 each). Both kinases were primarily detected in the synovial intimal lining, although positive cells were also identified in the sublining region (see Figure 2 for representative examples). No differences were noted between RA and OA as determined by image analysis (Table 1). Using anti-phospho-MKK3/6 antibodies, however, we observed phospho-MKK3 and -MKK6 expression in the intimal lining and sublining mononuclear cells primarily in RA samples. Image analysis demonstrated that both the relative areas of phospho-MKK3/6 and the intensity of staining were significantly higher in RA compared with OA (Table 1). The expression of MKK3, MKK6, and phospho-MKK3/6 is higher in lining compared with the sublining (P < 0.05). Double-staining experiments with CD55 and CD68 showed activated MKK3/6 expressed predominantly in synovial fibroblasts in the lining (see Figure 3 for representative examples). In sublining, macrophages expressed predominantly phospho-MKK3/6. Rare sublining T cells contained activated MKK3/6 (Figure 3), although most did not stain with the anti-phospho-MKK3/6 antibody.
Figure 1.
Expression of activated MKK3/6 in synovial tissue from patients with RA and OA. A: Western blot analysis was performed with tissue synovial extracts from RA patients (n = 5) and OA patients (n = 5). Extracts (200 μg) were separated on a 12% SDS-polyacrylamide gel electrophoresis gel, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-MKK3/6, MKK3, MKK6, and actin. Phospho-MKK3 and -MKK6 were distinguished on the basis of molecular weight. B: The quantitative densitometry analysis of phospho-MKK3/6 expression normalized for actin is shown as the mean ± SD. +, Positive control; *, P < 0.05.
Figure 2.
Immunohistochemistry of phospho-MKK3/6 in synovial tissue from RA and OA patients. Phospho-MKK3/6, MKK3, and MKK6 expressions are identified by immunohistochemistry as described in Material and Methods. Staining for MKK3, MKK6, and phospho-MKK3/6 were the highest in the intimal lining. Representative serial sections from a RA and an OA patient are shown. Table 1 shows the results of image analysis. Serial sections stained with control antibodies were negative (data not shown). Original magnifications, ×200.
Table 1.
Digital Image Analysis Results for the Expression of Phospho-MKK3/6, MKK3 and MKK6 in Synovial Tissue
| Lining
|
Sublining
|
|||
|---|---|---|---|---|
| % Stained area | Total expression (pixel units) | % Stained area | Total expression (pixel units) | |
| Phospho-MKK-3/6 | ||||
| OA (n = 7) | 3.4 ± 0.9 | 383 ± 188 | 0.6 ± 0.2 | 45 ± 20 |
| RA (n = 9) | 26.2 ± 5.5* | 5203 ± 1367* | 7.8 ± 1.3† | 1952 ± 434† |
| MKK6 | ||||
| OA (n = 6) | 32.3 ± 5.9 | 36 ± 9 | 5.4 ± 1.8 | 7 ± 2 |
| RA (n = 5) | 30.4 ± 7.8 | 37 ± 9 | 7.6 ± 1.6 | 11 ± 3 |
| MKK3 | ||||
| OA (n = 6) | 31.6 ± 5.9 | 30 ± 7 | 8.0 ± 2.5 | 8 ± 3 |
| RA (n = 6) | 28.9 ± 5.9 | 30 ± 7 | 10.0 ± 1.9 | 11 ± 2 |
Percent stained area equals percent of selected region positive for the particular protein.
Total expression equals mean optical density × percent stained area as described in Material and Methods.
Data are shown as mean ± SEM.
P < 0.01,
P < 0.005 for RA compared with OA.
Figure 3.
Immunohistochemistry double staining of activated MKK3/6 in synovial tissue from RA synovial tissue. To characterize cells expressing phospho-MKK3/6 (brown), tissue sections were stained with specific antibodies (blue) for fibroblasts (CD55), macrophages (CD68), and T cells (CD3) by double-staining immunohistochemistry as described in Material and Methods. Arrows indicate representative double-positive cells. The most intense double staining was observed with intimal lining fibroblasts, followed by macrophages. Rare positive T cells were present in lymphoid aggregates, but T-cell staining was generally weak for the phosphorylated kinases. Serial sections stained with control antibodies were negative (data not shown). Original magnifications, ×40.
Expression and Activation of MKK3 and MKK6 in FLS
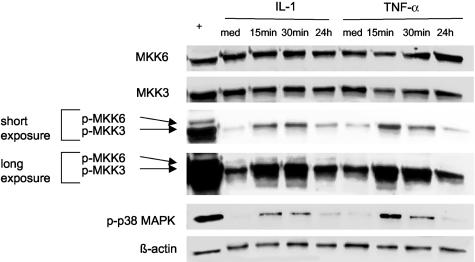
Because MKK3 and MKK6 activation was highest in the synovial intimal lining, we subsequently examined the expression of these cytokines in cultured synoviocytes. As anticipated, Western blot demonstrated that MKK3 and MKK6 are constitutively expressed in both RA and OA FLS (n = 3 each). The ability of IL-1 and TNF-α to induce phosphorylation of each of these kinases was investigated in a time-course study. FLS were stimulated with IL-1 (2 ng/ml) or TNF-α (10 ng/ml) for up to 24 hours and phosphorylation of MKK3/MKK6 was determined by Western blot analysis. As shown in Figure 4, modest MKK3 activation was detected under resting conditions and was significantly augmented by IL-1 and TNF-α treatment. A significant increase in MKK3 phosphorylation was detected within 15 minutes, with a 25-fold increase with IL-1 and a ninefold increase with TNF-α (P < 0.001 compared with medium alone). Of interest, MKK6 phosphorylation was low or undetectable in resting cells and a very modest increase was detected after cytokine stimulation (2.5-fold increase, P < 0.05). Note that the time course for activation for MKK3 and MKK6 is similar to p38. These data suggest that phosphorylation of MKK3 rather than MKK6 is the preferred activation pathway in FLS after cytokine stimulation. There were no significant differences observed between OA and RA FLS with regard of the timing or extent of phosphorylation (data not shown).
Figure 4.
Time course of IL-1 and TNF-α treatment on the protein expression of MKK3, MKK6, and phospho-MKK3/6 in FLS. Cultured FLS were stimulated with IL-1 (2 ng/ml) or TNF-α (10 ng/ml) for the indicated times. The levels of MKK3, MKK6, phospho-MKK3/6, and phospho-p38 (MAPK) were analyzed by Western blot analysis. UV-treated NIH3T3 is a positive control (+) for activation of MKK3 and MKK6. Note that an increase in phospho-MKK3 occurs within 15 minutes while minimal phospho-MKK6 is detected. A longer exposure is shown to demonstrate the faint phospho-MKK6 band. For comparison, phospho-p38 expression is shown in the same experiment. No change is observed in total MKK3 or MKK6 levels. This is representative of three independent experiments with similar results. Med, Medium.
MKK3 and MKK6 Form Stable Complexes with p38
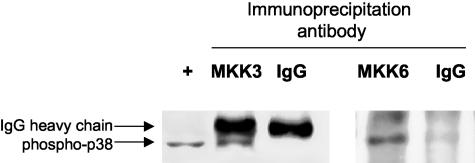
Because MKK3 and MKK6 have the capacity to activate p38 MAPK, we then determined if stable complexes between the kinases form in cultured FLS. Previous studies in FLS have demonstrated other complexes between MAPKKs and MAPKs, such as MKK4, MKK7, and JNK.22 Immunoprecipitation studies were performed using antibodies to either MKK3 or MKK6, followed by Western blot analysis to determine whether phospho-p38 is present in the complexes. As shown in Figure 5, phospho-p38 was readily detected in MKK3 and MKK6 immunoprecipitates but not in precipitates using control antibodies.
Figure 5.
MKK3 and MKK6 form stable complexes with p38 in FLS. RA FLS were stimulated with medium or 2 ng/ml of IL-1 for 15 minutes. Total protein was extracted and immunoprecipitated with antibodies to MKK3 or MKK6 or an IgG control. Western blot analysis was performed on immunoprecipitates to detect p38 MAPK. Representative example is shown. Note that IgG heavy chain is observed in the MKK3 immunoprecipitate and a faint 35- to 40-kd band is observed in the IgG immunoprecipitate in the MKK6 experiment. This is representative of two independent experiments with similar results.
MKK3 and MKK6 Function in Activated FLS
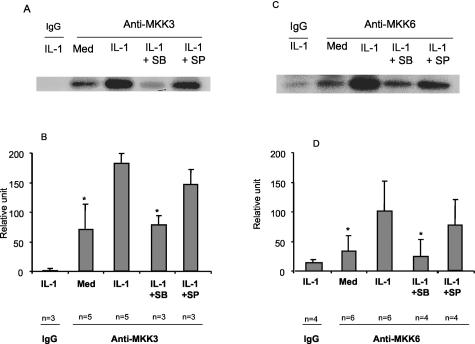
To evaluate kinase function, resting and IL-1-stimulated synoviocytes were lysed and MKK3 was recovered by immunoprecipitation. The immunoprecipitated MKK3 was incubated in the presence of the p38 substrate ATF-2 and 32P-ATP and resolved by SDS-polyacrylamide gel electrophoresis. Although ATF-2 is also a substrate for JNK, it is not directly phosphorylated by MKK3 or MKK6. Therefore, any kinase activity in the immunoprecipitates would require the presence of activated MAP kinase. As shown in Figure 6, IL-1 increased ATF-2 phosphorylation approximately fourfold in the MKK3 complexes compared with the control cells (P < 0.05). To confirm that p38 in the complex is responsible, selective inhibitors of JNK (SP600125) or p38 (SB203580) were added to the kinase reaction in some experiments. Only the SB203580 significantly decreased the ATF-2-phosphorylating activity indicating that p38 is responsible (2.5-fold decrease, P < 0.05). Similar experiments were performed with immunoprecipitated MKK6 (Figure 6, C and D). Although phosphorylated MKK6 was not noted by Western blot (see above), the more sensitive kinase assay was able to detect MKK6-mediated kinase activity. IL-1 also significantly increased ATF-2 phosphorylation, which was blocked by the p38 inhibitor (ninefold decrease, P < 0.05). Modest inhibition of phosphorylating activity was occasionally observed with the JNK inhibitor SP600125 (MKK6 in Figure 6C), although the differences were not statistically significant. Therefore, both MKK3 and MKK6 form stable complexes with p38 in FLS that can phosphorylate ATF-2.
Figure 6.
In vitro activation of MKK3 and MKK6. FLS were treated with IL-1 (2 ng/ml) and MKK3 and MKK6 activity was measured by immune complex kinase assay using ATF-2 protein as a substrate. The cell lysates were immunoprecipitated with anti-MKK3 or anti-MKK6 antibody or control IgG. A and C: Kinase assays demonstrated induction of ATF-2-phosphorylating activity in the MKK3 and MKK6 immunoprecipitates. Note that a faint band is observed in the IgG control lane for the MKK6 experiment. B and D: The quantitative densitometry data are presented as mean ± SD. The p38 inhibitor SB203580 completely blocked kinase activity of the immunoprecipitate. Although modest inhibition of phosphorylating activity was observed in some experiments using the JNK inhibitor SP600125 (MKK6 in C), overall the differences were not statistically significant. *, P < 0.05. SB, SB203580 (3 μmol/L); SP, SP600125 (10 μmol/L); Med, medium.
Discussion
RA is a chronic inflammatory disease marked by synovial hyperplasia with local invasion of bone and cartilage. This disorder is regulated by proinflammatory cytokines such as IL-1 and TNF-α that can activate a broad array of intracellular signal transduction mechanisms.23–25 Of the cytokine- and stress-activated pathways, MAP kinases are especially important in synoviocytes and chondrocytes because they can increase production of several mediators of inflammation and cartilage damage.26 The MAP kinase families phosphorylate a number of transcription factors such as activator protein-1 (AP-1), ATF-1 and ATF-2, with subsequent activation of matrix metalloproteinase and cytokine gene expression.27 p38 MAPK, in particular, can regulate cytokine production through a variety of transcriptional and translational mechanisms.28,29 Furthermore, p38 participates in other inflammation-related events, such as neutrophil activation,4 apoptosis,12 and nitric oxide synthase induction.30 Inhibition of p38 MAPK with the commonly used SB203580 reduces proinflammatory cytokine production in monocytes/macrophages, neutrophils, and T lymphocytes.31 In a rodent model of RA, p38 inhibition suppresses inflammation and bone destruction.6
p38 MAPK has two main upstream activators: MKK3 and MKK6.7,8,32,33 Studies in MKK3 knockout and MKK6 knockout mice demonstrate that both are essential for full p38 MAPK activation in vivo.34 MKK4 also phosphorylates p38 in vitro when overexpressed in mammalian cells.32,35 However, the role of MKK4 as an activator of p38 MAPK in vivo is unclear. p38 is activated in the rheumatoid synovium,5,36 although there is little information on the upstream kinases that contribute. The present study was designed to evaluate the potential role of two main upstream activators, MKK3 and MKK6 in RA. Western blot analysis demonstrated that both MKK3 and MKK6 are expressed in OA and RA synovial tissue, with no differences observed between OA and RA. This result was confirmed by quantitative immunohistochemistry, which identified MKK3 and MKK6 in the intimal lining and sublining region of synovial tissue. In contrast, phospho-MKK3/6 expression was markedly greater in RA than in OA synovium, especially in the intimal lining. High levels of activated MKKs were confirmed by Western blot analysis, with increases in both phospho-MKK3 and -MKK6. Because p38 activation contributes to bone destruction and synovial inflammation in animal models of arthritis,37 one can infer that similar pathways might be relevant in RA. If so, MKK3 and MKK6 are potential gateways to p38 activation in rheumatoid synovium and could enhance cytokine and protease production.
Double-staining experiments with specific markers demonstrated the expression of activated MKK3/6 expression predominantly in synovial fibroblasts in the RA synovial intimal lining where the destructive process is mediated. The regulation of MKK3 and MKK6 was therefore examined in cultured FLS. OA and RA FLS constitutively express MKK3 and MKK6, with no differences observed between cells derived from patients with the two diseases. Activation of MKK3 was rapidly increased by IL-1 or TNF-α, as determined by Western blot analysis and an anti-phospho-MKK antibody. In contrast to intact synovium where similar levels of phospho-MKK3 and -MKK6 were observed, cytokine stimulation only modestly increased MKK6 phosphorylation. These data suggest that MKK3 is the dominant p38-related MAPKK that is activated after cytokine stimulation. The observation is consistent with the fact that Mkk3, but not Mkk6, gene disruption reduces activation of p38 MAPK and inflammatory cytokine expression in TNF-α stimulated murine fibroblasts.17 Because phospho-MKK3 and -MKK6 levels are actually similar in RA synovium, the latter is probably expressed in macrophages and other cells in the tissue.
The role of the different MAPKKs depends on the specific MKK activated and may be cell type-specific. Although MKK6 activates all four family members of p38 (α, β, γ, and δ), MKK3 selectively phosphorylates p38α, γ, and δ.15 MKK6 is the principal activator of p38δ in epithelial cells,38 whereas MKK3 appears to be an important activator of p38α in murine peritoneal macrophages.10 In contrast, both the p38α and δ isoforms are activated by MKK3 in murine mesangial cells stimulated by TGF-β1.39 The relative expression of p38 isoforms in RA has not been fully explored, although p38α and δ are especially prevalent at sites of joint destruction.5,36
To facilitate signal transduction, the enzymes can be organized into functional units that include both upstream and downstream members of the family along with various scaffold proteins.40,41 For instance, we have recently described an activated tri-molecular complex in RA FLS that includes JNK and two MAPKKs (MKK4 and MKK7).22 Our immunoprecipitation experiments also demonstrate MKK3-p38 and MKK6-p38 complexes in synoviocytes as observed in some other cell lineages.41–43 The function of MKK3 and MKK6 in FLS was evaluated by determining the ability of the MKK-p38 complexes to phosphorylate ATF-2 in kinase assays. These studies clearly showed that the complexes are functionally active and that cytokine stimulation leads to activation of both the MKK and p38. Surprisingly, the MKK6 immunoprecipitate from cytokine-activated FLS also phosphorylated ATF-2 even though minimal amounts of phospho-MKK6 were detected. This is probably because of the much greater sensitivity of kinase assays compared with Western blots. The kinase activity of both complexes was blocked in vitro by the p38 inhibitor SB203580, indicating that the ATF-2 phosphorylating activity was mediated by p38. The JNK inhibitor modestly decreased kinase function in some experiments, although this was not statistically significant.
In conclusion, these studies demonstrate that MKK3 and MKK6 are important regulators of p38 in FLS and are activated in the synovium of patients. Because this pathway may be a critical regulator of joint destruction and inflammation, MAPKKs are potential therapeutic targets. MKK3 appears to be highly activated in cytokine-activated FLS and might be an especially attractive target for RA. Because alternative pathways for p38 activation are available in other cell types, MKK3 or MKK6 inhibitors could have distinct safety and efficacy profiles compared with a selective p38 inhibitor.
Acknowledgments
We thank Dr. William Bugbee for providing many of the clinical samples used in this study and David Boyle for valuable discussions and advice.
Footnotes
Address reprint requests to Gary S. Firestein, M.D., Division of Rheumatology, Allergy, and Immunology, UCSD School of Medicine, 9500 Gilman Dr., La Jolla, CA 92093. E-mail: gfirestein@ucsd.edu.
Supported in part by grants from the National Institutes of Health.
References
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Mori A, Miyata H, Akahane M, Ajisawa Y, Okudaira H. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J Biol Chem. 1998;273:24832–24838. doi: 10.1074/jbc.273.38.24832. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tetsuka T, Yoshida S, Watanabe N, Kobayashi M, Matsui N, Okamoto T. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000;465:23–27. doi: 10.1016/s0014-5793(99)01717-2. [DOI] [PubMed] [Google Scholar]
- Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, Zenz P, Redlich K, Xu Q, Steiner G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501–2512. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McLay LM, Halley F, Souness JE, McKenna J, Benning V, Birrell M, Burton B, Belvisi M, Collis A, Constan A, Foster M, Hele D, Jayyosi Z, Kelley M, Maslen C, Miller G, Ouldelhkim MC, Page K, Phipps S, Pollock K, Porter B, Ratcliffe AJ, Redford EJ, Webber S, Slater B, Thybaud V, Wilsher N. The discovery of RPR 200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg Med Chem. 2001;9:537–554. doi: 10.1016/s0968-0896(00)00331-x. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Stein B, Brady H, Yang MX, Young DB, Barbosa MS. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kamanaka M, Enslen H, Dong C, Wysk M, Davis RJ, Flavell RA. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 2002;3:785–791. doi: 10.1093/embo-reports/kvf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, Biemann HP, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Leng J, Bian D, Mahanivong C, Carpenter KA, Pan ZK, Han J, Huang S. Rac1-MKK3–p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem. 2002;277:48379–48385. doi: 10.1074/jbc.M209542200. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and H2O2. J Biol Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763–3768. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991;146:3365–3371. [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt OH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Hamann J, Wishaupt JO, van Lier RA, Smeets TJ, Breedveld FC, Tak PP. Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 1999;42:650–658. doi: 10.1002/1529-0131(199904)42:4<650::AID-ANR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Elices MJ, Tsai V, Strahl D, Goel AS, Tollefson V, Arrhenius T, Wayner EA, Gaeta FC, Fikes JD, Firestein GS. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994;93:405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarrajan M, Boyle DL, Chabaud-Riou M, Hammaker D, Firestein GS. Expression of the MAPK kinases MKK-4 and MKK-7 in rheumatoid arthritis and their role as key regulators of JNK. Arthritis Rheum. 2003;48:2450–2460. doi: 10.1002/art.11228. [DOI] [PubMed] [Google Scholar]
- Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Manning AM. Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum. 1999;42:609–621. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Kumar S, Votta BJ, Rieman DJ, Badger AM, Gowen M, Lee JC. IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J Cell Physiol. 2001;187:294–303. doi: 10.1002/jcp.1082. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler D, McLaughlin MH, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Perregaux DG, Dean D, Cronan M, Connelly P, Gabel CA. Inhibition of interleukin-1 beta production by SKF86002: evidence of two sites of in vitro activity and of a time and system dependence. Mol Pharmacol. 1995;48:433–442. [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Badger AM, Cook MN, Lark MW, Newman-Tarr TM, Swift BA, Nelson AH, Barone FC, Kumar S. SB 203580 inhibits p38 mitogen-activated protein kinase, nitric oxide production, and inducible nitric oxide synthase in bovine cartilage-derived chondrocytes. J Immunol. 1998;161:467–473. [PubMed] [Google Scholar]
- Young P, McDonnell P, Dunnington D, Hand A, Laydon J, Lee J. Pyridinyl imidazoles inhibit IL-1 and TNF production at the protein level. Agents Actions. 1993;39:C67–C69. doi: 10.1007/BF01972723. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Goff SP. c-Abl-induced apoptosis, but not cell cycle arrest, requires mitogen-activated protein kinase kinase 6 activation. Proc Natl Acad Sci USA. 1999;96:13819–13824. doi: 10.1073/pnas.96.24.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Minden A, Martinetto H, Claret FX, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- Neidhart M, Rethage J, Kuchen S, Kunzler P, Crowl RM, Billingham ME, Gay RE, Gay S. Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum. 2000;43:2634–2647. doi: 10.1002/1529-0131(200012)43:12<2634::AID-ANR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, Stroup GB, Webb E, Rieman DJ, Gowen M, Boehm JC, Adams JL, Lee JC. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175–183. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ma R, Flavell RA, Choi ME. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J Biol Chem. 2002;277:47257–47262. doi: 10.1074/jbc.M208573200. [DOI] [PubMed] [Google Scholar]
- Zanke BW, Rubie EA, Winnett E, Chan J, Randall S, Parsons M, Boudreau K, McInnis M, Yan M, Templeton DJ, Woodgett JR. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- Buchsbaum RJ, Connolly BA, Feig LA. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22:4073–4085. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Visconti R, Gadina M, Chiariello M, Chen EH, Stancato LF, Gutkind JS, O’Shea JJ. Importance of the MKK6/p38 pathway for interleukin-12-induced STAT4 serine phosphorylation and transcriptional activity. Blood. 2000;96:1844–1852. [PubMed] [Google Scholar]