Abstract
Mina53 is a novel Myc target gene that we previously demonstrated to be involved in cell proliferation. We studied, here, the expression of Mina53 in colon cancer to examine its possible role in carcinogenesis. We generated a specific monoclonal anti-human Mina53 antibody and found that colon tumor cell lines expressed Mina53 highly. We also found that expression of Mina53 was elevated in colon tumor tissues by immunoblotting analysis. Tissue sections of 23 surgical cases of adenocarcinoma and 1 case of adenoma were stained immunohistochemically, and the expression of Mina53 was found to be elevated in all of the adenocarcinomas compared to adjacent nonneoplastic tissues, which showed little staining. Deeply invading tumors as well as tumors that have invaded lymphatic vessels showed strong immunoreactivity against anti-Mina53 antibody. Mina53 was expressed in all pathological grades of cancer as well as in the adenoma. Staining patterns of Ki-67, a biomarker for cell proliferation, were similar to those of Mina53 in most cases, but the percentage of tumor cells stained by anti-Mina53 was higher. Although anti-Ki-67 antibody strongly stained some well-proliferating nonneoplastic cells including cells in the deeper part of the crypts and in lymphoid germinal centers, antibody to Mina53 rarely stained those cells. Suppression of mina53 expression severely suppressed proliferation of colon tumor cells in vitro. Together, our results indicate that the elevated expression of Mina53 is a characteristic feature in colon cancer, one that may have therapeutic applications.
Colon cancer is among the most frequent neoplasms in the world. Epithelial crypts of the colon are presumed to be the sites from which most neoplastic cells arise. It is currently thought that most colon cancers develop from pre-existing adenomas, although some may emerge de novo.1–4 Most colon cancers are known to progress through a series of gradual histological changes from premalignant and malignant stages to the metastatic state.1,5,6
Many investigations have been directed toward uncovering changes in the gene structure, gene expression, or the activity of gene products associated with colon cancer. Most studies have shown that loss of function of tumor suppressor genes as well as activation and abnormal expression of oncogenes are responsible for carcinogenesis. The members of the myc proto-oncogene family, c-, L-, and N-myc, are thought to be central regulators of cell growth, and deregulated expression of myc is associated with many cancers.7–10 C-myc is one of the well-studied oncogenes and its expression is associated with cell proliferation and is down-regulated in quiescent and differentiated cells. Studies have shown that c-myc is overexpressed in most human colon cancers,11,12 most of which harbor mutations in the tumor suppressor adenomatous polyposis coli (APC) gene.13–15 He and colleagues16 provided a molecular framework for understanding the previously enigmatic overexpression of c-myc in colon cancers by identifying c-myc as a target of the APC pathway. They demonstrated that c-myc is either induced by loss of function of the APC gene or suppressed by the functional APC gene product.
Despite intensive efforts to investigate the role(s) of c-myc in carcinogenesis, the mechanisms by which deregulation of c-myc gene expression contributes to carcinogenesis are still not fully resolved, and many aspects are still enigmatic.17 C-myc is a multifunctional gene, and its functions include cell division, cell growth, and apoptosis. C-myc appears to control the expression of several genes that mediate each of the above functions, some of which may contribute to carcinogenesis. Functional information about expression patterns of novel genes controlled by c-myc may therefore contribute to a better understanding of carcinogenesis induced by c-myc.
Recently we identified a novel gene, mina53, whose expression was demonstrated to be directly induced by c-myc.18 The mina53 gene encodes a 53-kd protein that is localized in the nucleus with part of the protein concentrated in the nucleolus. Specific inhibition of mina53 expression by the RNA interference (RNAi) method severely suppressed cell proliferation,18 suggesting that Mina53 may be implicated in carcinogenesis.
To address the question of whether Mina53 is expressed in human cancer and to evaluate its possible role in carcinogenesis, we generated a specific monoclonal antibody against human Mina53 protein and examined the expression of Mina53 in colon tumor cell lines and in surgically resected colon tumor tissues. Here we show that Mina53 is highly expressed in colon cancer and that Mina53 is involved in proliferation of colon tumor cells in vitro. Our results suggest that Mina53 may have a functional role in colon carcinogenesis and may be of use as a marker for colon cancer.
Materials and Methods
Anti-Mina53 Monoclonal Antibody
Recombinant human Mina53 was expressed in Escherichia coli and isolated as described previously.18 BALB/c mice were immunized via the sole with purified Mina53 emulsified in monophosphoryl-Lipid A + Trehalose dicorynomycolate adjuvant (Sigma-Aldrich Fine Chemicals, St. Louis, MO) and boosted after 2 weeks. Lymphocytes were isolated from lymph nodes of the hind limb and fused with mouse F01 myeloma cells using polyethylene glycol following the standard method. Cells were cultured along with cells from the thymus in 96-well plates in RPM1 1640 medium (Sigma) supplemented with 20% fetal calf serum, hypoxanthine/aminopterin/thymidine (ICN Biochemicals, Aurora, OH), and 5% Briclone hybridoma cloning medium (Archport, Dublin, Ireland). Approximately 2 weeks after fusion, the hybridoma culture media were screened for anti-Mina53 antibody activity using microtiter plates coated with recombinant Mina53. Cells in positive wells were cloned and culture media consistently positive after recloning were tested for specificity of the antibodies by Western blotting using HL60 and HeLa cell extracts. Hybridomas secreting specific antibodies were isotyped using a Zymed mouse MonoAB ID/SP kit (Zymed Laboratories, South San Francisco, CA) following the manufacturer’s instructions. One hybridoma secreting an IgG2a antibody, clone M532, was selected for this study. The antibody was produced as ascites fluid in prestane-preinjected mice and purified using a DE52 anion exchange resin (Whatman International, Kent, UK) after 50% ammonium sulfate fractionation. The IgG purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie brilliant blue staining.
Other Antibodies
Rabbit polyclonal anti-nucleolin (sc-13057) and anti-c-Myc (N-262) antibodies and goat anti-rabbit IgG-horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA), Alexa 488-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR), goat anti-mouse IgG-HRP and Cy3-conjugated anti-rabbit IgG (Zymed), anti-β-actin monoclonal antibody (AC-15) (Sigma), mouse monoclonal anti-Ki-67 (MIB-1) (DAKO, Glostrup, Denmark), and biotinylated rabbit anti-mouse IgG and biotinylated goat anti-rabbit IgG (Nichirei, Tokyo, Japan) were purchased.
Plasmids
cDNA for human mina53 was amplified by polymerase chain reaction with 5′-GCCCAAGCTTACCATGCCAAAGAAAGCAAAGCCTAC-3′, adding a HindIII site before the initiation methionine, and 5′-GCCCAAGCTTCTAGACTACTTGAATTAAACATTC-3′, adding a HindIII site after the stop codon as primers from pT/hmina53(465)18 The amplified 1.4-kb fragment was cloned into a pCAGGS mammalian expression vector (containing a chimeric promoter consisting of chicken actin and a CMV promoter)19 to produce pCAGGS/hmina53(465). pEGFP/hmina53(465) expressing green fluorescent protein (GFP)-Mina53 fusion protein was described previously.18
Cell Culture and Transfections
Two human colon tumor cell lines, SW620 and HT-29, and the human cervical carcinoma cell line HeLa were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. The human promyelocytic leukemia cell line HL60 was maintained in RPMI 1640 medium supplemented with 20% fetal calf serum. HL60 cells were cultured in the presence or absence of 10 nmol/L of phorbol 2-myristate 13-acetate (TPA) for 24 hours. HeLa cells were transfected with plasmids pCAGGS/hmina53(465) or pEGFP/hmina53(465) using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) to express Mina53 or GFP-Mina53 fusion protein, respectively. A small interference RNA molecule (siRNA) targeting human c-myc and a control siRNA (catalog no. 4604; Ambion Austin, TX) were purchased. The siRNA sequences targeting human mina53 and rat mina53 were described previously.18 The siRNA duplex specific for rat mina53 differed by five nucleotides of a 19-nucleotide sequence targeting human mina53 and has been shown not to affect mina53 expression in human HeLa cells.18 SW620 cells were transfected with siRNA basically as described previously.18 Briefly, 24 hours before transfection, cells were transferred to a 12-well plate coated with collagen type I (Asahi Technoglass, Chiba, Japan). Transfection was performed with 100 pmol of siRNA per well using OligofectAMINE (Invitrogen, Tokyo, Japan), according to their instruction, except that SW620 cells were cultured for 36 hours for transfection without serum. The number of cells was counted at specific time intervals.
Western Blotting and Indirect Immunofluorescence Staining
For Western blotting, cells were collected by treatment with trypsin and ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) and washed with PBS. Cells were then suspended in 0.125 of mol/L Tris-HCl buffer, pH 6.8, containing 3% sodium dodecyl sulfate, 50 mmol/L dithiothreitol, and 20% glycerol and boiled for 10 minutes before separation on a gradient sodium dodecyl sulfate-polyacrylamide gel (4 to 20%). Proteins were transferred to a polyvinylidene difluoride microporous membrane (Millipore, Bedford, MA), and nonspecific binding sites were blocked with 1% skim milk in PBS. After treatment with mouse monoclonal anti-Mina53 and HRP-conjugated goat anti-mouse IgG, signals were detected using an enhanced chemiluminescence Western blotting detection reagent system (Amersham Biosciences, Buckinghamshire, UK). The membrane was reprobed with a monoclonal anti-β-actin antibody, as described above, after treatment with a stripping buffer (Pierce, Rockford, IL). Some membranes were also treated with rabbit polyclonal anti-c-Myc and HRP-conjugated goat anti-rabbit IgG antibodies.
Human colon tumor specimens were surgically resected from four patients. Tissues were sliced into small pieces in PBS (10 μl per mg wet tissue). Solubilization buffer (0.125 mmol/L Tris-HCl, pH 6.8, 3% sodium dodecyl sulfate, 100 mmol/L dithiothreitol) was added (10 μl per mg wet tissue), boiled for 10 minutes, and centrifuged at 14,000 rpm for 10 minutes. The total protein concentration of the supernatant was determined using Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Five μg of total protein was electrophoresed and subjected to Western blotting with anti-Mina53, anti-c-Myc, or anti-β-actin antibodies as described above.
For indirect immunofluorescence staining, SW620 and HT-29 cells grown on glass coverslips were fixed in methanol for 10 minutes at −20°C. Mouse anti-Mina53 monoclonal and rabbit anti-nucleolin polyclonal antibodies were added and incubated for 120 minutes at 37°C. After three washes with 0.1% skim milk in PBS, Alexa 488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG were added, incubated for 120 minutes at 37°C, and washed three times with 0.1% skim milk in PBS. Finally, cells were embedded in Immunon (Thermo Shandon, Pittsburgh, PA) and observed with a fluorescence microscope.
Tissues for Immunostaining
Routinely processed formalin-fixed and paraffin-embedded specimens from 24 patients with primary colon neoplasia resected from 1988 to 1996 at the Department of Surgery, Kurume University Hospital, were used.20 The specimens included 23 cases of adenocarcinoma of the colon, in some of which tumor cells had invaded deeply into nonneoplastic tissues or into lymphatic vessels. There was also one case of adenoma. Tissue sections were classified after hematoxylin and eosin (H&E) staining according to the pathological grading system as well, moderately, and poorly differentiated. The characteristics of the tissues are outlined in Table 1.
Table 1.
Pathological Grade of Colon Tumors and Summary of Immunohistochemistry
| Patient | Grade | % Stained cancer cells
|
Staining intensity
|
Staining index
|
|||
|---|---|---|---|---|---|---|---|
| Mina53 | Ki-67 | Mina53 | Ki-67 | Mina53 | Ki-67 | ||
| 1 | Poor | 84.5 | 46.2 | 1 | 1 | 0.85 | 0.46 |
| 2 | Poor | 86.3 | 63.2 | 3 | 3 | 2.59 | 1.90 |
| 3 | Poor | 79.6 | 88.9 | 2 | 3 | 1.59 | 2.67 |
| * | 100.0 | 96.3 | 2 | 2 | 2.00 | 1.93 | |
| † | 0.0 | 31.9 | 0 | 2 | 0.00 | 0.64 | |
| 4 | Poor | 94.2 | 52.4 | 2 | 3 | 1.88 | 1.57 |
| 5 | Poor | 100.0 | 100 | 2 | 3 | 2.00 | 3.00 |
| 6 | Moderate | 94.3 | 81.6 | 2 | 2 | 1.89 | 1.63 |
| 7 | Moderate | 100.0 | 76.2 | 3 | 3 | 3.00 | 2.29 |
| 8 | Moderate | 97.5 | 87.4 | 2 | 3 | 1.95 | 2.62 |
| 9 | Moderate | 100.0 | 83.6 | 1 | 3 | 1.00 | 2.51 |
| 10 | Moderate | 92.4 | 75.0 | 2 | 2 | 1.85 | 1.50 |
| 11 | Moderate | 79.2 | 62.1 | 2 | 2 | 1.58 | 1.24 |
| 12 | Moderate | 89.0 | 54.2 | 1 | 2 | 0.89 | 1.08 |
| 13 | Moderate | 100.0 | 60.1 | 1 | 3 | 1.00 | 1.80 |
| 14 | Moderate | 95.5 | 84.8 | 3 | 3 | 2.87 | 2.54 |
| 15 | Well | 80.1 | 72.9 | 2 | 2 | 1.60 | 1.46 |
| 16 | Well | 100.0 | 63.5 | 2 | 2 | 2.00 | 1.27 |
| 17 | Well | 93.8 | 50.0 | 3 | 2 | 2.81 | 1.00 |
| 18 | Well | 100.0 | 100.0 | 3 | 3 | 3.00 | 3.00 |
| 19 | Well | 92.7 | 84.6 | 2 | 3 | 1.85 | 2.54 |
| 20 | Well | 78.7 | 74.0 | 2 | 3 | 1.57 | 2.22 |
| 21 | Well | 98.9 | 57.0 | 2 | 3 | 1.98 | 1.71 |
| 22 | Well | 92.7 | 43.1 | 3 | 3 | 2.78 | 1.29 |
| 23 | Well | 98.3 | 80.0 | 3 | 3 | 2.95 | 2.40 |
| 24 | Adenoma | 90.0 | 42.6 | 3 | 2 | 2.70 | 0.85 |
The area contained mucinous adenocarcinoma cells.
The cancer had a morphology similar to acinar cell carcinoma of the pancreas.
Immunostaining of Colon Tumor Tissues
Deparaffinized sections of 10% formalin-fixed, paraffin-embedded colon tumor tissues were immunostained by the streptavidin-biotin complex immunoperoxidase method.21 Sections mounted on slides were autoclaved for 20 minutes in 10 mmol/L of sodium citrate buffer, pH 6.0, for antigen retrieval. After pretreatment with 3% H2O2 in PBS and then with 1% skim milk and 5% rabbit serum in PBS, the primary antibody against Mina53 at a final concentration of 3.5 μg/ml in 1% skim milk or anti-Ki-67 antibody (used as recommended by the manufacturer) was reacted with tissues overnight at 4°C in a moist chamber. After three washes with 0.05% Tween 20 in PBS, sections were incubated sequentially with biotinylated rabbit anti-mouse IgG and then with HRP-streptavidin conjugate (Nichirei). Color was developed with 3,3-diaminobenzidine and H2O2 for 4 (Mina53) or 2 (Ki-67) minutes and then a water rinse was used to stop the reaction. After light counterstaining with hematoxylin, the slides were dehydrated, coverslipped, and observed with an Olympus AX80 microscope (Olympus Optical, Tokyo, Japan). A few sections were also stained with rabbit polyclonal anti-c-Myc antibody as described above.
Evaluation of Immunohistochemical Staining
Each section was scored on a scale from 0 to 3 by visual observation. The highest staining intensity was scored as 3, the lowest as 1, and no staining at all as 0. For estimation of the percentage of stained cells, images were captured with an Olympus digital camera (Olympus), processed with Photoshop, and printed out. The number of positive cells within representative fields was counted and expressed as the percentage of cells stained. The staining index was calculated as staining intensity multiplied by the average percentage of cells stained.
Results
Anti-Human Mina53 Monoclonal Antibody
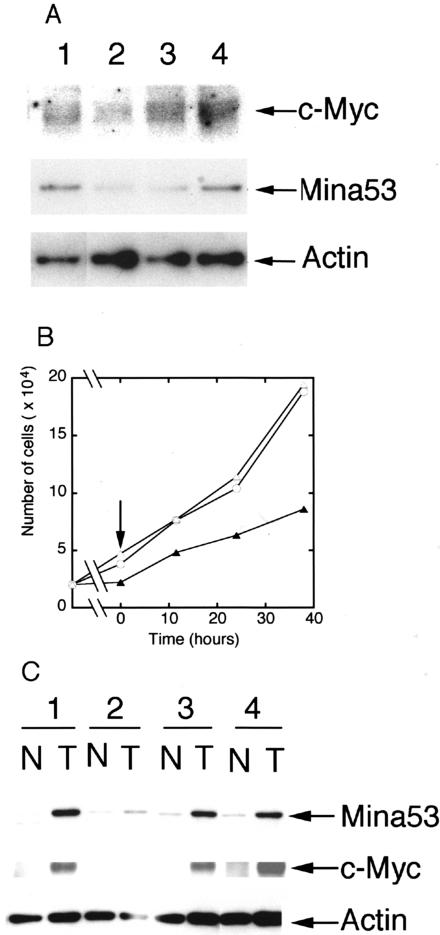
We generated a monoclonal antibody against recombinant human Mina53 protein. The initial screening assays by enzyme-linked immunosorbent assay resulted in a panel of monoclonal antibodies. One of the monoclonal antibodies, M532, recognized a single band with a molecular mass of 53 kd by Western blotting in the human cervical carcinoma cell line HeLa (Figure 1A, lane 1). M532 also recognized increased expression of Mina53 in HeLa cells transfected with a mammalian expression vector harboring mina53 cDNA (Figure 1A, lane 2). When Mina53 fused to green fluorescent protein (GFP) was expressed in HeLa cells, Western blotting analysis using M532 monoclonal antibody yielded a band with an expected molecular mass of 80 kd in addition to the endogenous Mina53 (Figure 1A, lane 3). In this experiment β-actin expression did not differ between lanes (Figure 1A, lanes 1 to 3, bottom), which confirmed that similar amounts of total protein were electrophoresed.
Figure 1.
Western blot analysis and subcellular localization of Mina53 protein using anti-Mina53 M532 monoclonal antibody. A: Western blot analysis for Mina53 in HeLa cells. Cell lysates were prepared from HeLa cells transfected with an empty vector pCAGGS (lane 1), plasmids expressing Mina53 (pCAGGS/hmina53, lane 2), or Mina53 fused to green fluorescent protein (GFP) (pEGFP/hmina53, lane 3), electrophoresed, and immunoblotted with anti-Mina53 M532 monoclonal antibody (top), or with anti-β-actin monoclonal antibody as a loading control (bottom), which confirmed that similar amounts of total protein were electrophoresed. B: Western blot analysis for Mina53 in HL60 cells and colon tumor cells. Cell lysates were prepared from HL60 cells cultured in the absence (lane 1) or presence (lane 2) of 10 nmol/L of TPA and from colon cancer cell lines HT-29 (lane 3) and SW620 (lane 4), electrophoresed, and immunoblotted with anti-Mina53 M532 monoclonal antibody (top) or with anti-β-actin monoclonal antibody as a loading control (bottom). C: The subcellular localization of Mina53 in colon tumor cells. The localization of Mina53 in colon tumor cell line SW620 was visualized by indirect immunofluorescence staining with anti-Mina53 M532 monoclonal antibody (a). Cells were also stained with rabbit polyclonal anti-nucleolin antibody (b). An overlapped image is also shown (c).
M532 also recognized a single band with a molecular mass of 53 kd by Western blotting in human promyelocytic leukemia cell line HL60 (Figure 1B, lane 1). HL60 cells are terminally differentiated by treatment with TPA in which the c-myc expression level is reduced.22,23 We showed previously that the expression of mina53 is reduced after treatment of HL60 cells with TPA using anti-Mina53 polyclonal antibody.18 Immunoblotting analysis using the M532 monoclonal antibody showed that treatment of cells with TPA reduced the signal of the 53-kd band (Figure 1B, lane 2). The specificity of the reduction was confirmed because TPA treatment did not significantly reduce β-actin expression (Figure 1A, lanes 1 and 2). These results indicate that the monoclonal antibody M532 recognizes specifically Mina53 protein.
Expression of Mina53 in Colon Tumor Cell Lines
The expression of Mina53 was examined in two colon tumor cell lines, SW620 and HT-29. Cell lysates were prepared from the cells in the proliferating phase and analyzed by immunoblotting using M532 antibody. The antibody recognized a single band of 53 kd in the two cell lines (Figure 1B, lanes 3 and 4). The results indicate that these cell lines express Mina53 and that antibody M532 specifically recognizes Mina53 protein in colon tumor cell lines with no cross-reactivity with other proteins. The expression level of Mina53 in these cell lines is much higher than that of Mina53 in HL60 cells experimentally reduced by TPA (Figure 1B, lanes 2 to 4). The levels of actin in the two colon tumor cell lines were not higher than that of HL60 cells treated with TPA (Figure 1B, lanes 2 to 4). These results suggest that the two colon tumor cell lines contain a higher level of Mina53 protein than terminally differentiated HL60 cells.
We previously demonstrated that Mina53 is localized in the nucleus and also concentrated in the nucleolus in HeLa cells.18 To investigate the localization of Mina53 in colon tumor cells, we performed double-immunofluorescence staining of cells using monoclonal antibody M532 and anti-nucleolin rabbit antibody. As shown in Figure 1C, M532 antibody stained specifically nuclei in SW620 cells with strong dotted staining in nucleoli that overlapped with the signals for nucleolin. The other cell line HT-29 showed a similar pattern of immunofluorescence staining (not shown). These results indicate that Mina53 locates in the nucleus with concentrated amounts in the nucleolus in the colon tumor cell lines, as we previously demonstrated in HeLa cells.18
Mina53 Is Involved in Proliferation of Colon Tumor Cells
To gain insight into the role of Mina53 in colon tumor cells, we specifically suppressed the expression of Mina53 in a colon tumor cell line SW620 by a specific siRNA for human mina53 (Figure 2A, lane 3). As shown in Figure 2B, reduction of Mina53 expression severely suppressed proliferation of SW620 cells. Treatment of cells with a nonspecific siRNA duplex and a specific siRNA for rat mina53 neither reduced expression of Mina53 nor suppressed proliferation of SW620 cells [Figure 2, A (lanes 1 and 4) and B]. The siRNA duplex specific for rat mina53 differed by five nucleotides of a 19-nucleotide sequence targeting human mina53 and had been shown to affect mina53 expression in rat cells but not in human HeLa cells.18 These results suggest that Mina53 is involved in proliferation of colon tumor cells.
Figure 2.
Correlations between c-myc, mina53, and cell proliferation. A: Effect of siRNA duplexes on expression of c-Myc and Mina53 in a colon tumor cell line SW620. Cell lysates were prepared from SW620 cells 24 hours after transfection with nonspecific siRNA duplex (lane 1), siRNA duplexes specific for c-myc (lane 2), human mina53 (lane 3), and rat mina53 (used as a negative control, lane 4), electrophoresed, and immunoblotted with anti-c-Myc (top) or M532 anti-Mina53 (middle) antibodies. The blotting membrane detecting Mina53 was reprobed with anti-β-actin antibody (bottom). B: Effect of siRNAs on proliferation of SW620 cells. SW620 cells (2 × 104) were transfected with siRNA duplexes specific for human mina53 (filled triangle), rat mina53 (used as a negative control, open triangle), and control siRNA duplex (open circle). Thirty-six hours later, serum was added (arrow), and numbers of cells were counted at the indicated time intervals after adding serum and expressed on the y axis. C: Expression of Mina53 and c-Myc in colon tissues. Total protein was extracted from paired nonneoplastic (N) and tumor (T) colon tissues from four patients, electrophoresed, and immunoblotted with M532 anti-Mina53 (top) or anti-c-Myc (middle) antibodies. The blotting membrane detecting Mina53 was reprobed with anti-β-actin antibody (bottom).
c-Myc Controls the Expression of Mina53 in Colon Tumor Cells
We previously demonstrated that mina53 is a direct Myc target gene.18 To examine the regulation of mina53 expression by c-Myc in colon tumor cells, the colon tumor cell line SW620 was treated with a specific siRNA for c-myc to suppress the expression of c-Myc. Specific suppression of c-Myc expression resulted in reduction of Mina53 expression (Figure 2A, lane 2). Treatment of cells with nonspecific control siRNAs reduced neither c-Myc nor Mina53 expression (Figure 2A, lanes 1 and 4). These results indicate that the expression of mina53 is regulated by c-Myc in colon tumor cells.
Elevated Expression of Mina53 in Human Colon Tumor Tissues Detected by Western Blotting Analysis
Tumor and adjacent nonneoplastic tissues derived from surgical specimens from four patients were analyzed by Western blotting for Mina53 and c-Myc proteins. In three cases, expression of Mina53 was clearly elevated in tumor tissues as compared to their nonneoplastic counterparts (Figure 2C, cases 1, 3, and 4). In these cases, expression of c-Myc was also clearly elevated in the tumor tissues as compared to their nonneoplastic counterparts. In one case in which c-Myc expression was hardly detected in either tumor or nonneoplastic tissues, the expression of Mina53 was only slightly elevated in the tumor tissue as compared to the nonneoplastic tissue (Figure 2C, case 2). The results showed a positive correlation between Mina53 and c-Myc levels in colon cancer. In all cases, the levels of β-actin were not significantly different. These results indicate that Mina53 expression is elevated in colon cancer and closely related to c-Myc expression, which is consistent with our conclusion that Mina53 is a Myc target gene.
Elevated Expression of Mina53 in Colon Tumor Tissues Detected by Immunohistochemical Analysis
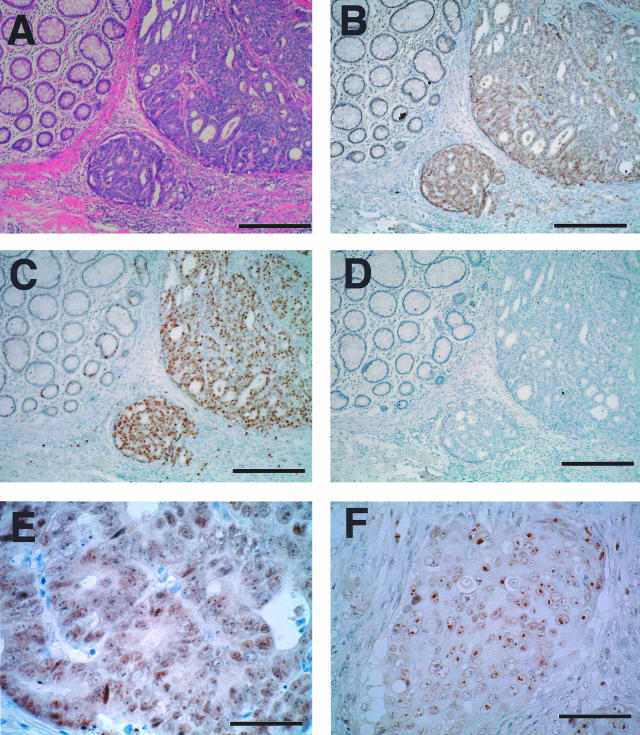
Monoclonal antibody M532 was used to detect Mina53 protein immunohistochemically in colon tumor tissues. H&E staining was used to demarcate the tumor areas. The section shown in Figure 3A contained moderately differentiated adenocarcinoma. Figure 3B shows marked staining for Mina53 in tumor areas, whereas most nonneoplastic epithelial cells around the tumors showed little staining. Staining is found mainly in nuclei (Figure 3, E and F) with dotted staining in nucleoli, a pattern similar to those obtained from the colon tumor cell line (Figure 1C). There was a lack of specific nuclear staining when the first antibody was omitted (Figure 3D) or when the section was incubated with the primary antibody in the presence of an excess amount of recombinant Mina53 protein (not shown).
Figure 3.
Immunohistochemical staining of Mina53 and Ki-67 proteins in colon tumor tissues. A: H&E staining of a section that contained a moderately differentiated adenocarcinoma. B: Serial section of A stained by anti-Mina53 antibody showing elevated expression of Mina53 in the tumor area but little staining in the adjacent nonneoplastic tissue. C: Serial section of A stained by anti-Ki-67 antibody. D: Control section of A in which the primary antibody was omitted. E: A high-power field of the section shown in B showed the characteristic nuclear localization of Mina53. F: Mina53 staining of a poorly differentiated adenocarcinoma. Scale bars: 300 μm (A–D); 50 μm (E); 75 μm (F).
In some sections of poorly and moderately differentiated adenocarcinoma tissues, tumor cells were found in lymphatic vessels just beneath the epithelium located away from the main neoplastic area. M532 antibody markedly stained these tumor cells, whereas the surrounding nonneoplastic cells were only weakly stained (Figure 4A). In addition, tumor cells that had penetrated into lymphatic vessels in deeper layers of the colon also showed strong immunoreactivity to Mina53 antibody, whereas surrounding nonneoplastic cells were not stained (Figure 4B). M532 antibody also stained deeply invading tumors (Figure 4C) and isolated poorly differentiated adenocarcinoma cells in fibrous stroma (Figure 4D), whereas nonneoplastic cells surrounding these tumor cells were not stained. These results suggest that Mina53 is highly expressed in tumors with invasive and metastatic potential.
Figure 4.
Immunohistochemical staining of Mina53 in tumors with invasive and/or metastatic potential, and comparison between Mina53, c-Myc, and Ki-67 staining patterns. A: Tumor cells that have penetrated into lymphatic vessels beneath the epithelium (arrows) were stained by anti-Mina53 M532 antibody. B: Tumor cells in lymphatic vessels in deeper layers of the colon were stained by anti-Mina53 antibody. C: Mina53 staining of a deeply invading tumor. D: Isolated tumor cells in fibrous stroma stained by anti-Mina53 antibody showing preferential expression of Mina53 in tumor cells as compared to nonneoplastic cells. E: H&E staining of a section that contained a tumor (arrow) in the vicinity of a germinal center (arrowheads). F: Serial section of E stained by M532 antibody showing expression of Mina53 in the tumor with little staining in the germinal center. G: Serial section of E stained by anti-Ki-67 antibody showing expression of Ki-67 in both tumor and germinal center. H: Serial section of E stained by anti-c-Myc antibody showing expression of c-Myc in the tumor with little staining in the germinal center. Scale bars: 75 μm (A, D); 150 μm (B); 300 μm (C, E–H).
Serial sections were stained with M532 and anti-c-Myc antibodies. The tissue section used here contained a tumor in the vicinity of a lymphatic follicle containing a lymphoid germinal center as revealed by H&E staining (Figure 4E). Figure 4, F and H, shows expression of Mina53 and c-Myc, respectively, in the tumor, but not in nonneoplastic cells surrounding these tumor cells. The germinal center showed little staining by both anti-Mina53 M532 and anti c-Myc antibodies, although the germinal center is known to contain many well-proliferating cells. Ki-67, a biomarker for cell proliferation, was highly expressed in the germinal center (Figure 4G), confirming that the germinal center contains many proliferating cells. These results showed that Mina53 staining patterns correlate well with those of c-Myc in colon tumor tissues.
Mina53 Expression by Pathological Grade of Colon Cancer
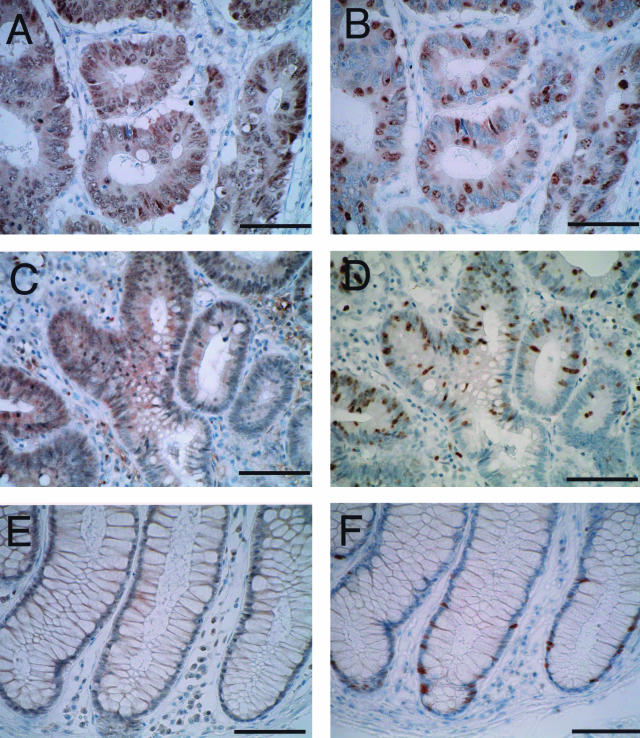
Mina53 was detected in specimens from all 23 patients with colon adenocarcinoma and 1 patient with adenoma. A summary of the immunohistochemical staining is presented in Table 1. Mina53 was expressed in all pathological grades of colon cancer. Well (Figure 5A), moderately (Figure 3B), and poorly (Figure 3F) differentiated adenocarcinomas were all markedly stained for Mina53 with average staining indexes of 2.28, 1.78, and 1.85, respectively (Table 1). These results suggest that there was no obvious correlation between the pathological grade of cancer and staining intensity, although there was a slight tendency for well-differentiated adenocarcinomas to show higher staining intensity than poorly and moderately differentiated ones (Table 1). In addition, Mina53 was highly expressed in the adenoma (Figure 5C), suggesting that the elevated expression of Mina53 is a general and relatively early event in colon carcinogenesis. As described above, staining was confined to the nucleus in all cases (Figure 3, E and F).
Figure 5.
Comparison of staining patterns between Mina53 and Ki-67 in well-differentiated adenocarcinoma, adenomatous, and nonneoplastic colon epithelium. Serial sections were stained by anti-Mina53 (left) or anti-Ki-67 (right) antibody. A and B: Staining of a well-differentiated adenocarcinoma for Mina53 (A) or Ki-67 (B). C and D: Staining of adenoma for Mina53 (C) or Ki-67 (D). E and F: Staining of epithelial cells in normal crypts for Mina53 (E) or Ki-67 (F). Scale bars, 75 μm (A–F).
Comparison of Staining Patterns between Mina53 and Ki-67 in Colon Tumor Tissues
Serial sections were also stained with anti-Ki-67 monoclonal antibody (MIB 1) to compare the staining pattern with that of Mina53. As reported before,24–26 anti-Ki-67 monoclonal antibody stained colon tumor tissues intensely (Figures 3C, 4G, and 5B). In most cases both antibodies against Ki-67 and Mina53 stained tumor cells similarly, as shown in Figure 3 (B and C), Figure 4 (F and G), Figure 5 (A and B), and Table 1.
When the percentages of tumor cells in a microscopic field stained for both Ki-67 and Mina53 were compared, differences were found between the two antibodies. In the majority of the cases, the percentage of Mina53-expressing cells was higher than that of Ki-67 (Figure 5, A and B). In a few cases, the percentage of Mina53-expressing cells was similar to that of Ki-67 (Table 1).
In one case (patient 3), the staining pattern was rather complex. Sections contained three different types of cancer. Two areas with poorly differentiated adenocarcinoma cells and mucinous adenocarcinoma cells showed similarly high percentages of positive cells for both Mina53 and Ki-67 (Table 1). In the third area, however, the tumor had a morphology similar to acinar cell carcinoma of the pancreas and was very weakly stained by Mina53 antibody but was strongly stained by Ki-67 antibody, although the percentage of positive cells was low as compared to the former two areas (Table 1). In the case of the adenoma (patient 24), almost all cells in the neoplastic areas were intensely stained by M532 antibody, whereas fewer cells were stained by anti-Ki-67 (Figure 5, C and D). Although there was one exception (patient 3), the results described above suggest that the percentage of Mina53-expressing cells is characteristically higher than that of Ki-67-expressing cells.
Comparison between Staining Patterns of Mina53 and Ki-67 in Nonneoplastic Colon Tissues
In some areas of nonneoplastic colon tissues, antibody against Ki-67 stained cells intensely, whereas M532 antibody stained those cells only weakly. Ki-67 staining was observed in nuclei of cells in the lower third of the crypts, the zone of active cell proliferation (Figure 5F). The staining intensity in nonneoplastic crypts did not differ significantly from cells found in the tumor areas (Figure 5, B and F). On the other hand, M532 antibody only faintly stained nuclei of these cells, and the intensity was far weaker than cells found in the tumor areas (Figure 5, A and E).
A lymphoid germinal center, which contains cells with high mitotic activity, was intensely stained by Ki-67 antibody (Figure 4G), but was rarely stained by M532 antibody (Figure 4F) as described before. These results suggest that Ki-67 is generally expressed in proliferating cells, whereas Mina53 is not always expressed in all proliferating cells.
Discussion
We previously isolated mina53 as a Myc target gene and showed a clear relationship between Mina53 expression and cell proliferation,18 which led us to suspect that Mina53 may be involved in the abnormal cellular growth observed in neoplastic diseases. In this study, we generated a monoclonal antibody against human Mina53 to study the expression of Mina53 in colon cancer. Western blotting analyses showed that the anti-Mina53 monoclonal antibody M532 specifically recognizes Mina53 protein.
We used antibody M532 to study the expression of Mina53 in colon tumor cell lines and colon tumor tissues. The two colon tumor cell lines showed prominent expression of Mina53. Our results showed that almost all colon tumor tissues examined exhibited elevated expression of Mina53. Mina53 was expressed in colon cancer of all pathological grades, well, moderately, and poorly differentiated, while most nonneoplastic cells showed little or no staining for Mina53. The elevated expression of Mina53 in tumor cells was observed irrespective of the location of the cells; that is, tumor cells in primary neoplasia, invading tumors, and tumors in lymphatic vessels as well as isolated tumor cells were prominently stained for Mina53 protein. We also observed that a higher percentage of tumor cells expressed Mina53 than Ki-67, a frequently used cell proliferation marker. Antibody to Ki-67 strongly stained tumor cells as well as some well-proliferating normal cells, including cells in the lower crypts and lymphoid germinal centers. On the other hand, antibody to Mina53 rarely stained cells in nonneoplastic areas. These results indicate that high expression of Mina53 can be regarded as a characteristic feature of colon cancer. Thus, the expression of Mina53 can be used as a marker for colon cancer and may be helpful in finding tumor cells that have invaded normal tissues. In addition, Mina53 staining may also serve as an additional prognostic marker. For example, detection of Mina53 staining in cells of lymph vessels may allow the selection of patients for systemic therapies.
In addition to the elevated expression of Mina53 in all pathological grades of colon cancer, the elevated expression of Mina53 was also observed in the adenoma. These results suggest that the elevated expression of Mina53 is a general event that occurs early in colon carcinogenesis and continues during the tumor progression. Thus, the high expression of Mina53 in colon cancer may be because of molecular impairment that occurs as an early event during carcinogenesis. With this assumption, impairment of the Wnt/APC/β-catenin pathway appears to be a good candidate. Somatic mutations in the APC gene were found in more than 60% of colon cancers and were suggested to be early events in colon carcinogenesis.14,15 The main function of APC protein is thought to be the regulation of free β-catenin in concert with glycogen synthase kinase-3β (GSK-3β) and axin proteins.27 Mutations of β-catenin were also found in some colon tumors lacking APC mutations.28,29 Loss of APC function, inactivation of axin,30 or activating β-catenin mutations31 results in cellular accumulation of β-catenin, which, when translocated to the nucleus, serves as an activator of T-cell factor (Tcf)-dependent transcription leading to increased expression of several specific target genes.32 He and colleagues16 identified c-myc as a target of the APC pathway and demonstrated that c-myc is induced by loss of function of the APC gene or by overexpression of β-catenin. Therefore, inactivating mutations of APC or axin or activating mutations of β-catenin would result in overexpression of c-myc. Mina53 staining patterns correlate well with those of c-Myc in colon tumor tissues. Suppression of c-myc expression by the specific siRNA in colon tumor cells reduced expression of mina53. These results suggest that c-Myc induced expression of Mina53 in colon tumor cells. Thus, overexpression of c-myc would subsequently result in the elevated expression of Mina53. It seems therefore that mina53 is a downstream, indirect target of the APC/β-catenin pathway, which has been implicated in colon carcinogenesis.
The expression pattern of Mina53 was compared with that of Ki-67, a widely used biomarker of cell proliferation. The percentage of Mina53-expressing cells was characteristically higher than that of Ki-67-expressing cells in most tumor tissues (Table 1). This may be because of the fact that mina53 is a Myc target gene. C-myc is expressed continuously in all phases of the cell cycle in proliferating cells,33,34 whereas Ki-67 is preferentially expressed in proliferating cells in late G1, S, M, and G2 phases.35,36 Because Mina53 is induced by c-Myc, it is reasonable that Mina53 is more widely expressed than Ki-67 in tumor tissues.
Cells in the crypts, which have been shown to grow well, were intensely stained by anti-Ki-67 antibody, but only weakly by anti-Mina53 antibody. Lymphoid germinal centers that contain nonneoplastic but proliferating cells were weakly stained by Mina53 antibody but strongly stained by Ki-67 antibody. Our previous18 and present studies show that Mina53 is an important factor for cell proliferation in cultured cell lines, human cervical carcinoma HeLa cells, a rat fibroblast cell, and colon cancer cells, all of which highly express c-myc. Thus, it is possible that Mina53 may play a role in cell proliferation only in some restricted types of cells, for example in cells with high expression of c-Myc. Alternatively, normal cells in the crypts and lymphoid germinal centers may require smaller amounts of Mina53 for cell proliferation or these cells may express a protein with a similar function as Mina53 protein. Either way, because tumor cells were intensely stained by anti-Mina53 antibody as compared to nonneoplastic cells in vivo, Mina53 may have some functions in carcinogenesis.
We also observed a lack of expression of c-Myc in lymphoid germinal centers, the staining pattern being similar to that observed for Mina53, which is consistent with our conclusion that mina53 is a Myc target gene. These observations, although surprising because c-Myc is known to be involved in cell proliferation, were similar to the results obtained recently by Klein and colleagues37 who demonstrated a lack of c-Myc expression in tonsillar germinal centers. They reported that expression of the other Myc family members, such as N-Myc or L-Myc, did not appear to compensate for the absence of c-Myc in germinal center B cells, because these cells also lacked expression of mRNA for these genes. Our observation that Myc target gene mina53 was poorly expressed in lymphoid germinal centers suggests that there is little Myc activity there, and is consistent with the observations of Klein and colleagues37 that N-Myc and L-Myc in addition to c-Myc were not expressed in cells in lymphoid germinal centers.
Another important issue that arises from this study is a treatment strategy for patients with colon cancers. Pharmacological strategies that target inactivation of oncogenes for the treatment of cancer are in development because activation of oncogenes has been shown to be associated with carcinogenesis. However, long-term use of medicines that are designed to inactivate oncogenes would be expected to produce serious toxicities because they also disrupt critical signal pathways in normal cells.38 The myc family oncogenes are thought to be central regulators of cell growth, and studies have shown that they are critical for normal biological processes, including organ development and regeneration.7–9,39,40 C-myc controls the expression of various genes, and specific Myc target genes are thought to be the actual players in each Myc function. This therefore calls for a thorough study of novel Myc target genes. Knowledge of the functions of these genes may help to develop medicines that are designed to inactivate specifically the functions involved in carcinogenesis with little effect on normal physiological processes. Although the specific role of Mina53 protein is not yet fully characterized, a specific siRNA duplex for human mina53 severely suppressed proliferation of colon cancer cells, suggesting that mina53 is a candidate target gene for colon cancer therapy.
In conclusion, we generated a specific anti-human Mina53 monoclonal antibody that will aid further studies on this novel Myc target gene. The antibody demonstrated increased expression of Mina53 in colon tumors as compared to nonneoplastic colon tissues. Our results suggest that the expression of Mina53 is an early event and a characteristic feature in colon cancer and that Mina53 may therefore play some role in colon carcinogenesis. We suggest that Mina53 can be used as a marker for colon cancer and that Mina53 may be exploited as a target for treatment of colon cancer.
Acknowledgments
We thank Dr. Munehiko Yamamoto, Department of Chemistry, Kurume University School of Medicine, for fruitful advice on producing monoclonal antibodies; Mr. Shigeo Kamimura for preparing serial sections of tissues; and Miss Yasuko Noguchi for her technical assistance.
Footnotes
Address reprint requests to Makoto Tsuneoka, Ph.D., Department of Forensic Medicine, Kurume University School of Medicine, Kurume 830-0011, Japan. E-mail: tsuneoka@med.kurume-u.ac.jp.
Supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan; and from the Novartis Foundation (Japan) for the Promotion of Science.
References
- Fearon ER, Vogelstein B. A genetic model for colon tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Kuramoto S, Oohara T. Flat early cancers of the large intestine. Cancer. 1989;64:950–955. doi: 10.1002/1097-0142(19890815)64:4<950::aid-cncr2820640430>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bedenne L, Faivre J, Boutron MC, Piard F, Cauvin JM, Hillon P. Adenoma-carcinoma sequence or “de novo” carcinogenesis? A study of adenomatous remnants in a population-based series of large bowel cancers. Cancer. 1992;69:883–888. doi: 10.1002/1097-0142(19920215)69:4<883::aid-cncr2820690408>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wada R, Matsukuma S, Abe H, Kuwabara N, Suda K, Arakawa A, Kitamura S. Histopathological studies of superficial-type early colon carcinoma. Cancer. 1996;77:44–50. doi: 10.1002/(SICI)1097-0142(19960101)77:1<44::AID-CNCR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colon cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Lüscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- Lüscher B. Function and regulation of the transcription factors of the Myc/Max/Mad network. Gene. 2001;277:1–14. doi: 10.1016/s0378-1119(01)00697-7. [DOI] [PubMed] [Google Scholar]
- Nesbit C, Tersakk J, Prochownik E. Myc oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- Stewart J, Evan G, Watson J, Sikora K. Detection of the c-myc oncogene product in colonic polyps and carcinomas. Br J Cancer. 1986;53:1–6. doi: 10.1038/bjc.1986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora K, Chan S, Evan G, Gabra H, Markham N, Stewart J, Watson J. C-myc oncogene expression in colon cancer. Cancer. 1987;59:1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cottrell S, Bicknell D, Kaklamanis L, Bodmer WF. Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet. 1992;340:626–630. doi: 10.1016/0140-6736(92)92169-g. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colon tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colon tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim Biophys Acta. 2002;1602:61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Tsuneoka M, Koda Y, Soejima M, Teye K, Kimura H. A novel Myc target gene, mina53, that is involved in cell proliferation. J Biol Chem. 2002;277:35450–35459. doi: 10.1074/jbc.M204458200. [DOI] [PubMed] [Google Scholar]
- Sunaga S, Maki K, Komagata Y, Ikuta K, Miyazaki JI. Efficient removal of lox-P flanked DNA sequences in a gene-targeted locus by transient expression of Cre recombinase in fertilized eggs. Mol Reprod Dev. 1997;46:109–113. doi: 10.1002/(SICI)1098-2795(199702)46:2<109::AID-MRD1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Fujitani N, Liu Y, Toda S, Shirouzu K, Okamura T, Kimura H. Expression of H type 1 antigen of ABO histo-blood group in normal colon and aberrant expressions of H type 2 and H type 3/4 antigens in colon cancer. Glycoconjugate J. 2000;17:331–338. doi: 10.1023/a:1007173722426. [DOI] [PubMed] [Google Scholar]
- Fujitani N, Liu Y, Okamura T, Kimura H. Distribution of H type 1–4 chains of the ABO(H) system in different cell types of human respiratory epithelium. J Histochem Cytochem. 2000;48:1649–1656. doi: 10.1177/002215540004801208. [DOI] [PubMed] [Google Scholar]
- Hozumi M. Fundamentals of chemotherapy of myeloid leukemia by induction of leukemia cell differentiation. Adv Cancer Res. 1983;38:121–169. doi: 10.1016/s0065-230x(08)60189-x. [DOI] [PubMed] [Google Scholar]
- Hickstein DD, Back AL, Collins SJ. Regulation of expression of the CD11b and CD18 subunits of the neutrophil adherence receptor during human myeloid differentiation. J Biol Chem. 1989;264:21812–21817. [PubMed] [Google Scholar]
- Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Jansson A, Sun XF. Ki-67 expression in relation to clinicopathological variables and prognosis in colon adenocarcinomas. APMIS. 1997;105:730–734. doi: 10.1111/j.1699-0463.1997.tb05078.x. [DOI] [PubMed] [Google Scholar]
- Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastorekova S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colon tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Polakis P. The oncogenic activation of β-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Roose J, Clevers H. TCF transcription factors: molecular switches in carcinogenesis. Biochim Biophys Acta. 1999;1424:M23–M37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Rabbitts PH, Watson JV, Lamond A, Forster A, Stinson MA, Evan G, Fischer W, Atherton E, Sheppard R, Rabbitts TH. Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J. 1985;4:2009–2015. doi: 10.1002/j.1460-2075.1985.tb03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitz W, Loidl P. Cell cycle dependent association of c-myc protein with the nuclear matrix. Oncogene. 1991;6:29–35. [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr, Miljkovic V, Cattoretti G, Califano A, Dalla-Favera R. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci USA. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6:2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- Sawai S, Shimono A, Wakamatsu Y, Palmes C, Hanaoka K, Kondoh H. Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse. Development. 1993;117:1445–1455. doi: 10.1242/dev.117.4.1445. [DOI] [PubMed] [Google Scholar]