Abstract
VEGF (vascular endothelial growth factor) is not only one of the most important angiogenesis factors, but is involved also in inflammatory processes. Recent studies have shown that VEGF as well as its receptor VEGFR-2 are expressed on osteoarthritic chondrocytes, but not on normal adult chondrocytes. Since mechanical overload is one of the causative factors for osteoarthritis, we studied its effect on VEGF expression on bovine cartilage disks that were compressed once with a strain of 50% and a strain rate of 1/second. Under these conditions, control disks (without pressure) were completely negative for VEGF expression as evidenced by immunocytochemical stainings as well as by enzyme-linked immunosorbent assay (ELISA) measurements. In contrast, 4 days after mechanical overload, the cartilage disks were positive in both detection methods. In addition, after mechanical overload chondrocytes were strongly immunopositive for hypoxia-inducible factor-1α (HIF-1α), the limiting protein of the dimeric transcription factor HIF-1 that is known to induce VEGF expression. Furthermore, the matrix metalloproteases MMP-1, MMP-3, and MMP-13, could be easily detected in pressure-treated disks by immunohistochemistry whereas staining in controls was low or undetectable. The tissue inhibitors of metalloproteinases (TIMP-1 and -2) could be detected in controls but not in samples treated with mechanical overload. To prove that increased MMP or decreased TIMP expression could be a result of the autocrine action of VEGF on chondrocytes, we repeated the experiments in the presence of a specific inhibitor for the kinase activity of the VEGFR-2. This inhibitor was effective to reduce mechanically induced MMP-1, -3, and -13 immunostaining and to restore TIMP expression. Taking together, these findings indicate that VEGF is induced in chondrocytes by mechanical overload and mediates destructive processes in osteoarthritis as an autocrine factor.
Vascular endothelial growth factor (VEGF-A, subsequently termed only VEGF) is known as an important mediator of angiogenesis.1–4 It is a 46 to 48 kd glycoprotein of two 121–206 residue subunits generated by alternative splicing from a single VEGF gene. VEGF is expressed during embryogenesis, but in the adult only in the menstrual cycle, during tissue remodeling, wound healing, as well as in malignant or certain inflammatory diseases. Hypoxia and various growth factors/cytokines enhance VEGF expression.1,2 A 28-base sequence has been identified in the 5′ promoter of the rat and human VEGF gene is essential for hypoxia-induced transcription.5 It reveals a high degree of homology and similar protein-binding characteristics for the hypoxia-induced transcription factor-1 (HIF-1) binding site within the erythropoietin gene.
VEGF acts mainly on endothelial cells by stimulating their proliferation, their migration, and the induction of various genes involved in tissue remodeling. However, a few other cell types also express VEGF receptors (VEGFR) including monocytes/specialized macrophages,6 osteoblasts,7 and osteoarthritic (OA) chondrocytes, but not resting chondrocytes.8–10 Whereas chemotactic effects of VEGF on monocytes and osteoblasts have been shown,6,7 little is known about VEGF expression and responses in chondrocytes.
Effects of VEGF are mediated by two signaling receptors that belong to the class III tyrosine kinase receptor family with seven extracellular immunoglobulin domains, one plasma membrane domain, and an intracellular tyrosine kinase domain: VEGFR-1 (flt-1, fms-like tyrosine kinase-1) and especially VEGFR-2 (KDR, kinase domain region/flk-1, fetal liver kinase-1). Furthermore, the co-receptors neuropilin-1 and -2 can bind the splice forms with different activities, but do not transduce signals on their own. Stimulation of VEGFR induces their dimerization, autophosphorylation, and induction of their kinase activity. Subsequently, kinases like mitogen-activated protein kinases (MAPK) or Akt kinase are phosphorylated and activated which finally results in the induction of transcription factors, proliferative, or chemotactic responses.1,2
In previous studies we and others have shown that OA chondrocytes produce VEGF, in particular the diffusible splice variants VEGF121 and VEGF189 and, moreover, express VEGFR-2 but not VEGFR-1.8–10 In contrast, normal adult chondrocytes are negative for both ligand and receptor. VEGF/VEGFR expression in chondrocytes appears to be associated with the OA process despite angiogenesis occuring only sometimes in the calcified cartilage. The factors that induce VEGF expression in cartilage and the possible effects of VEGF on cartilage are unknown.
Mechanical factors such as chronic overload (eg, in the varus knee) or a high sudden stress (high-intensity blunt impact trauma) play an important role for the pathogenesis of the osteoarthritic process. Epidemiological studies have suggested that participation in activities that expose joints to high levels of impact loading may increase the probability of joint degeneration.11,12 Traumatic joint injury has been demonstrated to be a risk factor for development of secondary osteoarthritis, but the mechanism by which this occurs is unknown. Trauma to the knee joint has been shown to induce cartilage degradation in vivo,13 and high-impact loads cause tissue injury14,15 and initiate cartilage degradation.16 Attempts to model this process in vitro have led to investigation of the effects of compressing cartilage tissue using loading conditions sufficient to produce acute injury.17–23
Using a well established in vitro injury model17–23 Kurz et al24 has shown that injurious compression of newborn bovine cartilage can induce cell death to a low extend, accompanied by cartilage swelling, release of matrix proteoglycan, and loss of the anabolic response to low-amplitude dynamic compression in the remaining cells. In the present paper, we use the same model to study the mechanisms that might be responsible for the degeneration of the tissue after mechanical injury.
Several in vitro studies have shown that the application of high mechanical stress as it occurs in a high-intensity impact trauma activates a wide array of cellular machinery and processes, including DNA synthesis, and apoptosis.24 Evidence that VEGF is up-regulated by mechanical stress was firstly provided by Li et al.25 They found a marked increase in VEGF mRNA in the ventricular wall of the heart after diastolic pressure had been increased.
The aim of the present study was to examine whether VEGF is induced by mechanical stress in cartilage disks. Since a recent study has shown that HIF-1α is up-regulated in the non-ischemic but mechanically stressed myocardium,26 we further examined the possibility that mechanical stress might also induce HIF-1α expression in chondrocytes. Many studies have shown that matrix metalloproteinases (MMPs) are highly up-regulated in OA cartilage, and it is also well know that these enzymes play also an important role for angiogenesis by facilating capillary growth via extracellular matrix (ECM) dissolution.27 Therefore, we further studied whether up-regulation of MMPs or down-regulation of tissue inhibitors of metalloproteinases (TIMPs) by mechanical stress might be mediated by VEGF.
Materials and Methods
Tissues and Application of Mechanical Stress
Cartilage disks (3 mm diameter × 1 mm thickness) were obtained from the supero-lateral quadrant of the femoro-patellar groove of 23 month-old cows and cultured under standard conditions as described previously.24 Two days after isolation mechanical injury was applied to groups of three to four cartilage disks in unconfined compression. A single controlled ramp-and-hold displacement to 50% final strain was applied with a velocity of 1 mm/second using an incubator-housed compression apparatus as described by Loening et al.28 This is a well-accepted model for the simulation of a blunt-impact trauma.24,28
Four days (for detection of HIF-1α, VEGF, and MMP) or 1 hour (for tyrosine phosphorylation) after a single compression, disks were fixed with 4% formaldehyde in phosphate-buffered saline, (PBS, pH 7.4), embedded in paraplast, and serially sectioned into 7-μm sections (about 10 sections per disk from the center part) for histological and immunhistochemical analysis, or were homogenized in 150 mmol/L NaCl, 20 mmol/L Tris/HCl-buffer, pH 7.4.
Histology and Immunohistochemistry
Deparaffinized 7-μm sections were stained with toluidine blue and examined by light microscopy. For immunohistochemistry, sections were dewaxed, incubated with testicular hyaluronidase (2 mg/ml in PBS, pH 5.0, for 30 minutes at room temperature) and pronase (1 mg/ml in PBS, pH 7.4, for 30 minutes at room temperature), immunostained with anti-VEGF (1:40 in Tris-buffered saline, 60 minutes; sc-7269 mouse monoclonal IgG2a, Santa Cruz Biotechnology, CA), or anti-VEGFR-1 (1:40; sc-316 rabbit polyclonal, Santa Cruz Biotechnology), or anti-VEGFR-2 (1:40; sc-6251 monoclonal IgG1, Santa Cruz Biotechnology), or anti-MMP-1 (1:20; 04–10-6798 mouse monoclonal, Biocarta, CA), or anti-MMP-3 (1:50; 04–10-6961 mouse monoclonal, Biocarta, CA), or anti-MMP-13 (1:50; sc-12363, goat polyclonal, Santa Cruz Biotechnology), or anti-TIMP-1 (1:50; 04–10-8480; mouse monoclonal, Biocarta, San Diego, CA), or anti-TIMP-2 (1:50; 04–10-8506; mouse monoclonal, Biocarta, CA), or anti-HIF-1 α (1:1500; mouse monoclonal, abcam, Cambridge, UK), followed by biotinylated secondary antibodies and a peroxidase-labeled streptavidin-biotin staining technique;29 nuclei were counter-stained with hemalum. Controls were performed either by omitting the primary antibody, or absorption of the primary antibody to recombinant human VEGF (2 μg/500 μl) overnight at 4°C. Co-staining was done according to standard protocols using the DAKO Envision system (diaminobenzidine (MMP-1 and -3) and Fast Red (VEGF) as chromogens; DAKO, Glostrup, Denmark).
Enzyme-Linked Immunosorbent Assay and Western Blots
For enzyme-linked immunosorbent assay (ELISA), aliquots of supernatant of injured and control disks (100 μl) were analyzed by a sandwich ELISA (R&D Systems, Minneapolis, MN) that detects all VEGF splice forms. Human recombinant VEGF165 (PreproTech, Rocky Hill, NJ) served as standard. For Western blots, samples were reduced in the presence of 10 mmol/L dithiothreitol, proteins separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% gels), transferred onto nitrocellulose membranes that were blocked and incubated with antibodies according to standard techniques as described.30 Signals were detected by chemoluminescence reaction (ECL-Plus; Amersham-Pharmacia, Uppsala, Sweden). Since the differences in amino acid residues between human and bovine VEGF are very low,31 the antibodies are cross-reactive.
Analysis of Kinase Phosphorylation
Cartilage disks were crushed in an achate mortar under liquid nitrogen, homogenized in cold PBS, and lysed with Triton-lysis-buffer (50 mmol/L Tris-HCl, pH 7.8, 100 mmol/L NaCl, 2 mmol/L EDTA, 1% Triton X-100, 2 mmol/L Na3VO4). The lysates were mixed vigorously (vortex mixer) and clarified in an Eppendorf centrifuge (15 minutes, 14,000 × g, 4°C). After protein determination from an aliquot, samples with equal amounts were boiled in 50 to 200 μl SDS-PAGE sample buffer, separated by SDS-PAGE (10%), transferred onto a polyvinylidene difluoride (PVDF) membrane that was blocked with 5% bovine serum albumin (BSA) overnight. The blots were incubated with anti-phosphorylated extracellular-signal related kinases ERK (1:250, mouse monoclonal; sc-7383, Santa Cruz, CA) and later with horseradish peroxidase-labeled anti-mouse IgG (1:30.000; DAKO, Glostrup, Denmark) and visualized by enhanced chemiluminescence (ECL System; Amersham).
Inhibition of the VEGFR
A potent and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinase activity is the 4-[(4′-chloro-2′-fluoro)phenylamino]-6,7-dimethoxyquinazoline32 (676475 Calbiochem). Mechanical injury and control was applied with (2 hours pre-incubation) or without inhibitor (500 nmol/L).
Statistics
Dunnet’s test has been used for the statistical analysis of the results. The level of significance was set at P < 0.05.
Results
High-Intensity Impact Stress Induces VEGF
To identify factors that induce VEGF expression in cartilage, we used bovine cartilage disks as a model. Since mechanical overload is the most likely factor for VEGF induction in OA, we simulated this situation in an incubator-housed compression apparatus.28 In this device controlled ramp-and-hold displacements to 50% final strain were applied at a rate of 1 mm/second. Controls were kept under the same conditions without pressure. As measured with an oxygen electrode, under conditions of pressure the oxygen concentration did not change.
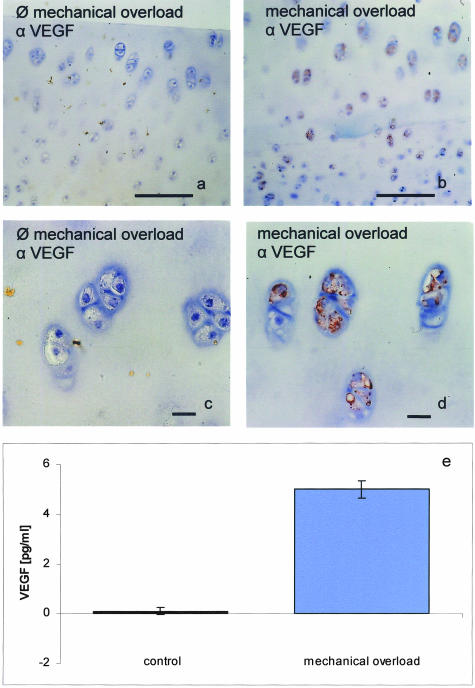
Under these conditions, control disks (without pressure) were completely negative for VEGF expression as evidenced by immunocytochemical stainings as well as by ELISA measurements of homogenized cartilage (Figure 1). In contrast, after mechanical overload, the cartilage disks were positive in both detection methods and the ELISA measurement showed that VEGF concentrations raised significantly after high-impact stress. VEGF could be immunostained in most chondrocytes, and the VEGF content of supernatant was raised to well-measurable concentrations. With the same method VEGF was measured in human OA cartilage.8 Thus, injurious compression is an inductive factor for VEGF expression in chondrocytes.
Figure 1.
Mechanical overload induces VEGF in bovine cartilage disks. Bovine cartilage exlants were obtained from the femoro-patellar groove of 23-month-old cows. Two days after isolation mechanical injury was applied to groups of three to four cartilage disks in unconfined compression. A single controlled ramp-and-hold displacement to 50% final strain was applied with a velocity of 1 mm/second using an incubator-housed compression apparatus. Four days after compression, the disks were fixed and immunostained for VEGF and nuclei were counterstained with Meyer’s hemalum. Strong immunoreactivity for VEGF can be detected in mechanically injured explants (b (overview) and d (detail)), but not in control explants (a (overview) and c (detail)). The specificity of the immunoreaction was checked by pre-adsorption of the antibody to recombinant VEGF. VEGF concentrations are strongly increased in supernatant of injured, but not in supernatant of control explants (e). Immunoreactive VEGF of the samples was determined by an ELISA detecting all soluble VEGF splice variants. Means ± standard deviations from n = 6 individual cows. Original magnification, ×150 (a and b); ×300 (c and d). Bar, 100 μm (a and b); bar, 10 μm (c and d).
High-Intensity Impact Stress Induces HIF
VEGF expression is regulated by several transcription factors including HIF, activator protein-1 (AP-1), AP-2, SP-1, and others. To evaluate whether HIF-1, the most common activator of VEGF expression, is involved in pressure-induced VEGF formation, we analyzed its expression by immunocytochemistry and by Western blot.
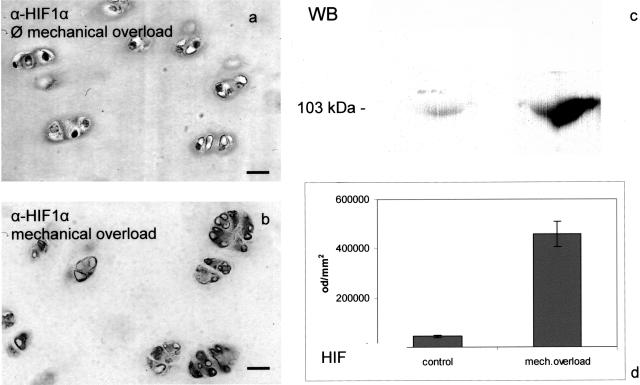
Chondrocytes were immunopositive for HIF-1α (the limiting protein of the dimeric HIF-1 complex, see Discussion) after mechanical overload, and in cartilage homogenates a band corresponding in molecular mass to bovine HIF-1α yielded a significantly stronger signal after mechanical overload than without pressure. Thus, the transcription factor HIF-1 is activated in cartilage by mechanical overload (Figure 2). The intensity of six Western blots was determined by optical density measurement using the analysis system PC BAS 2.0. Under these conditions only 6% of all cells were apoptotic,24 far more HIF- or VEGF-immunopositive.
Figure 2.
Mechanical overload induces HIF-1α in bovine cartilage disks. Mechanical injury was applied to bovine cartilage explants obtained from the femoro-patellar groove as descibed in Figure 1. Four days after compression, the disks were fixed and immunostained for HIF-1α and nuclei were counter-stained with Meyer’s hemalum. Strong immunoreactivity for HIF-1α can be detected in disks with mechanical overload (b) but not in control disks (a). HIF-1α protein can be detected by Western blot in disks treated with mechanical overload (lanes 2 and 4), but not in control disks (lanes 1 and 3). Samples were boiled for 5 minutes in sample buffer, proteins separated by SDS-PAGE and transferred onto nitrocellulose membranes that were stained with an HIF-1α antibody (c). The intensity of six Western blots was determined by optical density measurement using the analysis system PC BAS 2.0 (d). Original magnification, ×300 (a and b). Bar, 10 μm.
High-Intensity Impact Stress Induces MMP Expression and Reduces TIMP Expression
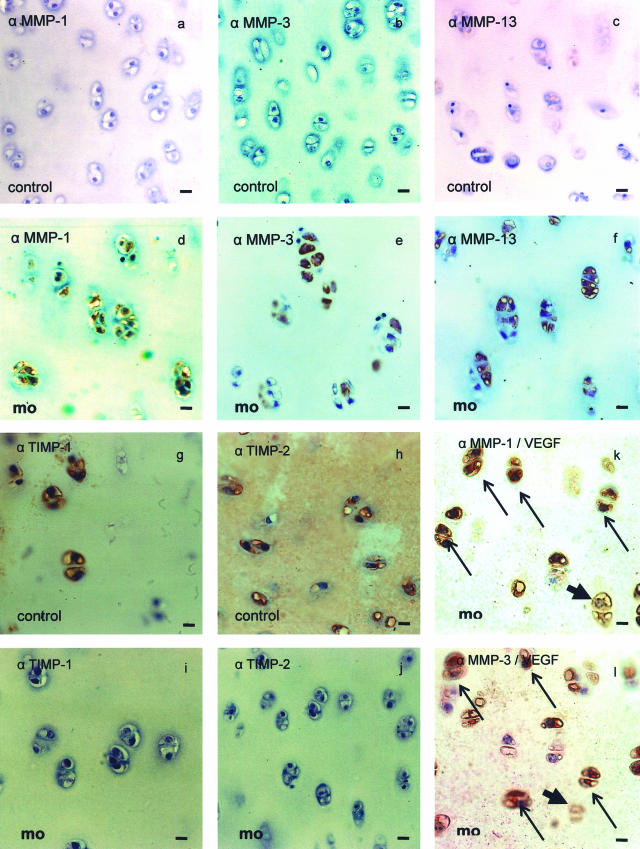
MMPs are important mediators of cartilage destruction in OA. Therefore, we investigated the effect of mechanical overload on bovine cartilage disks. As compared to controls, the proteases MMP-1, MMP-3, and MMP-13 (Figure 3, d to f) could be easily detected in mechanically injured explants by immunohistochemistry whereas staining in controls was low or undectable (Figure 3, a to c). Thus, MMPs that are able to destruct cartilage are induced in chondrocytes by mechanical overload. TIMP-1 and -2 are expressed in control explants (Figure 3, g and h) but not in mechanically injured explants (Figure 3, i and j). Co-localization of VEGF and MMP-1 and -3 revealed a co-expression in most chondrocytes after mechanical overload (Figure 3, k and l).
Figure 3.
MMPs are induced by mechanical overload in bovine cartilage disks. Bovine cartilage exlants were exposed to mechanical overload as described in Figure 1 and immunostained for MMP-1, -3, and -13. Nuclei in the sections were counterstained with Meyer’s hemalum. Strong immunoreactivity for MMP-1, -3, and -13 can be detected in disks with mechanical overload (mo) (d–f) but not in control disks (a–c). No immunoreactivity for TIMP-1 and -2 can be detected in disks with mechanical overload (mo) (i and j) but strong in control disks (g and h). Only few cells are solely positive for MMP-1 or MMP-3 (arrowheads, k and l); most cells are for VEGF (bold arrows) and MMP-1 or -3 positive (fine arrows, k and l). Original magnification ×300. Bar, 10 μm.
A VEGFR Kinase Inhibitor that Blocks Its Signal Transduction in Chondrocytes Is Able to Reduce MMP-1, -3, and -13 and to Restore TIMP Expression after Mechanical Overload
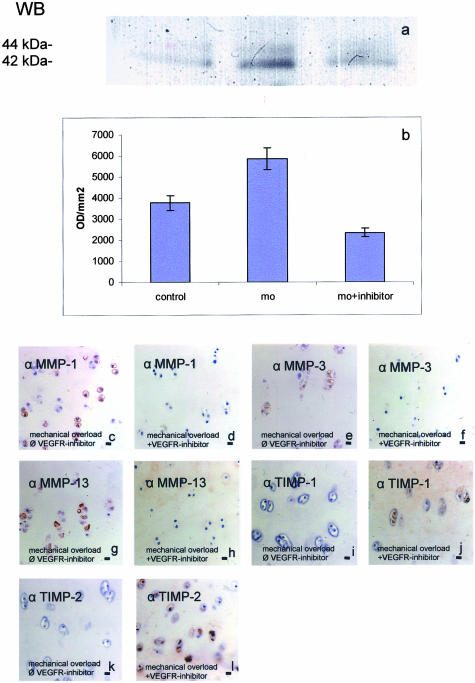
In the previous experiments we could show that mechanical overload induces VEGF as well as MMP expression in cartilage disks. To prove that MMP expression could be a result of the autocrine action of VEGF on chondrocytes, we repeated the experiments in the presence of a specific inhibitor for the kinase activity of the VEGFR-2. First we tested the efficiency of the inhibitor by analyzing the effect of recombinant VEGF on VEGFR signal transduction. VEGF phosphorylated the mitogen-activated protein kinases p44/p42 (extracellular-regulated kinases (ERK)) within 60 minutes (Figure 4a). This effect could be blocked by co-incubation with the VEGFR kinase inhibitor 4-[(4′-chloro-2′-fluoro)phenylamino)6,7-dimethoxyquinazoline at 0.5 μmol/L ((32); Calbiochem-676475; IC50 = 0.1 μmol/L for VEGFR-2). Thus, the VEGFR-2 kinase inhibitor is able to specifically block VEGF-induced effects in cartilage. The intensity of six Western blots was determined by optical density measurement using the analysis system PC BAS 2.0 (Figure 4b).
Figure 4.
Mechanical overload induces the phosphorylation of the MAPK ERK 1/2 which is suppressed by pre-incubation with 500 nmol/L 4-[(4′-chloro-2′-fluoro)phenylamino]6,7-dimethoxyquinazoline, an inhibitor of the VEGFR-2 kinase activity (a and b). Cartilage disks were exposed to mechanical overload alone or together with the inhibitor and after 1 hour lysed and analyzed for phosphorylated ERK 1 (p44) and ERK 2 (p42) by Western blotting using a specific antibody. Mechanical overload (MO) strongly induced the phosphorylation of ERK (lanes 2 and 3). Co-stimulation with the VEGFR-inhibitor reduced phosphorylation of ERK 1/2 (lane 4). The intensity of six Western blots was determined by optical density (OD) measurement using the analysis system PC Bas 2.0 (b). MMP-1, -3, and -13 are induced by mechanical overload in bovine cartilage disks (c, e, g). The VEGFR-2 kinase inhibitor is able to specifically block VEGF-induced effects in cartilage. When applied in an experiment with cartilage disks under mechanical overload, this inhibitor was effective to reduce MMP-1, -3, and -13 (d, f, h) immunostaining. TIMP-1 and -2 are reduced by mechanical overload (i and k). Co-incubation with the VEGFR-2 kinase inhibitor leads to an increase of TIMP-1 and -2 (j and l). Original magnification, ×150. Bar, 10 μm.
When applied in an experiment with cartilage disks under mechanical overload, this inhibitor was effective to reduce MMP-1, -3, and -13 immunostaining (Figure 4, c and d). Thus, MMP-1, -3, and -13 expression appears to be (at least partially) dependent on the activation of VEGFR-2 by the ligand. TIMP-1 and -2 are reduced by mechanical overload (Figure 4, i and k). Co-incubation with the VEGFR-2 kinase inhibitor leads to an increase of TIMP-1 and -2 (Figure 4, j and l).
Discussion
Osteoarthritis (OA) is an organ failure of articular cartilage which can be caused by a variety of mechanical, genetic, and/or biochemical factors. It involves an initial proliferation and clustering of the chondrocytes in cartilage fissures, followed by the proteolytic degradation of the extracellular collagen/proteoglycan matrix. OA develops most frequently in the absence of a known cause (primary OA), but it may also result from joint injury or from developmental, metabolic, and inflammatory disorders that destroy the articular surface causing secondary OA. Mechanical factors such as high-intensity impact joint loading are known to induce damage to the articular cartilage.11,12 Inflammatory factors appear to be responsible in the transformation of regular chondrocytes to OA chondrocytes which resemble, in part, hypertrophic chondrocytes.33,34 As soluble factors, pro-inflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) have been proposed to play a major role in transformation of normal to OA chondrocytes.33
We here show that VEGF, formerly recognized mainly as an angiogenesis factor, can act as a further destructive factor in OA cartilage. VEGF expression in chondrocytes is induced by high-intensity impact stress, and it acts in cartilage as an autocrine inductor of MMPs known to be responsible for cartilage destruction.35,36 We used an in vitro model for the application of high-intensity impact stress which has been stated by Loening et al.28
Interestingly, VEGF induction in chondrocytes by mechanical overload is linked to activation of the transcription factor HIF-1 which is known to bind to a hypoxia response element (HRE) in the human VEGF gene promoter.37 Activated HIF-1 is a trans-activating dimeric protein of composed two subunits, HIF-1α and HIF-1β, which belong to the family of basic helix-loop-helix transcription factors. Normally, HIF-1 activity depends on the amount of HIF-1α protein whereas HIF-1β is constitutively expressed regardless of oxygen tension. The HIF-1α subunit is unstable under normoxic conditions and not detectable, because it possesses an oxygen-dependent degradation domain that targets it for ubiquination and subsequent degradation in the proteasome. Interestingly, not only hypoxia is known to stabilize HIF-1α, but also mechanical stresses, eg, in cardiac myocytes.26 Also, the mechanical induction of VEGF has been reported in myocardial,25 mesangial,38 or smooth muscle cells.39,40 It appears that after an initial stimulation of stretch-activated ion channels, the PI3K (phosphatidylinositol-3-kinase)/Akt/FRAP (FKBP and rapamycin-associated protein) signal transduction pathways are involved in this process26,41 which stabilizes HIF-1α and, subsequently, the dimeric HIF transcription factor complex. Finally, the expression of VEGF is enhanced.
VEGF induction via stabilization of HIF-1α by mechanical stresses appears to be also a decisive factor for VEGF expression in chondrocytes after mechanical overload in cartilage slices, since we could observe their induction in our model in parallel. Other cytokines or growth factors that are also known to enhance VEGF expression in other cell types may further contribute to this process.
We and others have previously shown8–10 that OA chondrocytes do not only produce VEGF, but express also receptors for this peptide. We now elucidate some of the autocrine, destructive functions of VEGF in cartilage. After induction of VEGF in bovine cartilage disks by mechanical overload, MMP-1 (collagenase 1, EC 3.4.24.7), MMP-3 (stromelysin; EC 3.4.24.17), and MMP-13 (collagenase 3, EC 3.4.24.-) are increased while TIMP-1 and -2 are decreased. Their formation could be reduced in the presence of a VEGFR kinase inhibitor that blocks the signal transduction of VEGF. Recently, Enomoto et al10 showed that in cultivated OA chondrocytes, VEGF induced MMP-1 and -3 productions significantly. All MMPs are known to occur in OA and are able to degrade various collagens and proteoglycans.35,36 Thus, VEGF is an autocrine regulator of OA chondrocytes that induces MMPs and, therefore, contributes to their destructive potential (Figure 5). By decreasing TIMP-2 in cartilage explants after mechanical overload, VEGF could reduce the inhibitors of MMPs and the dephosphorylation of VEGF-R42 (Figure 5). Patwari et al43 could show that only the MMP-3 mRNA level (mRNA measured by Northern analysis) is increased 24 hours after injurious compression of cartilage explants. This may depend on time after injurious compression.
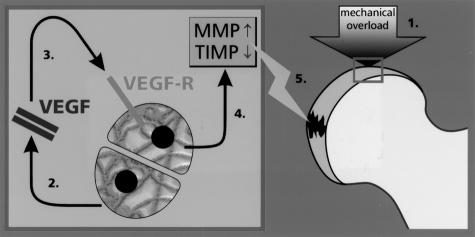
Figure 5.
Scheme of the role of VEGF in osteoarthritis. Mechanical overload induces the transcription factor HIF-1. The subsequent expression of VEGF activates the chondrocytes autocrinally for producing MMP-1, -3, and -13. TIMP-1 and -2, the inhibitors of MMPs, are reduced by mechanical overload. The increase of MMP and the decrease of TIMP contribute to the destruction of the articular cartilage.
The function of VEGF in OA chondrocytes resembles, in part, its role in hypertrophic chondrocytes. Here, VEGF is involved in endochondral ossification, the process whereby cartilage is replaced by bone and long bones are formed.44,45 In hypertrophic cartilage, VEGF couples cartilage remodeling/destruction with angiogenesis and deposition of bone. The two latter processes are only sometimes observed in OA or experimental models of mechanical overloading of cartilage (blood vessel formation in OA, osteophyte formation.46,47). Apart from these functions within the cartilage, VEGF may also be involved in inflammatory reactions in OA and contribute to symptoms such as pain and swelling by targeting the synovial membranes. Chronic inflammatory responses are often associated with the production of angiogenic factors that induce vascular proliferation, and in rheumatoid arthritis VEGF is highly expressed in synovial cells.29,48
In conclusion, after application of mechanical overload to bovine cartilage disks, VEGF and MMPs are induced in chondrocytes. Induction of VEGF involves the stabilization of the transcription factor HIF-1α that is a known promoter of VEGF expression. Furthermore, MMP-1, -3, and -13 induction and TIMP-1 and -2 inhibition is at least, in part, dependent on the autocrine action of VEGF on chondrocytes, since a VEGFR kinase inhibitor is able to reduce their formation. These data strongly suggest that VEGF plays an important autocrine or paracrine role in the progression of osteoarthitis.
Acknowledgments
We thank Frank Lichte, Miriam Lemmer, Marion Lorenzen, Inka Kronenbitter, Sonja Seiter, Karin Stengel, and Regine Worm for their expert technical assistance and Clemens Franke for the drawing (Figure 5).
Footnotes
Address reprint requests to Prof. Dr. Rolf Mentlein, Department of Anatomy, University of Kiel, Olshausenstraβe 40, 24106 Kiel, Germany. E-mail: rment@anat.uni-kiel.de.
Supported by a grant from the Deutsche Forschungsgemeinschaft DFG (PU 214/3–1).
References
- Neufeld G, Cohen T, Gengrinovitch S, Poltrak Z. Vascular endothelial growth factor and its receptors. EMBO J. 1999;13:9–22. [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Held-Feindt J. Angiogenesis factors in gliomas: a new key to tumour therapy? Naturwissenschaften. 2003;90:385–394. doi: 10.1007/s00114-003-0449-9. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol. 2002;132:93–98. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. Expression of the vascular endothelial growth factor in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pfander D, Kortje D, Zimmermann R, Weseloh G, Kirsch T, Gesslein M, Cramer T, Swoboda B. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis. 2001;60:1070–1073. doi: 10.1136/ard.60.11.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, Matsumoto H, Toyama Y, Okada Y. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol. 2003;162:171–181. doi: 10.1016/s0002-9440(10)63808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin EL, Paul IL. Response of joints to impact loading: I. In vitro wear. Arthritis Rheum. 1971;14:356–388. doi: 10.1002/art.1780140306. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Lane NE. Aging, sports, and osteoarthritis. Sports Med Arthrosc Rev. 1996;4:276–287. [Google Scholar]
- Thompson RC, Oegema TR, Lewis JL, Wallace LJ. Osteoarthrotic changes after acute transarticular load: an animal model. J Bone Joint Surg. 1991;73:990–1001. [PubMed] [Google Scholar]
- Pickvance EA, Oegema TR, Thompson RC. Immunolocalization of selected cytokines and proteases in canine articular cartilage after transarticular loading. J Orthop Res. 1993;11:313–323. doi: 10.1002/jor.1100110302. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Vener MJ, Griffiths HJ, Lewis JL, Oegema TR, Wallace L. Scanning electron-microscopic and magnetic resonance-imaging studies of injuries to the patellofemoral joint after acute transarticular loading. J Bone Joint Surg. 1993;75:704–713. doi: 10.2106/00004623-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130:278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- Steinmeyer J, Knue S. The proteoglycan metabolism of mature bovine articular cartilage explants superimposed to continuously applied cyclic mechanical loading. Biochem Biophys Res Commun. 1997;240:216–221. doi: 10.1006/bbrc.1997.7641. [DOI] [PubMed] [Google Scholar]
- Farquhar T, Xia Y, Mann K, Bertram J, Burton-Wurster N, Jelinski L, Lust G. Swelling and fibronectin accumulation in articular cartilage explants after cyclic impact. J Orthop Res. 1996;14:417–423. doi: 10.1002/jor.1100140312. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Borkey AL, Lust G. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. J Biomech. 1997;30:1–9. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg. 1977;59:1068–1076. [PubMed] [Google Scholar]
- Jeffrey JE, Thomson LA, Aspden RM. Matrix loss and synthesis following a single impact load on articular cartilage in vitro. Biochim Biophys Acta. 1997;1334:223–232. doi: 10.1016/s0304-4165(96)00097-9. [DOI] [PubMed] [Google Scholar]
- Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- Radin EL, Paul IL, Lowy M. A comparison of the dynamic force transmitting properties of subchondral bone and articular cartilage. J Bone Joint Surg. 1970;52:444–456. [PubMed] [Google Scholar]
- Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- Li J, Hampton T, Morgan JP, Simons M. Stretch-induced VEGF expression in the heart. J Clin Invest. 1997;100:18–24. doi: 10.1172/JCI119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1α in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:25–33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 1989;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, Nuttall ME, Hung HH, Blake SM, Grodzinsky AJ, Lark MW. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF165 of the angiogenic peptide vascular endothelial growth factor are expressed in the synovial tissue of patients with rheumatoid arthritis. J Rheumatol. 2001;28:1482–1486. [PubMed] [Google Scholar]
- Held-Feindt J, Krisch B, Mentlein R. Molecular and functional analysis of the somatostatin receptor subtype 2 (sst2) in human glioma cells. Mol Brain Res. 1999;64:101–107. doi: 10.1016/s0169-328x(98)00312-x. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Brace RA. Ovine vascular endothelial growth factor: nucleotide sequence and expression in fetal tissues. Growth Factors. 1998;16:11–22. doi: 10.3109/08977199809017488. [DOI] [PubMed] [Google Scholar]
- Hennequin LF, Thomas AP, Johnstone C, Stokes ES, Plé PA, Lohmann JJ, Ogilvie DJ, Dukes M, Wedge SR, Curwen JO, Kendrew J, Lambert-van der Brempt C. Design and structure-activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J Med Chem. 1999;42:5369–5389. doi: 10.1021/jm990345w. [DOI] [PubMed] [Google Scholar]
- Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- Goldring MB. Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther. 2001;1:817–829. doi: 10.1517/14712598.1.5.817. [DOI] [PubMed] [Google Scholar]
- Huebner JL, Otterness IG, Freund EM, Caterson B, Kraus VB. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998;41:877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Thomas S, Burt D, Lane S, Chusney G, Sacks S, Viberti G. Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc Natl Acad Sci USA. 1997;94:12112–12116. doi: 10.1073/pnas.94.22.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:897–903. doi: 10.1152/ajplung.00044.2001. [DOI] [PubMed] [Google Scholar]
- Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium. 2001;8:41–48. doi: 10.3109/10623320109063156. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Seo D-W, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei B-y, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, Cole AA, Lark MW, Grodzinsky AJ. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kolwalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification, and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Carlevaro MF, Cermelli S, Cancedda R, Descalzi-Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- Bullough PG, Jagannath A. The morphology of the calcification front in articular cartilage. J Bone Joint Surg. 1983;65:72–78. doi: 10.1302/0301-620X.65B1.6337169. [DOI] [PubMed] [Google Scholar]
- Radin EL, Parker HG, Paul IL. Pattern of degenerative arthritis: preferential involvement of distal finger-joints. Lancet. 1971;20:377–379. doi: 10.1016/s0140-6736(71)92213-6. [DOI] [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Spencer-Green G, Yeo K-T, Yeo T-K, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]