Abstract
Experimental allergic encephalomyelitis (EAE) and multiple sclerosis (MS) are characterized by strong sexual dimorphisms, many of which may be due to genetically controlled sex hormone effects on the immune system, the central nervous system (CNS), or both. In the present study we used 487 gonadectomized and 376 intact age-matched F2 mice generated through crosses of B10.S/SgMcdJ and SJL/J mice to assess the role of adult gonadal hormones in regulating clinical and histopathological quantitative traits (QT) associated with EAE in the context of genetic heterogeneity. We found that gonadectomy resulted in different effects, depending on the QT and the sex of the mouse. Ovariectomized mice on average had lower cumulative clinical disease scores, shorter duration of clinical signs, and increased peak disease scores. This trend was accompanied by a significant increase in the incidence of acute, progressive EAE which is more frequently seen in intact and orchiectomized males. Although spinal cord (SC) inflammation was the better predictor of clinical signs of EAE in both sexes, ovariectomized females had considerable reductions in nearly all histopathological QT in both the brain and SC. Orchiectomy resulted in modestly significant increases in disease severity and peak score and earlier onset of clinical signs. With the exception of SC demyelination and lesion scores, orchiectomy had no effect on histopathological QT. Importantly, gonadectomy reduced but did not completely abolish any of the sexually dimorphic clinical QT seen in intact mice. It did however, lead to a significant sexual dimorphism in incidence and severity not seen in intact mice. For histopathological QT, no sexual dimorphism was detected for brain lesions in either intact or gonadectomized mice. In contrast, SC histopathological QT exhibited significant sexual dimorphisms, which were impacted by gonadectomy. The results from this study indicate that within the context of genetic heterogeneity, circulating gonadal hormones influence both clinical and histopathological QT in this model of MS, but they do not solely account for the sexual dimorphisms seen in these traits. Thus, additional mechanisms must play a role in regulating gender differences in autoimmune disease of the CNS.
Autoimmune diseases such as rheumatoid arthritis (RA), Graves’ disease, systemic lupus erythematosus (SLE), myasthenia gravis, Sjögren’s syndrome, Hashimoto’s thyroiditis, and multiple sclerosis (MS) are sexually dimorphic in that they occur more frequently in women than in men.1,2 Additionally, in MS both the disease course and the response to interferon-β therapy tend to be more favorable in women than men.3,4 However, the prognostic significance of gender in MS is somewhat controversial, particularly with respect to early- and adult-onset forms of the disease.5–12
Gender differences in susceptibility to experimental allergic encephalomyelitis (EAE), the principal animal model for MS, have also been observed.13 Female SJL/J mice have more severe disease in both active and passive models, which is associated with increased concentrations of Th1 cytokine production by T cells. In contrast, males produce higher concentrations of Th2 cytokines and develop less severe disease.14,15 Additionally, SJL/J male mice have a developmental delay in EAE susceptibility not seen in female mice. This age-associated sexual dimorphism has been observed in multiple settings.15–18 Once males mature, they are susceptible to EAE but they do not exhibit the relapsing/remitting form of disease seen in females.19 Interestingly, this difference can be overcome by prepubertal orchiectomy of SJL/J males,20 suggesting that the clinical disease course in males is regulated by testicular hormones.
The sexual dimorphism seen in MS and EAE may be due to multiple factors, but gender-related differences in immune responsiveness and sex hormones are likely to play a significant role. Such roles may include genetically controlled gonadal hormone regulation of the immune response21–23 as well as brain and spinal cord (SC) development and function.24–31 In early-onset MS, the first symptoms generally develop postpubertally,1,6,10–12 and increased concentrations of sex hormones produced during pregnancy are associated with a significant reduction in the severity of disease, while clinical symptoms are often exacerbated postpartum, a time characterized by significant alterations in sex hormone levels.1,32,33 Importantly, both estrogen-34–37 and androgen-38–41 regulated responses have been shown to be genetically controlled. To assess the role of circulating adult gonadal hormones on the clinical and histopathological QT of EAE in male and female mice in the context of genetic heterogeneity, we used cohorts of intact and gonadectomized F2 intercross mice generated using H2s matched EAE-susceptible SJL/J and EAE-resistant B10.S/SgMcdJ mice.
Materials and Methods
Animals
Male and female SJL/J and B10.S/SgMcdJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). (B10.S/SgMcdJ × SJL/J) and (SJL/J × B10.S/SgMcdJ) F1 hybrid mice were bred in the animal facilities at the College of Veterinary Medicine, University of Illinois at Urbana-Champaign. F2 populations were similarly produced by intercrossing F1 hybrids in multiple directions. All animals were maintained in accordance with the Animal Welfare Act and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Animals were continuously bred over 3 years and randomly selected to generate immunization cohorts irrespective of age. Mice reported here were selected for parity in season and age at the time of immunization.
Induction and Evaluation of EAE
Emulsions of SJL/J mouse SC homogenate (MSCH) in complete Freund’s adjuvant (CFA) (MSCH-CFA) were prepared using CFA containing Mycobacterium tuberculosis H37Ra at a concentration of 1.0 mg/ml (Difco Laboratories, Detroit, MI). Emulsification was performed by traditional syringe extrusion. Two syringes were connected by an 18-gauge double-hub microemulsifying needle. One syringe contained the CFA and the other syringe contained an equal volume of phosphate-buffered saline (PBS) with 6.67 mg/ml MSCH. Spinal cord tissue for MSCH was harvested from female SJL/J mice, as previously described.42 The plunger of the syringe containing the MSCH was first depressed followed by the other plunger. The plungers were depressed alternately until the emulsion thickened and failed to separate.
Mice were immunized subcutaneously with a total of 0.3 ml of MSCH-CFA emulsion by injecting 0.15 ml at posterior sites on each flank. One week later, mice were similarly immunized at two anterior flank sites. All mice were weighed and scored for clinical QT on a daily basis from day 10 through day 60 postinjection. Severity scores from 0 to 4 were assigned as previously described: 0, no clinical expression of disease; 1, flaccid tail without hind limb weakness; 2, hind limb weakness; 3, complete hind limb paralysis and floppy tail; and 4, hind leg paralysis accompanied by a floppy tail and urinary or fecal incontinence.43 Clinical EAE profiles were established for each mouse based on the clinical QT scores according to previously defined criteria.44 Briefly, animals which demonstrated a progressive increase in clinical severity without remission, culminating in a score of 4 or death, were classified as acute progressive disease, while mice which tended to neither progress nor remit following onset of clinical signs, and remained below a severity score of 4, were classified as chronic disease. The severity index was calculated by dividing the cumulative clinical QT scores by the number of days with clinical signs of EAE.
CNS tissues were dissected from calvaria and vertebrae and fixed in 10% phosphate-buffered formalin (pH 7.4). Representative brain and SC samples were embedded in paraffin, sectioned at 5 μm, mounted on glass slides, and stained with hematoxylin and eosin (H & E) for lesion assessment. Luxol fast blue-periodic acid Schiff stain (LFB-PAS) was used to visualize demyelination. Lesion QT scores were assigned using a semiquantitative system based on the following criteria: mononuclear/lymphocytic cellular infiltration, polymorphonuclear cellular infiltration (primarily neutrophils), lesion severity, and extent of demyelination. Lesion scoring of brain was completed using three coronal sections as previously described.45 Lesion scoring of SC was completed using longitudinal sections of the entire SC.
Gonadectomy
Adult gonadectomized mice were continuously generated over a 3-year period and randomly selected to establish immunization cohorts irrespective of age. Ovariectomies were performed via two posterior flank incisions whereas orchiectomies were done with a single midline abdominal incision. Animals were allowed to recover for 2 weeks before immunization. Xylazine and ketamine were used for anesthesia. Additionally, buprenorphine (Buprenex) was administered before surgery for analgesia.
Statistical Analysis
Significance of differences in disease incidences was determined by χ2 analysis, and significance of differences for clinical and histopathological QT was carried out by multivariant analysis of variance or Student’s t-test. Significance was identified at P ≤ 0.05.
Results
The characteristics of the intact and gonadectomized F2 populations are outlined in Table 1. Overall, 376 intact mice and 487 gonadectomized mice with similar numbers of males and females were studied. In both cohorts, the mice were similarly distributed among the four parental combinations, and all were of adult age (>4 months old).
Table 1.
Baseline Characteristics of Intact and Gonadectomized F2 Populations
| Variable | Intact | Gonadectomized |
|---|---|---|
| Total number | 376 | 487 |
| Sex (number) | ||
| Male | 183 | 234 |
| Female | 193 | 253 |
| Age in weeks (mean ± SD) | 36.4 ± 6.9 | 39.4 ± 6.1 |
Intact females exhibited significantly greater cumulative diseases scores and longer duration of clinical signs than intact males (Table 2). In the females this correlated with a significantly larger proportion of the animals exhibiting the chronic (83%) rather than the acute, progressive (17%) form of EAE (Table 3). In contrast, affected intact F2 males had greater peak clinical disease scores, shorter duration of clinical signs, and greater weight loss than females (Table 2). These QT differences correlated with a more equal proportion of the intact males developing the acute, progressive (44%) and chronic (56%) forms of EAE (Table 3). These results replicate findings in our previous report on intact mice.44
Table 2.
Clinical Signs of EAE in Intact and Gonadectomized Female and Male F2 Mice
| Phenotype | No. affected females (total) | No. affected males (total) | χ2 | DF* | P |
|---|---|---|---|---|---|
| Incidence | 142/193 (73.5%) | 135/183 (73.7%) | 0.002 | 1 | 0.97 |
| Incidence (Gndx) | 181/253 (71.5%) | 190/234 (81.2%) | 6.25 | 1 | 0.01 |
| Intact vs. Gndx | χ2 = 0.22 | χ2 = 3.29 | |||
| DF = 1 | DF = 1 | ||||
| P = 0.63 | P = 0.07 |
| Phenotype | Females, mean ± SC(N) | Males, mean ± SD (N) | t | DF* | P |
|---|---|---|---|---|---|
| Day of onset | 18.2 ± 2.9 (142) | 18.6 ± 3.2 (135) | 1.18 | 275 | 0.24 |
| Day of onset (Gndx) | 18.1 ± 3.1 (181) | 17.7 ± 3.1 (190) | 1.19 | 369 | 0.23 |
| Intact vs. Gndx | t = 0.31 | t = 2.61 | |||
| DF = 321 | DF = 323 | ||||
| P = 0.76 | P = 0.009 | ||||
| Cumulative score | 66.9 ± 28.9 (142) | 45.1 ± 28.1 (135) | 6.35 | 275 | 8.9 E-10 |
| Cumulative score (Gndx) | 55.4 ± 32.0 (181) | 41.6 ± 28.8 (190) | 4.38 | 369 | 1.5 E-05 |
| Intact vs. Gndx | t = 3.35 | t = 1.103 | |||
| DF = 321 | DF = 323 | ||||
| P = 0.0009 | P = 0.27 | ||||
| Duration | 35.5 ± 13.4 (142) | 24.3 ± 16.0 (135) | 6.35 | 275 | 8.9 E-10 |
| Duration (Gndx) | 28.6 ± 15.9 (181) | 21.5 ± 16.5 (190) | 4.23 | 369 | 3.0 E-05 |
| Intact vs. Gndx | t = 4.16 | t = 1.52 | |||
| DF = 321 | DF = 323 | ||||
| P = 4.1 E-05 | P = 0.13 | ||||
| Severity index | 2.1 ± 0.5 (142) | 2.2 ± 0.6 (135) | 1.23 | 275 | 0.22 |
| Severity index (Gndx) | 2.2 ± 0.6 (181) | 2.4 ± 0.6 (190) | 2.71 | 369 | 0.007 |
| Intact vs. Gndx | t = 1.72 | t = 2.92 | |||
| DF = 321 | DF = 323 | ||||
| P = 0.09 | P = 0.004 | ||||
| Peak score | 3.0 ± 0.8 (142) | 3.2 ± 0.9 (135) | 2.60 | 275 | 0.01 |
| Peak score (Gndx) | 3.2 ± 0.8 (181) | 3.4 ± 0.8 (190) | 2.73 | 369 | 0.007 |
| Intact vs. Gndx | t = 2.54 | t = 1.94 | |||
| DF = 321 | DF = 323 | ||||
| P = 0.01 | P = 0.05 | ||||
| Weight loss | 3.0 ± 3.2 (193) | 4.2 ± 4.0 (183) | 3.20 | 374 | 0.002 |
| Weight loss (Gndx) | 3.2 ± 3.3 (253) | 4.0 ± 3.6 (234) | 2.62 | 485 | 0.009 |
| Intact vs. Gndx | t = 0.67 | t = 0.45 | |||
| DF = 444 | DF = 415 | ||||
| P = 0.50 | P = 0.66 |
DF, degrees of freedom.
Table 3.
Disease Subtypes in Intact and Gndx Female and Male F2 Mice
| Disease subtype | No. affected females (total) | No. affected males (total) | χ2 | DF* | P |
|---|---|---|---|---|---|
| Acute | 24/142 (17%) | 59/135 (44%) | 23.69 | 1 | 1.1 E-06 |
| Acute (Gndx) | 57/181 (31%) | 104/190 (55%) | 20.39 | 1 | 6.3 E-06 |
| Intact vs. Gndx | χ2 = 9.02 | χ2 = 3.84 | |||
| DF = 1 | DF = 1 | ||||
| P = 0.003 | P = 0.05 | ||||
| Chronic | 118/142 (83%) | 76/135 (56%) | 23.69 | 1 | 1.1 E-06 |
| Chronic (Gndx) | 124/181 (69%) | 86/190 (45%) | 20.39 | 1 | 6.3 E-06 |
| Intact vs. Gndx | χ2 = 9.02 | χ2 = 3.84 | |||
| DF = 1 | DF = 1 | ||||
| P = 0.003 | P = 0.05 |
DF, degrees of freedom.
The effects of gonadectomy on the various QT were studied separately in each sex cohort (Table 2). Factors that were significantly affected by the loss of ovaries in females include the cumulative disease score, duration of clinical signs, and average peak disease score. Ovariectomy resulted in a decrease in disease duration and cumulative disease score, but caused an increase in the peak disease score. The changes in these QT values were associated with a significant increase in ovariectomized mice exhibiting acute, progressive disease (31%) and a decrease in the incidence of the chronic (69%) form of disease (Table 3, Figure 1). Thus, ovariectomized female mice tend to be sicker for a shorter period of time when compared to non-ovariectomized mice.
Figure 1.
Disease profiles change to a more acute form of EAE in gonadectomized female and male mice. However, the sexual dimorphism in disease type is significant both before and after gonadectomy (P < 0.0001).
In contrast, orchiectomized mice had modestly significant differences in the average day of disease onset, which was earlier, and an increase in the severity index and average peak disease score (Table 2). Overall, however, the effect of orchiectomy was much less dramatic than ovariectomy. Nevertheless, a significant shift in the subtype of EAE in orchectomized mice from the chronic (45%) to the acute, progressive (55%) form of the disease was associated with these changes in clinical QT (Table 3, Figure 1).
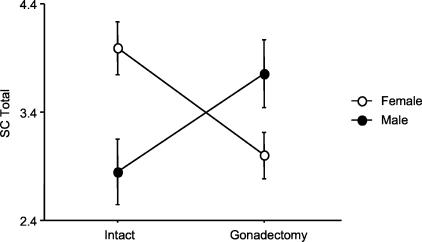
In the histological analyses, ovariectomy affected all brain inflammation QT: it decreased suppuration (a reflection of neutrophilic infiltration), mononuclear cell infiltration, demyelination, lesion severity, and total brain lesion score. Thus, the absence of female gonadal hormones inhibited brain inflammation and histological EAE. In contrast, no histopathological QT in the brain were affected by orchiectomy. In the SC, all female histopathological QT were also significantly reduced by ovariectomy (Table 5), whereas SC demyelination, lesion severity, and total lesion scores were all significantly increased by orchiectomy (Table 5, Figure 2). In fact, gonadectomy completely reversed the direction of the sexual dimorphism seen in the SC: intact males and ovariectomized females had higher lesion scores than the opposite sex-matched cohort (Figure 3).
Table 5.
Influence of Gender and Sex Hormones on Spinal Cord Histopathology
| Phenotype | Males, mean ± SD (N) | Females, mean ± SD (N) | t | DF* | P |
|---|---|---|---|---|---|
| Spinal cord suppuration | |||||
| Intact | 0.2 ± 0.6 (124) | 0.1 ± 0.4 (80) | 0.97 | 202 | 0.33 |
| Gndx | 0.1 ± 0.3 (144) | 0.2 ± 0.7 (110) | 1.94 | 252 | 0.05 |
| Intact vs. Gndx | t = 2.96 | t = 0.41 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.003 | P = 0.68 | ||||
| Spinal cord mononuclear cell infiltration | |||||
| Intact | 1.2 ± 0.8 (124) | 0.9 ± 0.9 (80) | 2.49 | 202 | 0.014 |
| Gndx | 0.8 ± 0.8 (144) | 1.1 ± 1.0 (110) | 2.18 | 252 | 0.03 |
| Intact vs. | t = 3.06 | t = 1.75 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.002 | P = 0.08 | ||||
| Spinal cord demyelination | |||||
| Intact | 1.4 ± 1.0 (124) | 1.0 ± 0.9 (80) | 2.90 | 202 | 0.004 |
| Gndx | 1.1 ± 1.0 (144) | 1.3 ± 1.1 (110) | 1.23 | 252 | 0.22 |
| Intact vs. Gndx | t = 2.03 | t = 2.05 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.04 | P = 0.04 | ||||
| Spinal cord lesion severity | |||||
| Intact | 1.3 ± 0.9 (124) | 0.9 ± 0.8 (80) | 3.06 | 202 | 0.0025 |
| Gndx | 1.0 ± 0.8 (144) | 1.2 ± 1.1 (110) | 1.95 | 252 | 0.05 |
| Intact vs. Gndx | t = 2.81 | t = 2.21 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.005 | P = 0.03 | ||||
| Total spinal cord lesion score | |||||
| Intact | 4.0 ± 2.7 (124) | 2.9 ± 2.7 (80) | 2.93 | 202 | 0.004 |
| Gndx | 3.0 ± 2.5 (144) | 3.8 ± 3.3 (110) | 2.07 | 252 | 0.04 |
| Intact vs. Gndx | t = 3.08 | t = 2.02 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.002 | P = 0.045 |
DF, degrees of freedom.
Figure 2.
Spinal cord total lesion scores in male and female mice exhibit a reversed sexual dimorphism in response to gonadectomy (P = 0.0005).
Figure 3.
Representative sections showing the reversed sexual dimorphism in spinal cord demyelination observed in male and female mice in response to gonadectomy. A: No demyelination. B and E: Moderate spinal cord demyelination representative of that seen in intact male mice and ovariectomized female mice, respectively. C and D: Severe spinal cord demyelination representative of that seen in orchiectomized males and ovariectomized female mice, respectively. Sections were stained with LFB-PAS. Scale bar, 100 μm.
These histopathological QT findings led us to compare CNS inflammation with clinical disease scores. The most highly positively correlated clinical and histopathological QT in both male and female mice was the clinical severity score and the SC total histopathology score, a correlation that is highly significant in gonadectomized mice, and not significant in the intact cohort. In contrast, brain histopathology scores had no correlation with any clinical QT in either sex and in either cohort. As expected from observing the mice, duration of clinical signs was highly negatively correlated with peak clinical score in all cohorts of mice (F = 138 in intact and F = 168 in gonadectomized mice, both P < 0.0001), indicating that shorter duration of clinical signs is associated with increased peak clinical scores.
The effects of gonadectomy on the sexual dimorphisms in the clinical and histopathological QT assessed in this study were variable. Gonadectomy resulted in a significant sexual dimorphism in disease incidence and severity which was not seen in intact mice (Table 2). For all other clinical QT and disease subtypes (Table 3) gonadectomy did not abolish the sexual dimorphisms seen in the intact population. In other words, all clinical phenotypes that exhibited a statistically significant sexual dimorphism in the intact cohort remained significantly dimorphic following adult gonadectomy. For each of the sexually dimorphic QT, intact females on average had greater trait values than intact males and this trend was maintained following gonadectomy.
None of the brain inflammatory QT exhibited a significant sexual dimorphism in either the intact or gonadectomized cohorts. In contrast, the only inflammatory SC QT that was not sexually dimorphic in the intact population was suppuration, a measure of neutrophilic inflammation (Table 5). However, following gonadectomy a significant sexual dimorphism in SC suppuration was observed while leading to the abolishment of the sexual dimorphism in SC demyelination seen in intact mice. All other SC histopathological QT exhibited significant sexual dimorphisms in both the intact and gonadectomized mice. Importantly, the maintenance of the sexual dimorphism in these SC inflammatory QT was due to a reversal in the significance of the QT between intact and gonadectomized males and females, ie, intact females > intact males versus ovariectomized females < orchiectomized males.
Discussion
The present study was optimized to assess how adult gonadal hormones regulate sexually dimorphic traits associated with EAE in the context of genetic heterogeneity. We chose mice of B10 origin (B10.S/SgMcdJ) mated to SJL/J mice to employ the prototypical strains showing sex differences. They were H2s matched, and the genetic control of EAE in progeny of these strains has been studied extensively.44–52 In this study we found a strong effect of gonadectomy on clinical and histopathological QT of EAE and evidence that factors other than just adult levels of circulating gonadal hormones play a significant role in regulating the sexual dimorphisms observed in these QT.
The current thinking on the role of gonadal hormones in autoimmune disease of the CNS is that the effects are due to sex hormones and that both estrogens and androgens have paradoxical effects. The classically held belief is that the sexual dimorphism seen in immune responses, and in certain autoimmune diseases in humans, is the result of the immunostimulatory effects of estrogens and the immunosuppressive effects of androgens.53,54 It is known that women are more often affected by MS, but they tend to have milder disease or a disease course characterized by remissions, whereas men on average tend to have a more progressive and severe form of MS, but with a lower incidence.2 It is not known whether the lower incidence and increased severity in men are due to lower levels of estrogen or higher levels of testosterone or neither. The effects of sex hormones are also paradoxical in EAE, in that SJL females have a higher incidence of EAE than young males, and their T cells are more capable of transferring disease to mice of either sex,55 but this sexual dimorphism is reversed in B10.PL mice.56 C57BL/6 mice show no sexual dimorphism at all in EAE induced by myelin oligodendrocyte glycoprotein.57 Ovariectomy of B10.PL TCR-transgenic female mice leads to greater severity of disease induced by the relevant MBP-peptide,58 but orchiectomy of B10.PL or SJL males also leads to increased disease severity.59 Administration of either exogenous estrogens or androgens has been shown to ameliorate disease in a variety of strains and EAE induction protocols.14,21,60–62 Clearly, there is no firm consensus on the role of sex hormone action in either exacerbating or preventing EAE.
The different clinical and histopathological presentations associated with EAE in male and female mice could be due to direct genetic effects of the sex chromosomes, to sex hormones or other gonadal hormones acting on the CNS or immune system, and/or to the genetic control of gonadal hormone-regulated responses in these tissues. It is likely that genetic background plays a role in controlling the sex hormone-regulated responses that affect disease parameters in EAE for the following reasons. We have clearly demonstrated that estrogen-regulated responses, including those that influence sexually dimorphic structures within the brain and the uterotropic inflammatory response, are genetically controlled.34–37 Androgen-regulated responses are similarly controlled by genotype.38–41 We have further shown that individual loci in mice not only control sexually dimorphic traits, they themselves are controlled by gender.45,50 One potential genetic contribution could come from estrogen receptor-α, -β, or androgen receptor polymorphisms. Indeed, an effect of human Esr1 polymorphisms on MS has been reported,63–65 although this claim is controversial.66
The most significant change after ovariectomy was a shift to more acute progressive disease, which made the female mice phenotypically more like intact and gonadectomized males. The male mice also exhibited this shift, although somewhat less strikingly. The appearance of more female mice with acute progressive EAE was associated with a concomitant change in clinical QT. Their mean duration was shorter, the peak score was higher, but morbidity over the course of the experiment (60 days) was lower because fewer females had long lasting clinical signs. However, not all females responded in this way. Fully 68.5% of ovariectomized female mice still showed chronic disease (usually without a relapsing-remitting pattern; data not shown). The intact and orchiectomized male mice also did not show the prototypic disease pattern. These data suggest that the response to the presence of gonadal hormones is likely to involve a set of polymorphic alleles at hormone-responsive genes that are segregating in the intercross population.
Concordant changes were also seen for CNS histopathology in female mice acquired at the end of the study or at sacrifice. Milder inflammatory lesions were seen in both the brain and the SC of ovariectomized females compared to the intact cohort. This overall reduction in inflammation may have contributed to the overall shortening of clinical signs. However, male mice showed an increase in the SC lesions, the parameter that correlated best with severity of clinical signs, and in fact they showed an earlier onset of clinical signs and greater severity and peak disease scores. This finding is consistent with previous reports about the protective effects of testosterone, where exogenous hormone ameliorated disease14 and orchiectomy of young SJL males reversed their proinflammatory cytokine deficiency.67 Interestingly, orchiectomized males also had reversed disease subtype distributions with more acute progressive disease than intact males. On the other hand, gonadectomy completely reversed the direction of the sexual dimorphism seen in the SC. Intact males, and ovariectomized females, had higher lesion scores than the opposite sex-matched cohort. Thus, reduction of either sex hormone led to an increase in acute progressive forms of EAE, but presumably by different routes in male versus female mice.
Despite the changes in the patterns of EAE seen primarily in female mice and the fact that most sexually dimorphic traits were reduced in significance, it remains the case that even in the gonadectomized cohort, significant sexual dimorphisms remain. These dimorphisms are most obvious in the different disease subtypes and in duration and cumulative score differences. These findings suggest that the source of the residual sexual dimorphism in the absence of adult gonadal hormones may be due to prenatal exposure to estrogens and androgens68 or is genetic in origin, either due to maternal or paternal effects, to the Y chromosome itself, or to steroid hormone-regulated genetically controlled responses. Such effects may take place at very early developmental stages, such as differences in the sexually dimorphic motor neuron structures in the SC,69–71 or may be active at the time of disease induction, such as the differential estrogen regulation of a key cytokine. Additionally, because all of the gonadectomized mice in this study were castrated as adults, the role of the loss of gonadal hormones at very early ages was not studied. These additional factors are presently under investigation.
Table 4.
Influence of Gender and Sex Hormones on Brain Histopathology
| Phenotype | Females, mean ± SD (N) | Males, mean ± SD (N) | t | DF* | P |
|---|---|---|---|---|---|
| Brain suppuration | |||||
| Intact | 0.4 ± 0.8 (124) | 0.3 ± 0.7 (80) | 0.89 | 202 | 0.37 |
| Gndx | 0.2 ± 0.5 (144) | 0.2 ± 0.5 (110) | 0.06 | 252 | 0.95 |
| Intact vs. Gndx | t = 2.55 | t = 1.13 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.01 | P = 0.26 | ||||
| Brain mononuclear cell infiltration | |||||
| Intact | 1.2 ± 0.8 (124) | 1.0 ± 0.9 (80) | 1.43 | 202 | 0.15 |
| Gndx | 1.0 ± 1.0 (144) | 0.9 ± 0.9 (110) | 0.97 | 252 | 0.33 |
| Intact vs. Gndx | t = 2.14 | t = 1.29 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.03 | P = 0.20 | ||||
| Brain demyelination | |||||
| Intact | 0.6 ± 0.8 (124) | 0.4 ± 0.6 (80) | 1.81 | 202 | 0.07 |
| Gndx | 0.3 ± 0.8 (144) | 0.3 ± 0.6 (110) | 0.37 | 252 | 0.71 |
| Intact vs. Gndx | t = 2.77 | t = 0.91 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.006 | P = 0.37 | ||||
| Brain lesion severity | |||||
| Intact | 1.2 ± 0.8 (124) | 1.0 ± 0.9 (80) | 1.48 | 202 | 0.14 |
| Gndx | 0.9 ± 0.9 (144) | 0.8 ± 0.8 (110) | 0.74 | 252 | 0.46 |
| Intact vs. Gndx | t = 2.57 | t = 1.27 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.01 | P = 0.21 | ||||
| Total brain lesion score | |||||
| Intact | 3.4 ± 2.7 (124) | 2.7 ± 2.8 (80) | 1.65 | 202 | 0.10 |
| Gndx | 2.4 ± 2.6 (144) | 2.2 ± 2.4 (110) | 0.71 | 252 | 0.48 |
| Intact vs. Gndx | t = 2.93 | t = 1.39 | |||
| DF = 266 | DF = 188 | ||||
| P = 0.004 | P = 0.17 |
DF, degrees of freedom.
Acknowledgments
We thank Amy Fillmore and Brian McFarland for assistance with these studies.
Footnotes
Address reprint requests to Dr. Cory Teuscher, C317 Given Medical Building, University of Vermont, Burlington, VT 05405. E-mail: c.teuscher@uvm.edu.
Supported by National Multiple Sclerosis Society Research Grant RG3129 (to C.T. and E.P.B.).
References
- Beeson PB. Age and sex association of 40 autoimmune diseases. Am J Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Hawkins SA, McDonnell GV. Benign multiple sclerosis? Clinical course, long term follow-up and assessment of prognostic factors. J Neurol Neurosurg Psychiatry. 1999;67:148–152. doi: 10.1136/jnnp.67.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon β-1a in MS, (SPECTRIMS) Study Group Randomized controlled trial of Interferon β-1a in secondary progressive MS: clinical results. Neurology. 2001;56:1496–1504. doi: 10.1212/wnl.56.11.1496. [DOI] [PubMed] [Google Scholar]
- Zaffaroni A, Ghezzi A. The prognostic value of age, gender, pregnancy and endocrine factors in multiple sclerosis. Neurol Sci. 2000;21:S857–S860. doi: 10.1007/s100720070026. [DOI] [PubMed] [Google Scholar]
- Simone IL, Carrara D, Tortorella C, Ceccarelli A, Livrea P. Early onset multiple sclerosis. Neurol Sci. 2000;21:S861–S863. doi: 10.1007/s100720070027. [DOI] [PubMed] [Google Scholar]
- Polliack ML, Barak Y, Achiron A. Late-onset multiple sclerosis. J Am Geriat Soc. 2001;49:168–171. doi: 10.1046/j.1532-5415.2001.49038.x. [DOI] [PubMed] [Google Scholar]
- Hammond SR, McLeod JG, Macaskill P, English DR. Multiple sclerosis in Australia: prognostic factors. J Clin Neurosci. 2000;7:16–19. doi: 10.1054/jocn.1998.0107. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- Simone IL, Carrara D, Tortorella C, Liguori M, Lepore V, Pellegrini F, Bellacosa A, Ceccarelli A, Pavone I, Livrea P. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology. 2002;59:1922–1928. doi: 10.1212/01.wnl.0000036907.37650.8e. [DOI] [PubMed] [Google Scholar]
- Trojano M, Liguori M, Bosco Zimatore G, Bugarini R, Avolio C, Paolicelli D, Giuliani F, De Robertis F, Marrosu MG, Livrea P. Age-related disability in multiple sclerosis. Ann Neurol. 2002;51:475–480. doi: 10.1002/ana.10147. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Pozzilli C, Liguori M, Marrosu MG, Milani N, Milanese C, Simone I, Zaffaroni M. Prospective study of multiple sclerosis with early onset. Mult Scler. 2002;8:115–118. doi: 10.1191/1352458502ms786oa. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- Kim S, Voskuhl RR. Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol. 1999;162:5561–5568. [PubMed] [Google Scholar]
- Stohlman SA, Matsushima GK, Casteel N, Frelinger JA. The defect in delayed-type hypersensitivity of young adult SJL mice is due to a lack of functional antigen-presenting cells. Eur J Immunol. 1985;15:913–916. doi: 10.1002/eji.1830150909. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Hinton DR, Kirkman L, Stohlman SA. Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur J Immunol. 1995;25:2318–2324. doi: 10.1002/eji.1830250830. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139–151. J Neurosci Res. 1996;45:680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Ifnla Res. 1998;48:290–301. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR. Gender issues and multiple sclerosis. Curr Neurol Neurosci Rep. 2002;2:277–286. doi: 10.1007/s11910-002-0087-1. [DOI] [PubMed] [Google Scholar]
- Darlington C. Multiple sclerosis and gender. Curr Opin Invest Drugs. 2002;3:911–914. [PubMed] [Google Scholar]
- Behl C. Oestrogen as a neuroprotective hormone. Natl Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- Gilmore DP. Sexual dimorphism in the central nervous system of marsupials. Int Rev Cytol. 2002;214:193–224. doi: 10.1016/s0074-7696(02)14006-x. [DOI] [PubMed] [Google Scholar]
- Behl C, Manthey D. Neuroprotective activities of estrogen: an update. J Neurocytol. 2000;29:351–358. doi: 10.1023/a:1007109222673. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Multiple ovarian hormone effects on brain structure and function. J Gender Specific Med. 1998;1:33–41. [PubMed] [Google Scholar]
- Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21:311–327. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- McEwen BS. How do sex and stress hormones affect nerve cells? Ann NY Acad Sci. 1994;743:1–16. doi: 10.1111/j.1749-6632.1994.tb55784.x. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;S3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Birk K, Ford C, Meltzer S. The clinical course of multiple sclerosis during pregnancy during pregnancy and puerperium. Arch Neurol. 1990;47:738. doi: 10.1001/archneur.1990.00530070026007. [DOI] [PubMed] [Google Scholar]
- Korn-Lubetzki I, Kahana G, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol. 1984;16:229. doi: 10.1002/ana.410160211. [DOI] [PubMed] [Google Scholar]
- Griffith JS, Jensen SM, Lunceford JK, Kahn MW, Zheng Y, Falase EA, Lyttle CR, Teuscher C. Evidence for the genetic control of estradiol-regulated responses: implications for variation in normal and pathological hormone-dependent phenotypes. Am J Pathol. 1997;150:2223–2230. [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, Griffith JS, Lyttle CR, Doerge RW, McNabb AW, Broadbent RE, Teuscher C. Interacting quantitative trait loci control phenotypic variation in murine estradiol-regulated responses. Endocrinology. 1999;140:556–561. doi: 10.1210/endo.140.2.6521. [DOI] [PubMed] [Google Scholar]
- Nakai M, Uchida K, Teuscher C. The development of male reproductive organ abnormalities after neonatal exposure to tamoxifen is genetically determined. J Androl. 1999;20:626–634. [PubMed] [Google Scholar]
- Lephart ED, Call SB, Rhees RW, Jacobson NA, Weber KS, Bledsoe J, Teuscher C. Neuroendocrine regulation of sexually dimorphic brain structure and associated sexual behavior in male rats is genetically controlled. Biol Reprod. 2001;64:571–578. doi: 10.1095/biolreprod64.2.571. [DOI] [PubMed] [Google Scholar]
- Palmer R, Gallagher PM, Boyko WL, Ganschow RE. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci USA. 1983;80:7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Catterall JF. Genetic regulation of androgen-induced accumulation of mouse renal β-glucuronidase messenger ribonucleic acid. Endocrinology. 1986;118:1081–1086. doi: 10.1210/endo-118-3-1081. [DOI] [PubMed] [Google Scholar]
- Melanitou E, Cohn DA, Bardin CW, Janne OA. Genetic variation in androgen regulation of ornithine decarboxylase gene expression in inbred strains of mice. Mol Endocrinol. 1987;1:266–273. doi: 10.1210/mend-1-3-266. [DOI] [PubMed] [Google Scholar]
- Tennent BJ, Shultz KL, Beamer WG. Genetic susceptibility for C19 androgen induction of ovarian granulosa cell tumorigenesis in SWXJ strains of mice. Cancer Res. 1993;53:1059–1063. [PubMed] [Google Scholar]
- Korngold R, Feldman A, Rorke LB, Lublin FD, Doherty PC. Acute experimental allergic encephalomyelitis in radiation bone marrow chimeras between high and low susceptible strains of mice. Immunogenetics. 1986;24:309–315. doi: 10.1007/BF00395536. [DOI] [PubMed] [Google Scholar]
- Teuscher C, Hickey WF, Korngold R. An analysis of the role of TNF in the phenotypic expression of actively induced experimental allergic orchitis and experimental allergic encephalomyelitis. Clin Immunol Immunopathol. 1990;54:442–453. doi: 10.1016/0090-1229(90)90057-w. [DOI] [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunologically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder TA, Greiner DL, Grunnet M, Goldschneider I. Relative susceptibility of SJL/J and B10. S mice to experimental allergic encephalomyelitis (EAE) is determined by the ability of prethymic cells in bone marrow to develop into EAE effector T cells. J Neuroimmunol. 1993;42:23–32. doi: 10.1016/0165-5728(93)90208-g. [DOI] [PubMed] [Google Scholar]
- Encinas JA, Lees MB, Sobel RA, Symonowicz C, Greer JM, Shovlin CL, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. Genetic analysis of susceptibility to experimental autoimmune encephalomyelitis in a cross between SJL/J and B10. S mice. J Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- Teuscher C, Butterfield RJ, Ma RZ, Zachary JF, Doerge RW, Blankenhorn EP. Sequence polymorphisms in the chemokines Scya1 (TCA-3), Scya2 (monocyte chemoattractant protein (MCP)-1), and Scya12 (MCP-5) are candidates for eae7, a locus controlling susceptibility to monophasic remitting/non-relapsing experimental allergic encephalomyelitis. J Immunol. 1999;163:2262–2266. [PubMed] [Google Scholar]
- Blankenhorn EP, Butterfield RJ, Rigby R, Cort L, Giambrone D, McDermott P, McEntee K, Solowski N, Meeker ND, Zachary JF, Doerge RW, Teuscher C. Genetic analysis of the influence of pertussis toxin on experimental allergic encephalomyelitis susceptibility: an environmental agent can override genetic checkpoints. J Immunol. 2000;164:3420–3425. doi: 10.4049/jimmunol.164.6.3420. [DOI] [PubMed] [Google Scholar]
- Encinas JA, Lees MB, Sobel RA, Symonowicz C, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. Identification of genetic loci associated with paralysis, inflammation and weight loss in mouse experimental autoimmune encephalomyelitis. Int Immunol. 2001;13:257–264. doi: 10.1093/intimm/13.3.257. [DOI] [PubMed] [Google Scholar]
- Fillmore PD, Brace M, Troutman SA, Blankenhorn EP, Diehl S, Rincon M, Teuscher C. Genetic analysis of the influence of neuroantigen-complete Freund’s adjuvant emulsion structures on the sexual dimorphism and susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 2003;163:1623–1632. doi: 10.1016/S0002-9440(10)63519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–993. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann NY Acad Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- Papenfuss TL, Rogers C, Gienapp I, Valo J, McClain M, Damico N, Whitacre CC. Sex differences in experimental autoimmune encephalomyelitis (EAE) are murine strain and neuroantigen dependent. EMBO J. 2003;17:C40. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunol Lett. 2002;81:25–29. doi: 10.1016/s0165-2478(01)00339-x. [DOI] [PubMed] [Google Scholar]
- Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 β-Estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Adlard K, Schuster JC, Unsicker L, Vandenbark AA, Offner HJ. Gender differences in protection from EAE induced by oral tolerance with a peptide analogue of MBP-Ac1–11. J Neurosci Res. 1999;55:432–440. doi: 10.1002/(SICI)1097-4547(19990215)55:4<432::AID-JNR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-α production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- Bebo B, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- Niino M, Kikuchi S, Fukazawa T, Yabe I, Tashiro K. Estrogen receptor gene polymorphism in Japanese patients with multiple sclerosis. J Neurol Sci. 2000;179(S1–2):70–75. doi: 10.1016/s0022-510x(00)00381-6. [DOI] [PubMed] [Google Scholar]
- Mattila KM, Luomala M, Lehtimaki T, Laippala P, Koivula T, Elovaara I. Interaction between ESR1 and HLA-DR2 may contribute to the development of MS in women. Neurology. 2001;56:1246–1247. doi: 10.1212/wnl.56.9.1246. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Fukazawa T, Niino M, Yabe I, Miyagishi R, Hamada T, Tashiro K. Estrogen receptor gene polymorphism and multiple sclerosis in Japanese patients: interaction with HLA-DRB1*1501 and disease modulation. J Neuroimmunol. 2002;128:77–81. doi: 10.1016/s0165-5728(02)00140-6. [DOI] [PubMed] [Google Scholar]
- Savettieri G, Cittadella R, Valentino P, Manna I, Andreoli V, La Russa A, La Porta G, Ruscica F, Ragonese P, Pirritano D, Bonavita S, Tedeschi G, Quattrone A. Lack of association between estrogen receptor 1 gene polymorphisms and multiple sclerosis in southern Italy in humans. Neurosci Lett. 2002;327:115–118. doi: 10.1016/s0304-3940(02)00410-x. [DOI] [PubMed] [Google Scholar]
- Wilcoxen SC, Kirkman E, Dowdell KC, Stohlman SA. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J Immunol. 2000;164:6237–6243. doi: 10.4049/jimmunol.164.12.6237. [DOI] [PubMed] [Google Scholar]
- Martin JT. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur J Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249:309–314. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Clemens LG. Perinatal modification of a sexually dimorphic motor nucleus in the spinal cord of the B6D2F1 house mouse. Physiol Behav. 1989;45:831–835. doi: 10.1016/0031-9384(89)90303-x. [DOI] [PubMed] [Google Scholar]