Abstract
Annexin A1 (ANXA1) protein expression was evaluated by Western blot in a series of 32 head and neck squamous cell carcinomas (HNSCCs) in a search for molecular alterations that could serve as useful diagnostic/prognostic markers. ANXA1 down-regulation was observed in 24 cases (75%) compared with patient-matched normal epithelium. In relation to clinicopathological variables, ANXA1 down-regulation was significantly associated with advanced T stages (P = 0.029), locoregional lymph node metastases (P = 0.038), advanced disease stage (P = 0.006), hypopharyngeal localization (P = 0.038), and poor histological differentiation (P = 0.005). ANXA1 expression was also analyzed by immunohistochemistry in paraffin-embedded sections from 22 of 32 HNSCCs and 8 premalignant lesions. All dysplastic tissues showed significantly reduced ANXA1 expression compared to a strong positive signal observed in adjacent normal epithelia (except basal and suprabasal cells). A close association was observed between ANXA1 expression and the histological grade in HNSCC. Well-differentiated tumors presented a positive ANXA1 signal in highly keratinized areas whereas moderately and poorly differentiated tumors exhibited very weak or negative staining. Our findings clearly identify ANXA1 as an effective differentiation marker for the histopathological grading of HNSCCs and for the detection of epithelial dysplasia.
Head and neck squamous cell carcinoma (HNSCC) is one of the most common types of tumors, afflicting 500,000 patients worldwide each year.1The survival rate for patients with HNSCC has remained unchanged in recent years despite advances in diagnosis and treatment. Our ability to prognosticate advanced cases of HNSCC is especially poor owing to variations in the biological behavior of the tumors and inadequacies of the present staging system. Novel markers that can distinguish differences in tumor condition and behavior are needed for the diagnosis and treatment of such cancers. The development of molecular profiling techniques for genomic and proteomic analysis has introduced a new approach to cancer research aimed at discovering differential gene and protein expression associated with cancer development and progression.
Annexins are a structurally related family of calcium- and phospholipid-binding proteins that have been implicated in a broad range of molecular and cellular processes,2 including the modulation of phospholipase A2 and kinase activities in signal transduction, the maintenance of cytoskeleton and extracellular matrix integrity, tissue growth and differentiation, inflammation, and blood coagulation. Human annexins and their cognate orthologues comprise the A subfamily of vertebrate annexins represented by 12 members, designated by the ANX symbol stem-suffixed with a subfamily classification A1 through A11 or A13.3 Annexin A1 (ANXA1) has long been considered a putative mediator of glucocorticoid immunosuppressive activity and a mouse gene knockout model appears to support this interpretation.4
The term annexinopathies has been used to define those human diseases in which abnormal levels and pleiotropic effects of annexins contribute to the pathogenesis,5 although annexins have yet to be directly implicated in the etiology of any genetic disease. Thus, overexpression of ANXA2 may contribute to the phenotype in a hemorrhagic form of acute promyelocytic leukemia, whereas underexpression of ANXA5 accompanies the anti-phospholipid syndrome and preeclampsia. The altered expression of annexins has also been associated with cell line transformation (http://genome-www.stanford.edu/nci60/),6 tumor progression,7 and metastasis,8,9 and interpreted to suggest either a homeostatic or possible tumor suppressive role for annexins. ANXA1 is overexpressed in breast cancer10 and hepatocellular carcinoma11 but markedly down-regulated in esophageal, prostate, and gastric carcinomas.6,8,12–15 ANXA2 is overexpressed in brain glial tumors16 and pancreatic carcinoma17 and down-regulated in prostate cancer.18 Possible tumor suppressor roles have been proposed for ANXA6 in melanoma19 and squamous cell carcinoma,20 for ANXA7 in melanoma,21 and for ANXA10 in hepatocellular carcinoma.22 ANXA4 has been associated with chemoresistance.23
The magnitude and specificity of annexin changes in various cancers underscores their potential value as molecular markers and their possible role in carcinogenesis itself. The above-cited studies identify ANXA1 as a promising candidate, but some contradictory findings in metastatic conditions and cultured cell lines require more direct analysis of primary tumor tissue to resolve the cellular conditions originating these changes. We therefore evaluated ANXA1 protein expression in a study set of invasive HNSCC tumors together with patient-matched normal epithelium and premalignant lesions from head and neck squamous epithelia using both Western blot and immunohistochemistry (IHC) analysis.
Materials and Methods
Tissue Specimens
Surgical tissue specimens from 32 patients with HNSCC who consecutively underwent resection of their tumors at the Hospital Central de Asturias were obtained for this prospective study, following institutional review board guidelines. Informed consent was obtained from each patient. None of them had received radio/chemotherapy before intervention. Biopsies were sharply excised, placed in sterile tubes, and frozen immediately in liquid nitrogen. Clinically normal adjacent mucosa was also collected. All tissue samples were stored at −80°C until analysis. A portion of the surgical tissue specimen was fixed in buffered formaldehyde, dehydrated in graded alcohol solutions, and embedded in paraffin for use in histological analysis and IHC studies.
The characteristics of the patients studied and the clinicopathological features of their tumors (site, pT stage, pN stage, disease stage, and histopathological grade) are shown in Table 1. The stage of disease was determined after the surgical resection of the tumor according to the current tumor-node-metastasis staging (TNM) system of the International Union Against Cancer. The histological grade was determined according to the degree of differentiation of the tumor (Broders’ classification). All patients were habitual tobacco and alcohol consumers.
Table 1.
Summary of Clinicopathological Parameters of HNSCC Patients and Their Tumors, with Corresponding ANXA1 Protein Expression Results as Number and Percentage of the Patient Population, with Statistical Assessment
| Feature | No. of patients | ANXA1 down-regulated (%) | P value* |
|---|---|---|---|
| Mean age at resection (median): | 59 (61) | ||
| Sex | |||
| Male | 31 (97%) | ||
| Female | 1 (3%) | ||
| Site | |||
| Oropharynx | 9 (28%) | 6 (67) | 0.038 |
| Supraglottic larynx | 12 (38%) | 10 (83) | |
| Glottic larynx | 4 (12%) | 1 (25) | |
| Hypopharynx | 7 (22%) | 7 (100) | |
| pT stage | |||
| T1–T2 | 6 (19%) | 2 (33) | 0.029 |
| T3 | 16 (50%) | 13 (81) | |
| T4 | 10 (31%) | 9 (90) | |
| pN stage | |||
| N0 | 13 (41%) | 7 (54) | 0.038† |
| N1–3 | 19 (59%) | 17 (89) | |
| Disease stage | |||
| I–II | 5 (16%) | 1 (20) | 0.006 |
| III | 8 (25%) | 6 (75) | |
| IV | 19 (59%) | 17 (89) | |
| Histopathologic grade | |||
| Well-differentiated | 16 (50%) | 8 (50) | 0.005 |
| Moderately-differentiated | 9 (28%) | 9 (100) | |
| Poorly-differentiated | 7 (22%) | 7 (100) | |
| Total | 32 | 24 (75) |
Chi-square test.
Fisher’s test.
Premalignant Lesions
Tissue was obtained from archival, paraffin-embedded blocks from the Hospital Central de Asturias. Representative sections from tissue were used for IHC study and the diagnosis was confirmed for each lesion by a pathologist (AHZ). The distribution of patients according to the histological nature of their lesions was: hyperplasia/hyperkeratosis (three cases), mild dysplasia (one case), moderate dysplasia (two cases) and severe dysplasia/carcinoma in situ (two cases).
Protein Extraction and Western Blotting
For protein extraction, freshly frozen tissue from each sample was microdissected by cryostat sectioning to ensure that it contained at least 75% epithelial tumor cells. Each sample was frozen and thawed three times and mechanically lysed in ice-cold lysis buffer containing 50 mmol/L HEPES pH 7.9, 250 mmol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 0.2% (v/v) Nonidet P-40, 10% glycerol plus a phosphatase- and protease-inhibitor mixture (25 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin).
Whole protein extract concentration of the supernatant was estimated by Bradford’s method using a protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein extract (100 μg per lane) were boiled in Laemmli sample buffer, separated on sodium dodecyl sulfate polyacrylamide gel (10%), and transferred to polyvinylidene difluoride membranes using a Semidry Trans Blot (Bio-Rad). Membranes were immunoblotted with mouse IgG anti-ANXA1 monoclonal antibody (1:4000 dilution) (Zymed Laboratories, San Francisco, CA). Anti-mouse IgG secondary antibodies were used at 1:5000 dilution. For protein load control, anti-β-actin mouse monoclonal antibody (Sigma-Aldrich, St. Louis, MO) was used. Anti-mouse IgG secondary antibody was used at 1:25,000 dilution. Both immunoreactive bands were visualized in one reaction by the enhanced chemiluminescence Western blotting analysis system (Amersham-Pharmacia-Biotech, Piscataway, NJ).
Immunohistochemical Study
The formalin-fixed, paraffin-embedded tissues were cut into 4-μm sections and dried on capillary-gap glass slides (ChemMate; BioTEK Solutions, Santa Barbara, CA). The sections were deparaffinized with standard xylene and hydrated through graded alcohols into water. Antigen retrieval was performed using proteinase K. Staining was done at room temperature on an automatic staining workstation (TechMate 1000, BioTEK Solutions) by using the Envision peroxidase mouse system (Envision Plus; DAKO, Carpinteria, CA). Slides were placed for 20 minutes in a 3% hydrogen peroxide blocking medium and then allowed to react with mouse IgG anti-annexin I monoclonal antibody (Transduction Laboratories, Lexington, KY) at 1:200 dilution for 30 minutes. Immunodetection was performed with the Envision system and diaminobenzidine chromogen as substrate (DAKO). Counterstaining with hematoxylin for 1 minute was the final step. After staining, the slides were dehydrated through graded alcohols and mounted with a coverslip using a standard medium. Appropriate positive controls were used (normal laryngeal epithelium). Negative controls with an omission of the antiserum from the primary incubation were also included. The slides were viewed randomly, without clinical data, by two of the authors.
Statistical Analysis
The molecular results data distributed among different clinical groups of tumors were tested for significance by the chi-square and Fisher’s exact tests with the help of the statistical software package SPSS (SPSS Inc., Chicago, IL). P < 0.05 values were considered statistically significant.
Results
Loss of ANXA1 Protein in HNSCC Tumors
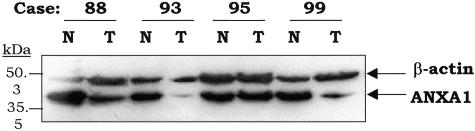
Immunoblot analysis of ANXA1 protein expression in patient-matched normal and tumor epithelium from 32 different patients with primary HNSCC was performed using a commercially available mouse monoclonal antibody against ANXA1. Complete or substantial loss of this 39-kd protein was observed in 24 cases (75%) of tumors examined, whereas strong expression of ANXA1 was detected in adjacent normal epithelium (Figure 1). Normalization for protein loading was achieved using anti-β-actin antibodies (Figure 1).
Figure 1.
Loss of ANXA1 protein expression in HNSCC determined by Western blotting. Whole protein extracts (100 μg) from tumor (T) and patient-matched normal (N) epithelia were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The blots were probed sequentially with monoclonal anti-ANXA1 antibody (1:4000 dilution) and anti-β-actin antibody (1:5000 dilution) and the immunoreactive bands were visualized in one enhanced chemiluminescence reaction.
Table 1 presents the association of ANXA1 down-regulation with clinicopathological parameters. Loss of ANXA1 was found at each primary head and neck anatomical site with statistically significant differences (P = 0.038). We found an association of normal ANXA1 expression with glottic laryngeal localization (three of four) whereas all hypopharynx tumors (seven of seven) showed a marked reduction of ANXA1 protein levels. Loss of ANXA1 correlated with advanced T and N stages, being more frequent in large tumors (90%) than in small HNSCCs (33%) (P = 0.029) and also in HNSCCs with locoregional lymph node metastasis (89%) above N0-stage tumors (54%), with differences attaining statistical significance (P = 0.038). Down-regulation of ANXA1 was significantly associated with patients in advanced disease stage, 89% in stage IV versus 20% in stage I and II (P = 0.006). With regard to histopathological grade, a relationship was observed between ANXA1 protein levels and histological differentiation. Normal ANXA1 expression was observed in well-differentiated tumors whereas ANXA1 expression was down-regulated in all moderate and poorly differentiated tumors (P = 0.005).
ANXA1 Expression Correlates with Histological Differentiation Grade in HNSCC
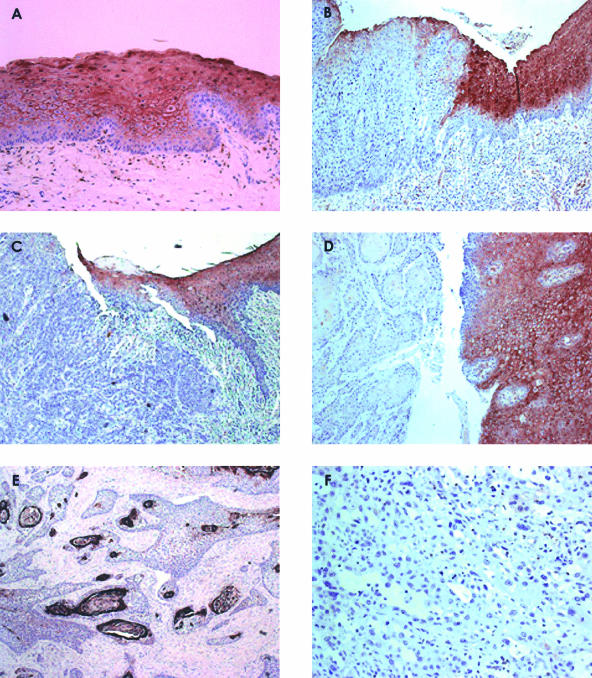
We next performed IHC analysis in 22 of the HNSCC specimens to confirm the observations from Western blot analysis and to localize the sites of ANXA1 expression. All sections selected for study contained both normal and malignant epithelia. ANXA1 expression was detected in all cell layers of normal epithelium, except basal and suprabasal cells, with strong positive signal toward the most external layers of epithelia (Figure 2A). ANXA1 staining was preferentially membrane-localized, although both nuclear and cytoplasmic staining were noted in the superficial layers. We also observed positive staining in both endothelial and infiltrated neutrophils, whereas stroma cells showed negligible ANXA1 expression. In contrast, cancer tissue specimens showed a markedly reduced ANXA1 expression, as compared with the corresponding normal epithelia (Figure 2, C and D). We detected an abnormal distribution of ANXA1 that correlated significantly with histopathological grade in HNSCC. We observed ANXA1 staining only in cancerous cells surrounding the horn pearl in the highly keratinized areas of well-differentiated HNSCC (Figure 2E), whereas tumor cells with poor differentiation exhibited negative staining (Figure 2F).
Figure 2.
Immunohistochemical analysis of ANXA1. A: All cell layers (except basal and suprabasal layers) were positively stained in normal epithelium. B: ANXA1 immunostain distinguished between normal and dysplastic epithelia. The left side shows markedly reduced ANXA1 expression in dysplastic compared to normal epithelium (top right). C and D: ANXA1 staining distinguished between normal epithelium and invasive HNSCC tumors. The left side shows absence of immunoreactivity in infiltrated tumor cells whereas normal epithelium exhibited strong ANXA1 signal (top and right sides). E: ANXA1 staining strongly correlated with histological differentiation grade in HNSCC. ANXA1 was irregularly expressed (as patches) in well-differentiated HNSCC. F: Poorly differentiated tumors were consistently negative for ANXA1 immunostaining. Original magnifications: ×200 (A–C); × 100 (D); ×50 (E); ×400 (F).
Loss of ANXA1 Expression in Early Stages of Tumorigenesis
IHC analysis was performed on a study set of eight premalignant lesions (distributed as described in Materials and Methods) to determine whether the loss of ANXA1 expression occurred early in the development of tumorigenesis in HNSCC. All selected sections included normal epithelia as internal control. A marked reduction of ANXA1 expression was detected as weak staining on the most superficial layers of dysplastic tissue, in marked contrast to the strong ANXA1 signal detected in corresponding normal epithelium (Figure 2B). ANXA1 staining was also negative in hyperplasia with hyperkeratosis of squamous epithelia.
Discussion
Much of the complex fundamental biology of HNSCC remains poorly understood, despite intensive study. Like other epithelial neoplasms, head and neck carcinogenesis appears to evolve through a multistep process involving biomolecular changes, ensuing premalignant lesions, and consequent invasive cancer.24 Thus, epithelial carcinogenesis has been divided into three phases of initiation, promotion and progression that involve genetic alteration, dysregulated epithelial differentiation, abnormal proliferation and altered regulatory effects associated with the abnormal expression of cellular factors that regulate growth and development. The identification of molecular alterations associated with these events could yield insight into the mechanisms of initiation and progression of neoplasia and provide new tools for diagnosis, treatment, and prevention. Annexins are commonly dysregulated in cancers6 and their frequent down-regulation has suggested a possible homeostatic or tumor suppressor role.7,20,25,26 We prioritized ANXA1 for follow-up analysis on the basis that this protein is normally well expressed in a wide range of organs and tissues, is specifically implicated in epithelial differentiation and growth regulation,27,28 and is markedly down-regulated in certain cancers7,8,26 including esophageal squamous cell carcinomas.12,14,15 ANXA1 is a pleiotropic 39-kd protein, member of the annexin superfamily, of principal interest as one of the mediators of the anti-inflammatory actions of glucocorticoids. Subsequent research has shown that the protein plays a major regulatory role in cell-growth regulation and differentiation, neutrophil migration, central nervous system responses to cytokines, neuroendocrine secretion, and mediation of apoptosis.28 ANXA1 has also been shown to be a substrate for epidermal growth factor receptor kinase, a recognized indicator of poor prognosis and reduced survival in head and neck cancer.
DNA and protein microarray studies highlight the changes in ANXA1 expression in various cancers and identify other genes with similar expression profiles6 that may help to elucidate regulatory changes relevant to both ANXA1 function and tumor classification. These include genes with possible accessory roles in microtubules (MAP1B), intercellular adhesion and communication (GJA1, ITGB1), signaling (CNGB, AMSH, NRG1), protease activity (PSMD1, CAPN2), and tissue differentiation (RTN4). The fact that ANXA1 is a downstream target of IL-629 could partially explain cytokine action in tumor biology.
Because the qualitative and quantitative changes may also depend on the state of tissue differentiation, metastatic condition, or in vitro cell culture,8,9 we felt it important to visually demonstrate ANXA1 tissue localization in primary tumors where differentiation status could be directly compared with normal tissue to demarcate the lesions. We established that ANXA1 was significantly reduced in primary HNSCC tumors as a whole, although a normal expression level was maintained in well-differentiated epithelia. Our results focus on a key question as to whether annexin changes are a mere consequence of changes in proliferation rate or metastatic propensity. There are conflicting reports that ANXA1 may be further down-regulated or up-regulated in metastatic cell lines.8,9 Results from the mouse gene knockout studies indicate that ANXA1-deficient cells exhibit a slower growth rate but, paradoxically, ANXA1 itself may have an anti-proliferative role in mediating some effects of glucocorticoids.28
Our results provide original evidence for the reduced expression of ANXA1 protein in HNSCC development. The loss of ANXA1 protein expression is an early event in the initiation of premalignant lesions in HNSCC, analogous to the observations by Paweletz and colleagues12 in esophageal tumors. The high frequency of ANXA1 down-regulation in HNSCC tumors (24 of 32), the early stage at which the protein is lost, and the fact that ANXA1 is altered in multiple tumors of diverse cellular lineage all suggest that ANXA1 could be fundamentally important in human tumorigenesis. Experiments are needed to establish the pathogenic role of ANXA1 in HNSCC, but annexins A1 and A2 have already been associated with tumor suppression and inhibition of cell migration in prostate cancer.30 The loss of ANXA1 expression in HNSCC is presumably a consequence rather than an etiological factor in that it correlates with the loss of epithelial differentiation and abnormal proliferation inherent in tumorigenesis. Our observation that reduced ANXA1 protein expression is closely associated with histological changes (ie, alterations in differentiation and proliferation) supports this notion. Even within the same tissue section, it is possible to distinguish between normal epithelium with strong positive ANXA1 signal and individual cells exhibiting phenotypic changes characteristic of head and neck tumorigenesis that show negative staining. In normal epithelia of head and neck, ANXA1 expression was detected only in differentiated and nonproliferating cells, with negative staining in the proliferative layers of epithelia (basal and suprabasal). We observed a dramatic loss of ANXA1 expression in dysplastic epithelia compared to normal mucosa, indicating the utility of this molecular test for the detection of epithelial dysplasia. The transition from normal epithelium to hyperplasia and dysplasia is associated with an increased growth fraction and cells proliferating beyond the basal layer, respectively, as detected using markers of cell proliferative activity, such as Ki-67 and proliferating cell nuclear antigen.31 Interestingly, the ANXA1 expression patterns observed in both normal and epithelial dysplasia are opposite to those described for Ki-67 and proliferating cell nuclear antigen markers whose expression correlates with severity of laryngeal lesions.31
In relation to histopathological features, we observed by Western blotting that ANXA1 down-regulation correlates with the biological aggressiveness of HNSCC. Thus, we found that the loss of ANXA1 is significantly more frequent in: 1) large tumors, with higher proliferative index; 2) hypopharyngeal tumors, hypopharynx being the most lethal site of HNSCC, whereas glottic tumors retain ANXA1 expression; 3) tumors with nodal metastases, one of the most important determinants of prognosis in head and neck cancer; and 4) poorly differentiated tumors that correspond to more aggressive tumors with higher growth rate and recurrence compared to well-differentiated tumors. However, the data obtained from IHC clearly indicated that ANXA1 expression is closely associated with epithelial differentiation status. This suggests that the association of ANXA1 down-regulation with severity parameters could be indirect, because some of these parameters are probably not entirely independent. For instance, the association between the retention of ANXA1 expression and glottic laryngeal localization could be influenced by the fact that glottic tumors are usually more differentiated and smaller because they are more likely to be discovered in early stages
On the other hand, some differences in the level of ANXA1 expression were detected depending on the technique used. Thus, we observed retention of ANXA1 expression in 50% of well-differentiated tumors by Western blot. We cannot rule out that these apparent differences could be because of differences in the sensitivity between Western and IHC, the use of whole protein extracts from the tissue samples in Western, or the presence of contaminating neutrophils.
The cause of reduced ANXA1 protein expression is not known. Possible mechanisms include genomic deletions, truncating mutations of the ANXA1 gene, hypermethylation of the promoter with the subsequent loss of transcription, alteration of one or more proteins that regulate ANXA1 transcription such as IL-6,29 or alterations in posttranslational processing of the protein by proteolysis or phosphorylation. Defects of intracellular transport or protein storage that lead to reduced intracellular levels of ANXA1 may also be responsible. DNA deletion on chromosomal arm 9q near the ANXA1 gene (9q21.13, 67.6 Mb) has been reported in several neoplasms, including esophageal32 and nasopharyngeal carcinomas,33 and although not as common in HNSCC,34,35 there are isolated reports of loss of heterozygosity in this region associated with dysplasia and invasiveness of HNSCC.36 The fact that cultured HNSCC cells maintain their expression of ANXA19 suggests that regulatory alterations may be the underlying cause. Promoter hypermethylation is a defect commonly observed in cancer and is responsible for reduced expression of multiple important regulatory genes.37 However, because ANXA1 does not contain CpG islands in the promoter region or coding sequence,29,38 methylation is unlikely to be a significant factor in the reduced ANXA1 protein expression in HNSCC. Follow-up studies to determine the molecular mechanism of ANXA1 down-regulation in HNSCC are warranted.
The early detection of premalignant mucosal lesions in the head and neck is an important aspect of patient management that has a major impact on overall survival rates. Because disease stage at the time of diagnosis is the most important prognostic factor in the treatment of HNSCC, the identification and early treatment of small cancers correlate with excellent survival statistics. The ability to identify clinically important therapeutic targets or biomarkers for early detection of cancer will ultimately rely on the ubiquity with which the protein of interest changes with respect to the population norm. Proteins with the best chances of clinical utility will be those proteins whose expression patterns consistently change, not only between different patients, but also within the patient-matched sets, will most likely reflect the most important candidates for additional investigation in large validation studies. The qualitative evaluation of biological features is an important aspect for predicting the clinical course of disease. Because the histological diagnosis of HNSCC has been based, until now, on the traditional examination of hematoxylin and eosin-stained specimens, supplementary techniques that use specific, disease-relevant markers could enable a more objective assessment of head and neck pathology. Our findings clearly identify ANXA1 as an effective differentiation marker, providing the first demonstration of the potential utility of ANXA1 immunostain for the histopathological grading of HNSCC and for the detection of epithelial dysplasia. The method of immunostaining is applicable to routine-fixed, paraffin-embedded tissues and may serve as a useful diagnostic tool when the pathogenic role of ANXA1 is better understood.
Acknowledgments
We thank Aurora Fernandez Garcia for technical assistance.
Footnotes
Address reprint requests to Juan Pablo Rodrigo, Servicio de Otorrinolaringologia, Hospital Central de Asturias, C/ Celestino Villamil s/n, 33006 Oviedo (Asturias) Spain. E-mail: jrodrigo@hcas.sespa.es.
Supported by Fondo de Investigacion Sanitaria (grant 00/0171 to J. P. R.), the Consejería de Educación y Cultura del Principado de Asturias (plan I+D+I 2001–2004 to M. V. G.), the Ministerio de Ciencia y Tecnologia (PB98-1529 and BMC2002-00827 to M. P. F. and R. O. M.), and Obra Social Caj Astur (to J. M. G. P. and M. V. G.).
References
- Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18:4779–4786. [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Morgan RO, Jenkins NA, Gilbert DJ, Copeland NG, Balsara BR, Testa JR, Fernandez MP. Novel human and mouse ANXA10 are linked to the genome duplications during early chordate evolution. Genomics. 1999;60:40–49. doi: 10.1006/geno.1999.5895. [DOI] [PubMed] [Google Scholar]
- Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. EMBO J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- Rand JH. The annexinopathies: a new category of diseases. Biochim Biophys Acta. 2000;1498:169–173. doi: 10.1016/s0167-4889(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA. Dysregulation of the annexin family protein family is associated with prostate cancer progression. Am J Pathol. 2003;162:255–261. doi: 10.1016/S0002-9440(10)63816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–895. [PubMed] [Google Scholar]
- Wu W, Tang X, Hu W, Lotan R, Hong WK, Mao L. Identification and validation of metastasis-associated proteins in head and neck cancer cell lines by two-dimensional electrophoresis and mass spectrometry. Clin Exp Metastasis. 2002;19:319–326. doi: 10.1023/a:1015515119300. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151–156. doi: 10.1023/a:1018452810915. [DOI] [PubMed] [Google Scholar]
- Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, Maeba T, Sogawa K, Konishi R, Taniguchi K, Hatanaka Y, Hatase O, Nishioka M. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24:72–81. doi: 10.1053/jhep.1996.v24.pm0008707286. [DOI] [PubMed] [Google Scholar]
- Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J, Wang QH, Hu N, Linehan WM, Taylor PR, Liotta LA, Emmert-Buck MR, Petricoin EF., III Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60:6293–6297. [PubMed] [Google Scholar]
- Kang JS, Calvo BF, Maygarden SJ, Caskey LS, Mohler JL, Ornstein DK. Dysregulation of annexin I protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Clin Cancer Res. 2002;8:117–123. [PubMed] [Google Scholar]
- Xia SH, Hu LP, Hu H, Ying WT, Xu X, Cai Y, Han YL, Chen BS, Wei F, Qian XH, Cai YY, Shen Y, Wu M, Wang MR. Three isoforms of annexin I are preferentially expressed in normal esophageal epithelia but down-regulated in esophageal squamous cell carcinomas. Oncogene. 2002;21:6641–6648. doi: 10.1038/sj.onc.1205818. [DOI] [PubMed] [Google Scholar]
- Zhou G, Li H, DeCamp D, Chen S, Shu H, Gong Y, Flaig M, Gillespie JW, Hu N, Taylor PR, Emmert-Buck MR, Liotta LA, Petricoin EF, III, Zhao Y. 2D differential in-gel electrophoresis for the identification of esophageal scans cell cancer-specific protein markers. Mol Cell Proteomics. 2002;1:117–124. doi: 10.1074/mcp.m100015-mcp200. [DOI] [PubMed] [Google Scholar]
- Roseman BJ, Bollen A, Hsu J, Lamborn K, Israel MA. Annexin II marks astrocytic brain tumors of high histologic grade. Oncol Res. 1994;6:561–567. [PubMed] [Google Scholar]
- Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM. Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14:2575–2579. doi: 10.1093/carcin/14.12.2575. [DOI] [PubMed] [Google Scholar]
- Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q. Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- Francia G, Mitchell SD, Moss SE, Hanby AM, Marshall JF, Hart IR. Identification by differential display of annexin-VI, a gene differentially expressed during melanoma progression. Cancer Res. 1996;56:3855–3858. [PubMed] [Google Scholar]
- Theobald J, Hanby A, Patel K, Moss SE. Annexin VI has tumour-suppressor activity in human A431 squamous epithelial carcinoma cells. Br J Cancer. 1995;71:786–788. doi: 10.1038/bjc.1995.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka TR, Ito A, Asada H, Watabe K, Nishiyama K, Nakamoto K, Itami S, Yoshikawa K, Ito M, Nojima H, Kitamura Y. Annexin VII as a novel marker for invasive phenotype of malignant melanoma. Jpn J Cancer Res. 2000;91:75–83. doi: 10.1111/j.1349-7006.2000.tb00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Lin CY, Peng SY, Jeng YM, Pan HW, Lai PL, Liu CL, Hsu HC. Down-regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am J Pathol. 2002;160:1831–1837. doi: 10.1016/S0002-9440(10)61129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EK, Tahir SK, Cherian SP, Collins N, Ng SC. Modulation of paclitaxel resistance by annexin IV in human cancer cell lines. Br J Cancer. 2000;83:83–88. doi: 10.1054/bjoc.2000.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- Srivastava M, Bubendorf L, Srikantan V, Fossom L, Nolan L, Glasman M, Leighton X, Fehrle W, Pittaluga S, Raffeld M, Koivisto P, Willi N, Gasser TC, Kononen J, Sauter G, Kallioniemi OP, Srivastava S, Pollard HB. ANX7, a candidate tumor suppressor gene for prostate cancer. Proc Natl Acad Sci USA. 2001;98:4575–4580. doi: 10.1073/pnas.071055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shridhar V, Sen A, Chien J, Staub J, Avula R, Kovats S, Lee J, Lillie J, Smith DI. Identification of underexpressed genes in early- and late-stage primary ovarian tumors by suppression subtraction hybridization. Cancer Res. 2002;62:262–270. [PubMed] [Google Scholar]
- Violette SM, King I, Browning JL, Pepinsky RB, Wallner BP, Sartorelli AC. Role of lipocortin I in the glucocorticoid induction of the terminal differentiation of a human squamous carcinoma. J Cell Physiol. 1990;142:70–77. doi: 10.1002/jcp.1041420110. [DOI] [PubMed] [Google Scholar]
- Croxtall JD, Gilroy DW, Solito E, Choudhury Q, Ward BJ, Buckingham JC, Flower RJ. Attenuation of glucocorticoid functions in an Anx-A1−/− cell line. Biochem J. 2003;371:927–935. doi: 10.1042/BJ20021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. Human annexin 1 is highly expressed during the differentiation of the epithelial cell line A 549: involvement of nuclear factor interleukin 6 in phorbol ester induction of annexin 1. Cell Growth Differ. 1998;9:327–336. [PubMed] [Google Scholar]
- Liu JW, Shen JJ, Tanzillo-Swarts A, Bhatia B, Maldonado CM, Person MD, Lau SS, Tang DG. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22:1475–1485. doi: 10.1038/sj.onc.1206196. [DOI] [PubMed] [Google Scholar]
- Krecicki T, Jelen M, Zalesska-Krecicka M, Rak J, Szkudlarek T, Jelen-Krzeszewska J. Epidermal growth factor receptor (EGFR), proliferating cell nuclear antigen (PCNA) and Ki-67 antigen in laryngeal epithelial lesions. Oral Oncol. 1999;35:180–186. doi: 10.1016/s1368-8375(98)00100-6. [DOI] [PubMed] [Google Scholar]
- Yen CC, Chen YJ, Chen JT, Hsia JY, Chen PM, Liu JH, Fan FS, Chiou TJ, Wang WS, Lin CH. Comparative genomic hybridization of esophageal squamous cell carcinoma: correlations between chromosomal aberrations and disease progression/prognosis. Cancer. 2001;92:2769–2777. doi: 10.1002/1097-0142(20011201)92:11<2769::aid-cncr10118>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fang Y, Guan X, Guo Y, Sham J, Deng M, Liang Q, Li H, Zhang H, Zhou H, Trent J. Analysis of genetic alterations in primary nasopharyngeal carcinoma by comparative genomic hybridization. Genes Chromosom Cancer. 2001;30:254–260. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1086>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Squire JA, Bayani J, Luk C, Unwin L, Tokunaga J, MacMillan C, Irish J, Brown D, Gullane P, Kamel-Reid S. Molecular cytogenetic analysis of head and neck squamous cell carcinoma: by comparative genomic hybridization, spectral karyotyping, and expression array analysis. Head Neck. 2002;24:874–887. doi: 10.1002/hed.10122. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yu GP, McCormick SA, Mo J, Datta B, Mahimkar M, Lazarus P, Schaffer AA, Desper R, Schantz SP. Genetic differences detected by comparative genomic hybridization in head and neck squamous cell carcinomas from different tumor sites: construction of oncogenetic trees for tumor progression. Genes Chromosom Cancer. 2002;34:224–233. doi: 10.1002/gcc.10062. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Dasgupta S, Roy A, Sengupta A, Roy B, Roychowdhury S, Panda CK. Sequential deletions in both arms of chromosome 9 are associated with the development of head and neck squamous cell carcinoma in Indian patients. J Exp Clin Cancer Res. 2003;22:289–297. [PubMed] [Google Scholar]
- Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- Morgan RO, Fernandez MP. A BC200-derived element and Z-DNA as structural markers in annexin I genes: relevance to Alu evolution and annexin tetrad formation. J Mol Evol. 1995;41:979–985. doi: 10.1007/BF00173179. [DOI] [PubMed] [Google Scholar]