Abstract
Tissue factor (TF), a transmembrane receptor for plasma factor VII(a), is the main initiator of the coagulation cascade. It has also been implicated in noncoagulant processes, including inflammation. The function of the TF cytoplasmic domain was studied in mice in which 18 of the 20 cytoplasmic amino acids were deleted. This mutation (TFδCT/δCT) is not associated with alterations in blood coagulation. Arthritis was induced by intra-articular injection of methylated bovine serum albumin (mBSA) in mice preimmunized with mBSA. Arthritis severity was significantly reduced in TFδCT/δCT mice compared to wild-type mice, including reductions in synovitis, synovial exudate, cartilage degradation, and bone damage. A marked reduction in synovial interleukin (IL)-1β and IL-6 mRNA was also observed. Serum anti-mBSA IgG1, but not IgG2a, was increased in mutant mice. Cutaneous delayed-type hypersensitivity and antigen-induced T-cell proliferation were reduced in TFδCT/δCT compared to wild-type mice. A significant down-regulation of lipopolysaccharide-induced IL-1, tumor necrosis factor, IL-6, macrophage migration inhibitory factor, and matrix metalloproteinase-13 mRNA was observed in immunized, but not in naive TFδCT/δCT macrophages ex vivo. These data suggest a significant role for the cytoplasmic domain of TF in the regulation of the immunoinflammatory responses, a murine arthritis model, and macrophage function.
Tissue factor (TF), a transmembrane receptor for the serine protease coagulation factor VII(a),1 is the most potent initiator of the extrinsic coagulation cascade.2 After vascular injury, the extracellular domain of TF binds circulating factor VIIa, activating factor X, which converts prothrombin to thrombin and leads to fibrin deposition. TF is normally present on the surface of certain cell types located outside the vasculature and its expression is tightly regulated.3 Its expression can also be induced in monocytes in response to certain stimuli, including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF).4 In addition to its essential role in hemostasis, TF has also been implicated in noncoagulant processes, including embryological development,5–7 tumor angiogenesis,8 tumor metastasis,9–11 sepsis, and inflammation.12
A growing body of evidence supports the involvement of TF-induced coagulation in inflammatory arthritis. Increased expression of TF and thrombin has been demonstrated in the synovium of patients with rheumatoid arthritis (RA) and in animal models of RA.13–16 Moreover, reduced levels of coagulation factors, with increased thrombin activity and thrombin-anti-thrombin complexes, have been found in RA synovial fluids.17,18
More recently, it has been shown that intra-articular injection of either recombinant TF or TF/FVIIa complex in mice induces remarkable cellular infiltration of synovium, and cartilage and bone destruction, indicating direct proinflammatory properties of TF.19,20 The function of the cytoplasmic domain of TF, which consists of only 21 residues in humans and 20 residues in mice, is primarily unknown. Melis and colleagues21 generated mice in which 18 of the 20 amino acids of the cytoplasmic tail are deleted (TFδCT/δCT). Unlike embryos with a full-length deletion of TF, which have defective vascular development and die in utero,22 cytoplasmic tail mutant mice display normal development and normal coagulation.21 These mice allow examination of the function of the cytoplasmic domain in noncoagulant functions of TF, such as inflammation. We investigated the role of the TF cytoplasmic domain in the development of arthritis. We report a significant reduction of disease severity in TFδCT/δCT mice accompanied by reduced synovial cytokine mRNA expression and impairment of the adaptive immune response.
Materials and Methods
Animals
Mice with deletion of 18 of the 20 amino acids of the cytoplasmic domain of TF (TFδCT/δCT) were generated by the Cre-lox recombination technique, as described previously.21 The deletion was introduced in exon 6 along with a floxed neoR selection cassette in intron 5 using homologous recombination in embryonic stem cells. TFδCT/δCT animals have normal fertility, embryonic and postnatal development, and coagulation and are of 25% Swiss/25% 129S/50% MF-1 background.21 Mice were bred and housed under specific pathogen-free conditions together with strain-identical wild-type (TF+/+) mice, which were used as controls. Previous experiments have shown that there was no difference between littermates and strain-identical TF+/+ and TFδCT/δCT mice in response to endotoxemia or immunization (unpublished observations). All experiments were approved by the Monash University Animal Ethics Committee.
Induction and Assessment of Arthritis
Antigen-induced arthritis (AIA) in mice was established as described previously.23 Briefly, mice were immunized on day 0 with 200 μg of methylated bovine serum albumin (mBSA) (Sigma Chemical Co., Castle Hill, Australia) emulsified in 0.2 ml of Freund’s complete adjuvant injected subcutaneously into the flank skin. At day 7, the mice received 100 μg of mBSA/0.1 ml Freund’s complete adjuvant by intradermal injection at the base of the tail. Arthritis was induced at day 21 by intra-articular injection of 30 μg of mBSA in 10 μl of sterile saline into the left knee, the right knee being injected with sterile saline alone.
Arthritis was analyzed histologically at day 28 after the first immunization. Knee joints were dissected and fixed in 10% buffered formalin for 7 days. Fixed tissues were decalcified for 3 weeks in 15% ethylenediaminetetraacetic acid (EDTA), dehydrated, and embedded in paraffin. Sagittal sections (5 μm) of the knee joint were stained with Safranin-O and counterstained with fast green/iron hematoxylin. Histological sections were scored from 0 to 3 for five parameters: Synovitis was defined as hypercellularity of the synovium including pannus formation. Joint space exudate was identified as leukocytes, discretely or in aggregates, in the joint space. Soft tissue inflammation was defined as leukocyte infiltration assessed in the infrapatellar fat pads, joint capsule, and the area adjacent to the periosteal sheath. Cartilage degradation was defined as the loss of Safranin-O staining of articular cartilage (0 = full stained cartilage, 3 = totally unstained cartilage). Bone damage was defined as the extent and depth of the subchondral bone invasion by pannus. A total score was generated from the sum of these five parameters.
Induction of Cutaneous Delayed-Type Hypersensitivity (DTH) to mBSA
Mice developing arthritis were challenged 24 hours before the end of the experiment by intradermal injection of 50 μg of mBSA in 20 μl of saline into one hind footpad. A similar volume of saline was injected into the contralateral footpad as a control. Footpad swelling was quantified 24 hours later using a micrometer (Mitutoyo, Kawasaki-shi, Japan). DTH was recorded as the difference in skin swelling between mBSA and saline-injected footpads, and expressed as change in footpad thickness (μm).
T-Cell Activation and Cytokine Production
To measure T-cell proliferation, spleens were removed at day 28 after the first immunization and a single cell suspension prepared in Dulbecco’s modified Eagle’s medium containing 5% fetal calf serum and 0.05% 2-mercaptoethanol. Cells (1 × 105 /200 μl) were cultured in triplicate in the presence or absence of mBSA (0.1, 1.0, 10, and 50 μg/ml) or phytohemagglutinin (1 μg/ml) in 96-well plates for 48 hours (37°C, 5% CO2.) The T-cell proliferation response was determined by measuring [3H]-thymidine incorporation during the final 18 hours. The cells were harvested and radioactivity incorporation into the DNA was measured with a Wallac 1409 liquid scintillation counter (Pharmacia, Turku, Finland).
To measure cytokine production, splenocytes (2 × 106 cells/500 μl) were incubated for 48 hours (37°C, 5% CO2) in 48-well tissue culture plates (0.5 ml/well) in the presence or absence of mBSA at a concentration of 0.1, 1, and 10 μg/ml. Interferon (IFN)-γ and IL-4 concentrations in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) as previously described.24 Primary and secondary monoclonal antibodies (mAbs) used were rat anti-mouse IFN-γ (R46A2; PharMingen, San Diego, CA) and biotinylated XMG1.2 (PharMingen) for the IFN-γ-ELISA, and rat anti-mouse IL-4 (11B11; American Type Culture Collection, Rockville, MD), and biotinylated BVD6 (DNAX Research Institute, Palo Alto, CA) for the IL-4 ELISA. The detection limits of the assays were 12 pg/ml and 15 pg/ml, respectively.
Antibody Response
Serum levels of anti-mBSA IgG were determined by ELISA as previously described.25 Briefly, polyvinyl microtiter plates were coated with 100 μl of mBSA (100 μg/ml) for 24 hours at 4°C. The plates were blocked with 2% casein (Sigma Chemical Co.) in phosphate-buffered saline (PBS) with 0.05% Tween-20 (PBS-T, Science Supply Australia, Seven Hill, NSW, Australia) at room temperature for 1 hour. After washing, 100 μl of serum samples were added at various dilutions in 2% casein/PBS-T and incubated for 24 hours at 4°C. The plate was then washed, and rabbit anti-mouse-IgG-biotinylated (1:2000; DAKO) or anti-isotype-specific (IgG1, IgG2a; DAKO) antibodies were incubated at room temperature for 2 hours. After further washing, the streptavidin-horseradish peroxidase conjugate (1:2000) was incubated for 30 minutes, which was then detected using the tetramethylbenzidine (TMB) peroxidase substrate with hydrogen peroxide (optical density at 450 nm determined).
Peripheral Blood (PB) Leukocyte Counts and Subsets
PB (100 μl) taken by cardiac puncture was collected at day 28 after the first immunization into 1.5-ml tubes containing 100 μl of citrate buffer (3.3%). PB leukocyte phenotyping was performed by labeling with monoclonal antibodies against leukocyte subset antigens. In brief, 100 μl of anti-coagulated blood was labeled with phycoerythrin-conjugated GR-1 (neutrophils), fluorescein isothiocyanate-conjugated M 1/70 (macrophages), B220 (B cells), with phycoerythrin-conjugated anti-CD4 or apocyanithin-conjugated anti-CD8 for 30 minutes. After lysis of red blood cells by Coulter Q-prep (Coulter Corp., Hialeah, FL), single cell suspensions were analyzed by flow cytometry (Mo-flo flow cytometer; Cytomation, Fort Collins, CO). Total PB leukocyte counts were performed using a Neubauer hemocytometer and light microscopy.
Peritoneal Macrophage Culture and Reactive Oxygen Species (ROS) Production
Resident peritoneal cells were obtained by washing the peritoneal cavity with ice-cold saline. A concentration of 1 × 106 cells/ml were cultured in 10% fetal calf serum/RPMI at 37°C overnight. After washing, adherent cells were starved with serum-free RPMI for 2 hours and stimulated with lipopolysaccharide (LPS) (1 μg/ml, Sigma) for a further 3 hours. Cells were then resuspended in Trizol reagent (Gibco BRL, Melbourne, Australia) for RNA extraction.
Phorbol myristate acetate (PMA)-induced ROS production was detected as previously described.26 In brief, 100 ng/ml of PMA (Sigma, Melbourne Australia) was added to macrophage suspensions (0.5 × 106 cells/ml). The cell suspension was then incubated at 37°C for 10 minutes. Dihydrorhodamine 123 (200 ng/ml) was added to the cell suspension and incubated for a further 10 minutes at 37°C. These samples were then placed on ice and immediately analyzed by flow cytometry.
Extraction and Real-Time Polymerase Chain Reaction (PCR) Analysis of Synovial and Peritoneal Macrophage mRNA
Total RNA was extracted from synovial tissue and peritoneal cells using Trizol reagent (Life Technologies, Inc., Grand Island, NY), according to the manufacturer’s protocol. Total RNA (0.5 to 1 μg) was reverse-transcribed using Superscript II reverse transcriptase (Life Technologies, Inc.) and oligo-(dT)18.
PCR amplification was performed on a LightCycler (Roche, Castle Hill, NSW, Australia) using SYBR Green I as a double-strand DNA-specific binding dye and continuous fluorescence monitoring as previously described.29 Murine IL-1β, IL-6, and β-actin PCR products (purified on agarose gel electrophoresis using the QIAEX II gel extraction system (Qiagen, Clifton Hill, Australia)) were used as the assay standards. The level of expression of each mRNA and their estimated crossing points were determined relative to the standard preparation using the LightCycler computer software.
For PCR, 5 μl each of the standard and sample cDNA dilutions were added to individual capillary tubes. Amplification (40 cycles) was performed in a total volume of 10 μl containing primer concentrations of 3 pmol and 1 μl of dNTP mix, Taq, reaction buffer and SYBR Green I dye as supplied in the LightCycler DNA Master SYBR Green I kit (Roche Diagnostics, Castle Hill, Australia). The following primer-specific nucleotide sequences of IL-1β (5′-CCCAAGCAATACCCAAAGAA-3′ and 5′-CATCAGAGGCAAGGAGGAAA-3′), IL-6 (5′-TTCCATCCAGTTGCCTTCTT-3′ and 5′-ATTTCCACGATTTCCCAGAG-3′), TNF (5′-GCCTCTTCTCATTCCTGCTT-3′ and 5′-CACTTGGTGGTTTGCTACGA-3′), macrophage migration inhibitory factor (MIF) (5′-TGACTTTTAGCGGCACGAAC-3′ and 5′-GACTCAAGCGAAGGTGGAAC-3′), matrix metalloproteinases (MMP)-13 (5′-GATGAAACCTGGACAAGCA-3′ and 5′-GATAGGGCTGGGTCACACTT-3′) and β-actin (5′-TGTCCCTGTATGCCTCTGGT-3′ and 5′-GATGTCACGCACGATTTCC-5′) were used. Melting curve analysis was performed at the end of each PCR reaction. Control reactions for product identification consisted of analyzing the melting peaks (°C) and determining the length of the PCR products (bp) by agarose gel electrophoresis. Relative quantification of target mRNA expression was calculated and normalized to β-actin expression. The results are presented as the fold induction of mRNA expression relative to the amount present in control samples.
Statistical Analysis
Data were analyzed using the Mann-Whitney two-sample rank test to determine the level of significance between means of groups for histological scores, or the Student’s t-test for comparison of continuous variables. Results are expressed as the mean ± SEM. A P value <0.05 was considered statistically significant.
Results
The Effect of TF Cytoplasmic Tail Mutation on the Development of AIA
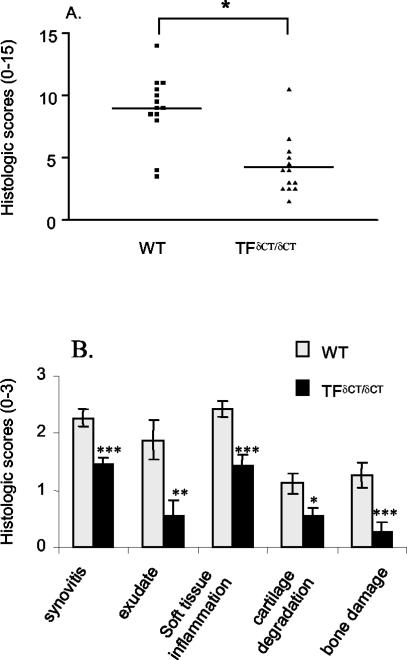
We first examined the effect of TF cytoplasmic tail mutation on the expression of arthritis. In comparison to saline injection (Figure 1; A to C), severe AIA developed in TF+/+ control mice on mBSA injection (n = 13; Figure 1, D to F, and Figure 2), involving extensive synovial lining hypercellularity, soft tissue inflammation, joint space exudation, cartilage degradation, and bone damage. In contrast to control animals, mBSA injection in TFδCT/δCT animals induced significantly reduced arthritis severity (n = 13, total score, P < 0.001; Figure 2A). Examination of individual aspects of synovial pathology exhibited significantly reduced synovitis (P < 0.0005), joint space exudate (P < 0.01), soft tissue inflammation (P < 0.0005), cartilage degradation (P < 0.05), and bone damage (P < 0.005) in TFδCT/δCT mice (Figure 2B).
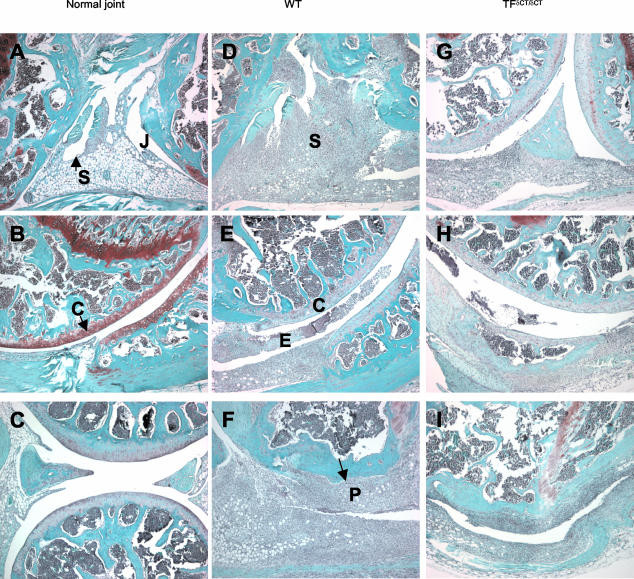
Figure 1.
Histological manifestations of AIA in TFδCT/δCT and TF+/+ (WT) mice. Mice were given intra-articular injection of either mBSA (30 μg) or saline on day 21 after the first immunization. On day 28, the severity of arthritis was assessed and scored as described in Materials and Methods. Safranin-O-stained sections of knee joint with saline injection (A–C), and mBSA injection of TF+/+ (WT) (D–F) and TFδCT/δCT mice (G–I). S, synovium; J, joint space; E, exudate; C articular cartilage; P, pannus formation. Original magnifications, ×50.
Figure 2.
A: Reduction of AIA in TFδCT/δCT mice. Arthritis was assessed at day 28 on a scale of 0 to 3 for five histopathological features (total score = 15) as described. Results are expressed as mean ± SEM of at least 13 mice in each group (*, P < 0.001 for TFδCT/δCT mice versus TF+/+ (WT) controls). B: Individual histological features of AIA in TFδCT/δCT mice. Arthritis was scored by histological analysis on a scale of 0 to 3 for synovitis, joint space exudate, soft tissue inflammation, cartilage damage, and bone damage. Results are expressed as means ± SEM [*, P < 0.05; **, P < 0.01; ***, P < 0.005 for TFδCT/δCT mice versus TF+/+ (WT) controls].
DTH, T-Cell Proliferation, and Cytokine Production
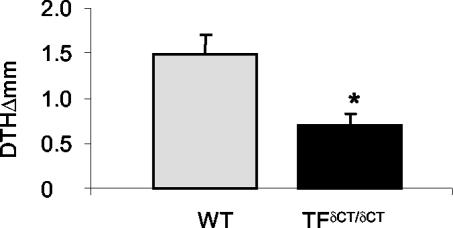
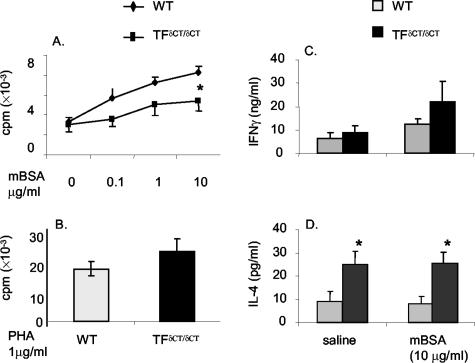
We next investigated whether the differences in arthritis were accompanied by differences in the systemic immune response. The T-cell-dependent immune response after induction of arthritis was analyzed. TF+/+ and TFδCT/δCT mice both developed cutaneous DTH after cutaneous challenge with mBSA. However, the DTH reaction was markedly reduced in TFδCT/δCT mice (Figure 3). Accordingly, mBSA-induced T-cell proliferation was observed in cells from both TF+/+ and TFδCT/δCT mice (Figure 4A), but the proliferative response was significantly weaker in TFδCT/δCT cells when compared to TF+/+ cells (P < 0.05). To confirm the specificity of the proliferation response, the effect of phytohemagglutinin on proliferation was used as a control. No difference in proliferative response to phytohemagglutinin was observed between TFδCT/δCT and TF+/+ mice (Figure 4B).
Figure 3.
Cutaneous DTH in TFδCT/δCT mice. Sensitized mice were challenged with mBSA into footpads and footpad swelling was measured after 24 hours. Cutaneous DTH was significantly reduced in TFδCT/δCT mice. Results are expressed as means ± SEM of seven mice in each group [*, P < 0.05 compared with TF+/+ (WT) mice].
Figure 4.
mBSA-specific spleen T-cell proliferation. Spleen cells were cultured in the absence or presence of the indicated amount of mBSA (A) and phytohemagglutinin (B) for 48 hours. Cultures were pulsed with [3H]-thymidine for the final 18 hours and the incorporated radioactivity was measured. Results are expressed as means ± SEM of eight mice in each group [*, P < 0.05 for TFδCT/δCT mice versus TF+/+ (WT) controls]. mBSA-specific T-cell cytokine expression. Spleen cells were cultured in the absence or presence of mBSA for 48 hours. Supernatants were analyzed by ELISA for IFN-γ (C) and IL-4 (D). Results are expressed as means ± SEM of eight mice in each group (*, P < 0.005 for vehicle versus mBSA treatment).
In naïve mice, IFN-γ, but not IL-4, was detectable in the supernatants of splenocytes isolated from both TFδCT/δCT and TF+/+ controls (37 ± 4 ∼ 38 ± 11 pg/ml, n = 5 each group). Incubation with mBSA had no effect on naïve mice splenocyte IFN-γ and IL-4 production. In immunized mice, increased basal splenocyte IFN-γ was detected from both TFδCT/δCT and TF+/+ controls (Figure 4C). For both groups, there was a nonsignificant trend toward increased IFN-γ in response to mBSA. However, there was no difference in the basal or induced production of IFN-γ between the two groups. In contrast, there was a significant increase in basal IL-4 levels in TFδCT/δCT cells from immunized mice compared to TF+/+ controls (Figure 4D, P < 0.05). No increase in splenocyte IL-4 production was observed in response to mBSA.
Antibody Levels
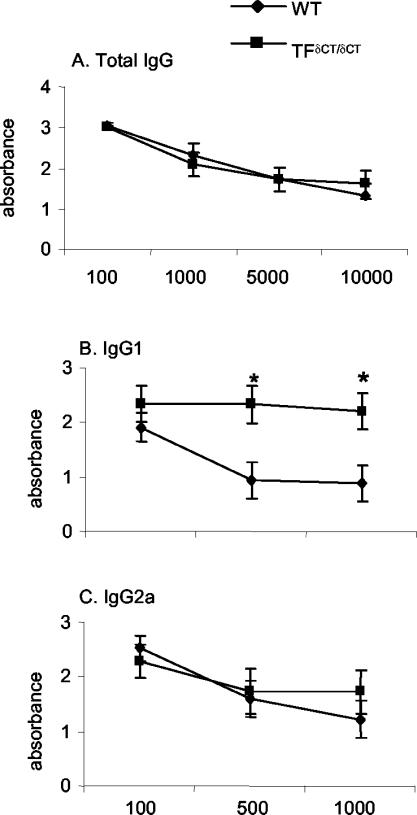
Next, the antibody response to mBSA was examined to evaluate the B-cell-dependent component of the immune response. There was no difference in the total serum anti-mBSA IgG levels between TF+/+ and TFδCT/δCT mice (Figure 5A). A significant increase in serum anti-mBSA IgG1 was however observed in TFδCT/δCT mice (P < 0.05, Figure 5B), whereas no significant difference in anti-mBSA IgG2a levels between two groups could be found (Figure 5C).
Figure 5.
Anti-mBSA IgG levels. Serum was collected at day 28 after first immunization from arthritic mice. The anti-mBSA antibody titers were measured by ELISA as described. A: Levels of total IgG in TFδCT/δCT and TF+/+ (WT) mice. Levels of IgG subclasses: B, IgG1; C, IgG2a in TFδCT/δCT and TF+/+ (WT) mice. Results are expressed as means ± SEM of serial dilution of serum (at least 13 mice in each group) [*, P < 0.05 for TFδCT/δCT mice versus TF+/+ (WT) controls].
Phenotype of PB Leukocytes
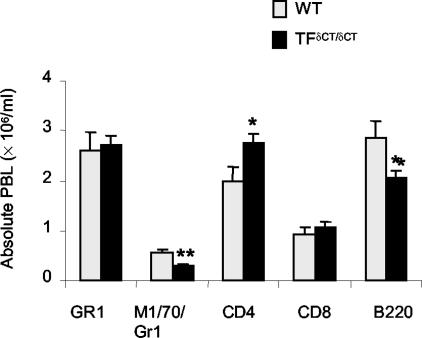
There was no difference in total PB leukocyte counts between affected TF+/+ and TFδCT/δCT mice. However, flow cytometric analysis revealed differences in PB leukocyte subsets (Figure 6): TFδCT/δCT mice had elevated numbers of CD4+ T cells (P < 0.05), and reduced numbers of B cells (B220+, P < 0.05) and monocytes (M1/70+/Gr1−, P < 0.01), when compared to TF+/+ mice.
Figure 6.
PB leukocytes in arthritic mice. PB was collected at day 28 after first immunization and leukocytes were phenotyped by labeling with various monoclonal antibodies and analyzed by flow cytometry. Numbers of total PB leukocytes (A) and absolute counts of leukocyte phenotypes (B) in TFδCT/δCT and TF+/+ (WT) mice. Results are expressed as means ± SEM of eight mice in each group [*, P < 0.05 and **, P < 0.01 for TFδCT/δCT mice versus TF+/+ (WT) controls].
Peritoneal Macrophage Counts and ROS Production
Peritoneal cells from naïve and immunized mice were counted. Significantly lower resident macrophage numbers were observed from naïve TFδCT/δCT (2.3 × 10−6 ± 0.2, n = 11) than from TF+/+ mice (4.4 × 10−6 ± 0.4, n = 11, P < 0.01). There was no significant difference in peritoneal cell counts between groups of immunized mice (4.3 × 10−6 ± 0.6 ∼ 5.7 × 10−6 ± 0.7, respectively), but cell counts were significantly increased in immunized TFδCT/δCT mice compared to naïve mice (P < 0.01). ROS production measured by flow cytometry measures ROS in a cell-by-cell basis and is unaffected by the total cell count. Significantly reduced ROS-positive cell counts were observed after PMA stimulation in TFδCT/δCT (25.6 ± 3.9, n = 7) compared to TF+/+ mice (44 ± 4.4, n = 7; P < 0.05).
Synovial and Peritoneal Cell Cytokine Gene Expression
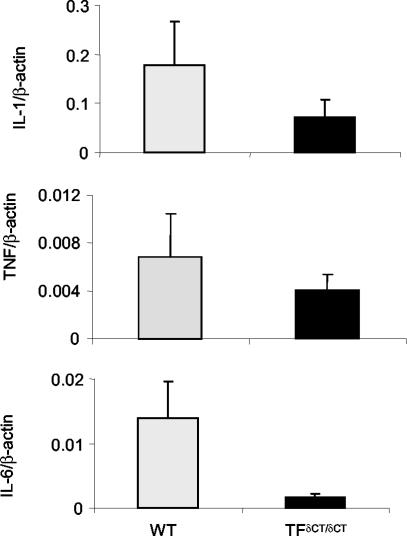
We next investigated the local mechanisms operative in the differences in arthritis between TF+/+ and TFδCT/δCT mice. IL-1β, TNF, and IL-6 mRNA were readily detected in the synovium of TF+/+ mice with AIA (Figure 7). In accordance with the attenuated inflammatory response, a significant reduction of IL-1, TNF, and IL-6 mRNA was observed in the synovium of TFδCT/δCT mice (Figure 7). The fold reduction in mRNA expression, relative to control animals, was 2.4-fold for IL-1β, 1.6-fold for TNF, and eightfold for IL-6.
Figure 7.
Synovial cytokine mRNA levels from TFδCT/δCT and TF+/+ (WT) mice with AIA. Total RNA extracted from arthritic knee joints of mice was reverse-transcribed. cDNA samples were amplified using real-time quantitative PCR and SYBR Green detection as described. The relative amounts of IL-1β, TNF, and IL-6 to β-actin determined from standard curve are expressed by ratio in TF+/+ (WT) (n = 5) and TFδCT/δCT mice (n = 4). Results are expressed as means ± SEM.
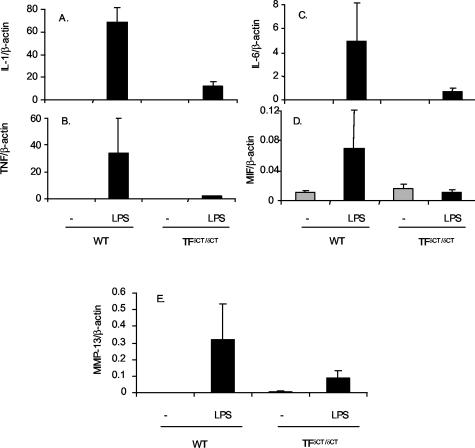
To assess whether alteration in proinflammatory gene expression was restricted to the site of antigen injection, we also examined cytokine gene expression in peritoneal cells. This also permitted the examination of a wider range of genes than that examinable in synovial RNA samples. In peritoneal cells obtained from immunized TF+/+ mice, increased IL-1, TNF, IL-6, and MIF mRNA were observed in response to LPS (Figure 8). LPS also induced the expression of MMP-13. However, unlike TF+/+ cells, LPS did not induce cytokines or MMP-13 mRNA in TFδCT/δCT cells (Figure 8). The fold reduction in LPS-induced mRNA expression, relative to TF+/+ animals, was 5.7-fold for IL-1β, 20-fold for TNF, 6.4-fold for IL-6, 6.3-fold for MIF, and 2.4-fold for MMP-13. There was no difference in LPS-induced IL-1 or IL-6 mRNA in resident peritoneal cells obtained from naïve mice (data not shown).
Figure 8.
Peritoneal macrophage cytokine mRNA levels from TFδCT/δCT and TF+/+ (WT) mice with AIA. Total RNA extracted from peritoneal cells of mice was reverse-transcribed. cDNA samples were amplified using real-time quantitative PCR and SYBR Green detection as described. The relative amounts of IL-1β, TNF, IL-6, MIF, and MMP-13 to β-actin determined from standard curve are expressed by ratio in TF+/+ (WT) (n = 4) and TFδCT/δCT mice (n = 4). Results are expressed as means ± SEM.
Discussion
Although it has long been recognized that the coagulation system is activated during inflammation, the role of the cytoplasmic domain of TF, which has no role in coagulation, remains to be elucidated. The cytoplasmic domain may influence cell activation via downstream signaling components. For example, it is involved in FVIIa-induced production of ROS in macrophages,26 TF-mediated tumor metastasis,10,11 and modulation of vascular endothelial growth factor production.28 A role in signal transduction is also suggested by observations including phosphorylation of the cytoplasmic tail by protein kinase C,29,30 and interaction with actin-binding protein 280.31 However, the cytoplasmic domain is not required for TF protein synthesis or for FVIIa-dependent activation of the p44/42 MAP-kinase pathway.32,33 Understanding of the signal transduction through the cytoplasmic domain of TF in the process of inflammation is incomplete.
This is first time the involvement of cytoplasmic domain of TF in the immune response and the development of experimental arthritis has been explored. We here report that deletion of the cytoplasmic domain of TF in mice resulted in attenuation of the immune response and an arthritis model. Reductions in AIA, accompanied by the suppression of proinflammatory gene expression, ROS production, cutaneous DTH, and antigen-specific T-cell proliferation, suggest the importance of the TF cytoplasmic domain in maintaining the normal immune system.
The reduction of arthritis severity in TFδCT/δCT mice compared with TF+/+ controls was observed in relation to all aspects of joint pathology, including articular cartilage and bone damage. The histopathological changes in joint inflammation in TFδCT/δCT mice were accompanied by attenuation of proinflammatory cytokine expression in the synovium. IL-1β, TNF, and IL-6 have been implicated in the pathology of RA, and are considered to enhance infiltration of inflammatory cells into synovium, increase permeability of blood vessels, and participate in cartilage and bone destruction.34 Recent evidence has demonstrated that transgenic mice overexpressing hIL-1α develop spontaneous inflammatory arthritis, whereas IL-1 receptor knockout mice are resistant to serum transfer arthritis.35,36 The role of TNF in the pathogenesis of RA is supported by observations including severe arthritis in TNF-overexpressing transgenic mice,37 and the clinical effects of anti-TNF therapy in human RA.38 Targeted genetic disruption of IL-6 markedly reduced arthritis severity in the murine AIA model.25
The reduced proinflammatory cytokine expression in inflamed sites and in peritoneal cells from immunized TFδCT/δCT mice suggests that modulation of cytokine gene expression may represent a mechanism by which TF modulates the inflammatory response independent of the coagulation pathway. Importantly, MMP-13 (collagenase −3), which is present at elevated levels in cartilage, synovial membranes, and synovial fluid of patients with RA,39 was also down-regulated in immunized TFδCT/δCT cells. This suggests that the cytoplasmic domain of TF may contribute to joint injury in RA directly through the regulation of destructive enzymes as well as via the regulation of inflammation. The absence of detectable differences in naïve mouse peritoneal cell cytokine gene expression is consistent with the increased sensitivity of cellular immune responses in mice under antigen challenge. TF has been shown to exacerbate systemic inflammation in response to low-dose endotoxin by a coagulation-independent mechanism, in which a TF pathway inhibitor completely inhibited activation of coagulation but not leukocyte activation, chemokine release, endothelial cell activation, or the acute phase response.40 These data indicate that TF plays a critical role in inflammation, which is independent of its role in the coagulation pathway.
In addition to a role in the control of proinflammatory gene expression, the current study suggests that the cytoplasmic domain of TF modulates antigen-specific T-cell responses and the Th1/Th2 balance. Cutaneous DTH and T-cell proliferative responses to antigen were significantly reduced in TFδCT/δCT mice. Increased serum anti-mBSA IgG1 was observed in TFδCT/δCT mice, consistent with a change in Th1/Th2 balance, as IgG1 is a Th2-polar IgG subtype in mice.41 Increased antigen-induced IL-4 production observed in TFδCT/δCT T cells is also consistent with a shift in favor of a Th2 immune response. The mechanism through which TF cytoplasmic domain could influence Th1/2 polarity is unknown, but the reduced inflammatory response in the arthritis model could be partially mediated by these alterations in Th1/Th2 polarity, and studies in other T-cell-dependent models of inflammation are required to address this question.
Although there was no difference in total numbers of leukocytes in both TF+/+ and mutant arthritic mice, decreased PB monocyte/macrophage counts were observed in TFδCT/δCT mice. Similarly, reduced resident peritoneal cell counts and decreased macrophage ROS induced by PMA were observed in TFδCT/δCT mice, indicating further aspects of macrophage responses are dependent on the cytoplasmic domain of TF. The reason for the increase in CD4+ T cell counts in TFδCT/δCT mice is unclear.
In summary, these studies are the first to examine the effects of TF intracellular domain in a model of chronic inflammation. Busso and colleagues42 recently reported a reduction in AIA in mice treated with functionally inactivated factor VIIa. These results suggested the importance of the VIIa-TF interaction in the inflammatory response, but did not resolve the potential for coagulation-independent intracellular processes to regulate this effect. Mutation of the cytoplasmic domain of TF reduced the severity of AIA, associated with decreased proinflammatory cytokine and MMP-13 production, reduced cutaneous DTH, and reduced T cell activation. These data indicate that TF acts as a modulator of systemic and local immune responses and macrophage function through its cytoplasmic domain. Development of TF ligand inhibitors specific for the cytoplasmic domain may be valuable treatment strategies in arthritis and inflammatory diseases.
Acknowledgments
We thank Ms. April Dacumos for processing joint sections.
Footnotes
Address reprint requests to A/Prof. Eric F Morand, Centre for Inflammatory Diseases, Monash University Department of Medicine, Monash Medical Centre, Locked Bag 29, Clayton, Victoria 3168, Australia. E-mail: eric.morand@med.monash.edu.au.
Supported by the National Health and Medical Research Council, Australia (program grant no. 143789).
Work was performed at the Department of Medicine, Centre for Inflammatory Diseases, Monash University Monash Medical Centre, Clayton, Victoria, Australia.
References
- Ruf W, Edgington TS. Structural biology of tissue factor, the initiator of thrombogenesis in vivo. EMBO J. 1994;8:385–390. [PubMed] [Google Scholar]
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Kolsto AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern DM, Nawroth PP. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci USA. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BM, Ruf W. Requirement for binding of catalytically active factor VIIa in tissue factor-dependent experimental metastasis. J Clin Invest. 1998;101:1372–1378. doi: 10.1172/JCI930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg ME, Konigsberg WH, Madison JF, Pawashe A, Garen A. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc Natl Acad Sci USA. 1995;92:8205–8209. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Luther T, Albrecht S, Magdolen V, Muller WA. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 1998;92:4167–4177. [PubMed] [Google Scholar]
- Weinberg JB, Pippen AM, Greenberg CS. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991;34:996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Brown FE, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM, Hunt JA, Dunwiddie C, Nutt EM. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992;63:155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]
- Marty I, Peclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107:631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N, Peclat V, Van Ness K, Kolodziesczyk E, Degen J, Bugge T, So A. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102:41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmassi F, de Negri F, Morale M, Song KY, Chung SI. Fibrin degradation in the synovial fluid of rheumatoid arthritis patients: a model for extravascular fibrinolysis. Semin Thromb Hemost. 1996;22:489–496. doi: 10.1055/s-2007-999049. [DOI] [PubMed] [Google Scholar]
- Nakano S, Ikata T, Kinoshita I, Kanematsu J, Yasuoka S. Characteristics of the protease activity in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 1999;17:161–170. [PubMed] [Google Scholar]
- Bokarewa MI, Morrissey J, Tarkowski A. Intra-articular tissue factor/factor VII complex induces chronic arthritis. Inflamm Res. 2002;51:471–477. doi: 10.1007/pl00012414. [DOI] [PubMed] [Google Scholar]
- Bokarewa MI, Morrissey JH, Tarkowski A. Tissue factor as a proinflammatory agent. Arthritis Res. 2002;4:190–195. doi: 10.1186/ar405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis E, Moons L, De Mol M, Herbert JM, Mackman N, Collen D, Carmeliet P, Dewerchin M. Targeted deletion of the cytosolic domain of tissue factor in mice does not affect development. Biochem Biophys Res Commun. 2001;286:580–586. doi: 10.1006/bbrc.2001.5425. [DOI] [PubMed] [Google Scholar]
- Martin DM, Boys CW, Ruf W. Tissue factor: molecular recognition and cofactor function. EMBO J. 1995;9:852–859. doi: 10.1096/fasebj.9.10.7615155. [DOI] [PubMed] [Google Scholar]
- Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977;20:841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR. Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol. 1997;27:530–537. doi: 10.1002/eji.1830270226. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413–3420. [PubMed] [Google Scholar]
- Drummond AE, Dyson M, Thean E, Groome NP, Robertson DM, Findlay JK. Temporal and hormonal regulation of inhibin protein and subunit mRNA expression by post-natal and immature rat ovaries. J Endocrinol. 2000;166:339–354. doi: 10.1677/joe.0.1660339. [DOI] [PubMed] [Google Scholar]
- Abe K, Shoji M, Chen J, Bierhaus A, Danave I, Micko C, Casper K, Dillehay DL, Nawroth PP, Rickles FR. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci USA. 1999;96:8663–8668. doi: 10.1073/pnas.96.15.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioncheck TF, Roy S, Vehar GA. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J Biol Chem. 1992;267:3561–3564. [PubMed] [Google Scholar]
- Car BD, Slauson DO, Dore M, Suyemoto MM. Endotoxin-mediated bovine alveolar macrophage procoagulant induction is dependent on protein kinase C activation. Inflammation. 1990;14:681–689. doi: 10.1007/BF00916371. [DOI] [PubMed] [Google Scholar]
- Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol. 1998;140:1241–1253. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen BB, Freskgard PO, Nielsen LS, Rao LV, Ezban M, Petersen LC. Factor VIIa-induced p44/42 mitogen-activated protein kinase activation requires the proteolytic activity of factor VIIa and is independent of the tissue factor cytoplasmic domain. J Biol Chem. 1999;274:21349–21354. doi: 10.1074/jbc.274.30.21349. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Sorensen BB, Slofstra SH, Van den Brande JH, Stam JC, van Bergen en Henegouwen PM, Richel DJ, Petersen LC, Peppelenbosch MP. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. J Biol Chem. 2002;277:27065–27072. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Cytokine networks in rheumatoid arthritis: implications for therapy. Agents Actions Suppl. 1995;47:37–51. doi: 10.1007/978-3-0348-7343-7_3. [DOI] [PubMed] [Google Scholar]
- Niki Y, Yamada H, Seki S, Kikuchi T, Takaishi H, Toyama Y, Fujikawa K, Tada N. Macrophage- and neutrophil-dominant arthritis in human IL-1 alpha transgenic mice. J Clin Invest. 2001;107:1127–1135. doi: 10.1172/JCI11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196:77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, Lopez-Otin C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997;40:1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- de Jonge E, Dekkers PE, Creasey AA, Hack CE, Paulson SK, Karim A, Kesecioglu J, Levi M, van Deventer SJ, van der Poll T. Tissue factor pathway inhibitor does not influence inflammatory pathways during human endotoxemia. J Infect Dis. 2001;183:1815–1818. doi: 10.1086/320723. [DOI] [PubMed] [Google Scholar]
- Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Busso N, Morard C, Salvi R, Peclat V, So A. Role of the tissue factor pathway in synovial inflammation. Arthritis Rheum. 2003;48:651–659. doi: 10.1002/art.10869. [DOI] [PubMed] [Google Scholar]