Abstract
Intracellular accumulations of filamentous material composed of tau proteins are defining features of sporadic and familial neurodegenerative disorders termed “tauopathies.” In Alzheimer’s disease, the most common tauopathy, tau pathology is predominantly localized within neurons; however, robust glial pathology occurs in other tauopathies. Although the pathogenesis of tauopathies remains primarily unknown, molecular chaperones such as heat-shock proteins (HSPs) are implicated in these tau disorders as well as other neurodegenerative diseases characterized by the accumulation of insoluble protein aggregates such as α-synuclein in Parkinson’s disease and polyglutamine in Huntington’s disease. We analyzed a variety of tauopathies with antibodies to a panel of HSPs to determine their role in the pathogenesis of these disorders. Although HSPs are not found in neuronal tau inclusions, we demonstrate increased expression of the small HSP αB-crystallin in glial inclusions of both sporadic and familial tauopathies. αB-crystallin was observed in a subset of astrocytic and oligodendrocytic tau inclusions as well as the neuropil thread pathology in cellular processes, but the co-expression of αB-crystallin with tau inclusions was relatively specific to tauopathies with extensive glial pathology. Thus, increased αB-crystallin expression in glial tau inclusions may represent a response by glia to the accumulation of misfolded or aggregated tau protein that is linked to the pathogenesis of the glial pathology and distinct from mechanisms underlying neuronal tau pathology in neurodegenerative disease.
Filamentous inclusions composed of the microtubule-associated protein tau are the neuropathological hallmark of a class of both sporadic and familial disorders that are collectively referred to as tauopathies.1 Tau is a low-molecular weight microtubule-associated protein that is abundant in the central nervous system where it is expressed predominantly in axons.2 Tau regulates the assembly and stability of microtubules and this microtubule binding function of tau is negatively regulated by phosphorylation.3 However, in tauopathies such as Alzheimer’s disease (AD), the tau protein in the filamentous aggregates is abnormally hyperphosphorylated and, biochemically, highly insoluble.1,3
In many tauopathies, including sporadic disorders such as corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) as well as the familial tauopathy frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), there is not only tau pathology in neurons but also robust tau pathology in astrocytes and oligodendrocytes,4 wherein tau is normally expressed at very low levels.5,6 Furthermore, there is also extensive tau pathology within the processes of neurons and glia in the form of neuropil threads in both gray and white matter.7 The pathogenic mechanisms underlying the in vivo aggregation of tau are primarily unknown, although distinct mechanisms may underlie this aggregation in neurons versus glia.8
Heat-shock proteins (HSPs) are implicated in the pathogenesis of a variety of neurodegenerative disorders including Huntington’s disease and Parkinson’s disease.9 HSPs are a large group of proteins that are highly conserved across species and function as molecular chaperones playing a critical role in protein stabilization, folding, and assembly.10,11 In Huntington’s disease, HSP70 was demonstrated to be neuroprotective against polyglutamine toxicity, whereas in Parkinson’s disease, HSP70 suppressed α-synuclein neurotoxicity in dopaminergic neurons.12,13 Furthermore, the expression of the small HSPs HSP27 and αB-crystallin (αBC), normally present at low levels in astrocytes and oligodendrocytes14,15 is induced in other neurodegenerative disorders, such as Alexander’s disease,14 Creutzfeldt-Jacob disease,15 and AD.16,17 However, in AD the enhanced expression of αBC is restricted to reactive astrocytes and microglia.16,17
In some tauopathies, αBC is expressed in a subset of degenerating achromatic or ballooned neurons that are only variably immunoreactive for tau proteins.18 Yet, it remains uncertain whether other HSPs are induced in tauopathies. In this study we analyzed a variety of disorders with tau pathology for the induction of HSPs. We demonstrate the increased expression of αBC in glia of both sporadic and familial tauopathies. The enhanced expression of αBC was specific to those disorders with prominent glial pathology thereby suggesting distinct pathogenic mechanisms for tau aggregation in neurons versus glia in neurodegenerative tauopathies.
Materials and Methods
Patients
Brain tissue was obtained from the brain bank at the Center for Neurodegenerative Disease and AD Center at the University of Pennsylvania School of Medicine. Fixed and frozen brain tissues from patients with the neuropathological diagnoses of CBD, PSP, FTDP-17, AD, and schizophrenia, as well as normal control brains, were analyzed histochemically and biochemically. Pathological diagnoses conformed with the established diagnostic criteria used for CBD,19 PSP,20 FTDP-17,21 and AD22 were used. All of the AD patients examined in this study were given a clinical diagnosis of probable AD22 and demonstrated extensive neurofibrillary pathology consistent with Braak stage V-VI.23 Brain regions examined included affected neocortex and basal ganglia from five CBD, six PSP, two FTDP-17 (N279K and intron 10 + 16), six schizophrenia, and four normal patients and affected neocortex and hippocampus from eight AD patients. Occipital lobe/visual cortex was used as a relatively unaffected control brain region. Demographic information for the patients analyzed is presented in Table 1.
Table 1.
Demographic Information of Patients Used for This Study
| Neuropathological diagnosis | No. of cases analyzed | Age, years (range) | Gender (M:F) | PMI, hours (range) |
|---|---|---|---|---|
| Normal | 4 | 63 (43–92) | 3:1 | 15 (5–30) |
| Schizophrenia | 6 | 77 (49–95) | 2:4 | 13 (7–20) |
| CBD | 5 | 68 (62–74) | 2:3 | 8 (5–15) |
| PSP | 6 | 76 (71–82) | 2:4 | 12 (2–20) |
| FTDP-17 | 2 | 56 (49–62) | 0:2 | 5.5 (5–6) |
| AD | 8 | 76 (59–96) | 3:5 | 13 (5–24) |
M, male; F, female; PMI, post-mortem interval.
Antibodies
Primary monoclonal antibodies (mAbs) for HSPs and dilutions (indicated in parentheses) including those specific for: HSP90 (1:200),24 HSP70 (1:200),25 Hsc70 (1:800),26 HSP60 (1:500),25 HSP40 (1:5000),27 αBC (1:1000 to 2500),28 HSP27 (1:200)29 were purchased from StressGen Biotechnologies Corp. (Victoria, Canada). Other mAbs specific for HSP2730 and HSP7031 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and used to confirm the results obtained with StressGen mAb. Immunoreactivity to αBC was also detected with a rabbit polyclonal antibody (1:200) purchased from StressGen Biotechnologies Corp. Antibodies used to assess tau pathology included the mAbs PHF-1 (1:2000), generously provided by Peter Davies (Department of Pathology and Neuroscience, Albert Einstein College of Medicine, NY),32 Tau14 (1:3000),33 and Tau46 (1:1000)33 as well as a rabbit polyclonal antiserum made to the N-terminal 12 amino acids of tau (Ntau, 1:250) (BabCo/Covance, Denver, PA). A rabbit polyclonal antibody specific for the astrocyte-specific intermediate filament glial fibrillary acidic protein (GFAP) was purchased from DAKO (1:10,000) (Carpinteria, CA).
Histochemistry and Immunohistochemistry
Tissue obtained at the time of autopsy was fixed in 10% formalin, paraffin-embedded, and cut into 6-μm-thick sections. In all cases, fixation time was limited to 30 hours. Immunohistochemistry was performed as previously described using the avidin-biotin complex (ABC) method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine as chromogen.34 Tau pathology and αBC immunoreactivity in all cases was assessed semiquantitatively by two individuals as absent (0), mild (1+), moderate (2+), or marked (3+) as shown in Table 2. Double-labeling immunofluorescence studies were performed by co-incubating sections with antibodies specific for tau and αBC. After extensive washes, sections were labeled using Alexa Fluor 488- and 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR), washed, and coverslipped with Vectashield-DAPI-mounting medium (Vector Laboratories). The sections were viewed with an Olympus BX51 (Tokyo, Japan) microscope equipped with bright-field and fluorescence light sources. Both bright-field and fluorescent images were obtained from the same field using a ProGres C14, Jenoptik camera (Laser Optik Systeme, Germany).
Table 2.
Tau and Corresponding αBC Immunoreactivity in Tauopathy Patients
| Diagnosis | No. | Frontal lobe
|
Occipital cortex
|
Basal ganglia
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gray matter
|
White matter
|
Gray matter
|
White matter
|
Globus pallidus
|
Striatum
|
||||||||
| Tau | αBC | Tau | αBC | Tau | αBC | Tau | αBC | Tau | αBC | Tau | αBC | ||
| CBD | 1 | 3 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 2 |
| CBD | 2 | 3 | 3 | 3 | 3 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 3 |
| CBD | 3 | 3 | 3 | 3 | 3 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 3 |
| CBD | 4 | 3 | 1 | 3 | 3 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 2 |
| CBD* | 5 | 3 | 2 | 3 | 3 | 0 | 1 | 0 | 0 | NA | NA | NA | NA |
| PSP | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| PSP | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 2 |
| PSP | 3 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 3 | 2 |
| PSP | 4 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 3 |
| PSP | 5 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 1 | 2 | 1 |
| PSP | 6 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 3 | 3 | 3 | 3 |
| FTDP-17, E10 + 16 | 1 | 3 | 3 | 3 | 3 | 1 | 2 | 1 | 2 | 3 | 3 | 3 | 2 |
| FTDP17, N279K | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 2 | 3 | 2 |
| SCH | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| SCH | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| SCH | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| SCH | 4 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| SCH | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 |
| SCH | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Frontal lobe, mid-frontal gyrus; occipital lobe, calcerine cortex; NA, not available; E, exon.
0, absent; 1, mild density; 2, moderate density; 3, high density.
Superior temporal gyrus was substituted for mid-frontal cortex because of tissue availability.
Tau Preparations and Western Blot Analysis
Fresh, frozen brain tissue was obtained from the mid-frontal lobe, striatum (caudate and putamen), and globus pallidus for biochemical analysis. Tissue was obtained from the hemisphere contralateral to that used for the immunohistochemical analysis. Gray and white matter was dissected from the mid-frontal cortex. Biochemical analysis of tau and αBC was performed as previously described.35 Briefly, sequential extraction of tau proteins was performed using buffers with increasing abilities to solubilize proteins as follows: 1) high-salt Tris-buffered saline (HS-TBS) (0.75 mol/L NaCl, 50mmol/L Tris buffer, pH 7.4, 2 mmol/L ethylenediaminetetraacetic acid); 2) 1% Triton in HS-TBS; 3) RIPA buffer (0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 2mmol/L ethylenediaminetetraacetic acid, 150 mmol/L NaCl, 50 mmol/L Tris buffer, pH 8.0); 4) SDS; and 5) 70% formic acid (FA). Extractions were performed at a concentration of 2.0 ml of extraction buffer per g of starting tissue except for FA, which was used at 0.5 ml/g of tissue. Each extraction step was repeated twice, samples were spun at 45,000 rpm for 30 minutes at 4°C and supernatants were collected. For Western blot analysis, nitrocellulose replicas were prepared from 7.5% or 15% SDS-polyacrylamide gel electrophoresis slab gels and probed with antibodies specific for tau or αBC as indicated. For all Western blots, 5 to 10 μl of extract was used corresponding to 2.5 to 5 μg of tissue, and bound mAbs were detected with horseradish peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA). Immunoreactive proteins were revealed using enhanced chemiluminescence (NEN Life Science, Boston, MA). Quantitative Western blot analysis was performed as previously described36 using a mixture of Tau14 and Tau46 or mAb αBC followed by 2 mCi/ml of I125-labeled goat anti-mouse IgG (IgG; New England Nuclear, Boston, MA) as secondary antibodies. The radiolabeled blots were exposed to Phosphorimager plates, and the protein bands were visualized and quantified with ImageQuant software (Molecular Dynamics Inc., Sunnyvale, CA).
Results
Increased αBC Immunoreactivity in Affected Neocortex and Basal Ganglia of Tauopathies
To investigate the possibility of a relationship between molecular chaperones and tauopathies, we immunostained brain sections with a variety of different tau pathologies using a panel of antibodies to HSPs including HSP90, HSP70, Hsc70, HSP60, HSP40, αBC, and HSP27. Although the majority of the antibodies revealed little or no change in HSP immunoreactivity relative to control brains, the HSP αBC, and to a lesser extent, HSP27, and HSP60, demonstrated enhanced immunostaining. To further characterize the expression of HSPs in tauopathies, we examined selected brain regions including mid-frontal and visual cortex and basal ganglia from a large panel of tauopathy and control brains (Table 1) with antibodies to these HSPs as well as to tau. Although there was a modest increase in αBC immunostaining in cells with the morphology of reactive astrocytes, in severely affected neocortex, there was a marked increase in both the quantity and intensity of the αBC immunoreactivity, particularly in white matter of CBD and FTDP-17 patients as compared to schizophrenia and normal patients (Figure 1). In PSP, there was a modest increase in αBC immunoreactivity relative to control brains that correlated with mild and variable neocortical tau pathology (Figure 1 and Table 2). Similarly, there was enhanced αBC expression relative to control normal adult and schizophrenia patients in the globus pallidus and, to a lesser extent striatum (caudate and putamen) in CBD, PSP, and FTDP-17, all of which are tauopathies with prominent tau pathology in the basal ganglia (Figure 2 and Table 2). In contrast, there was no increase in αBC immunostaining in the unaffected occipital lobes that lacked tau pathology in all patients examined (Table 2). Moreover, semiquantitative analysis of tau and αBC immunostaining demonstrated a correlation between αBC expression and the amount of tau pathology (Figure 3).
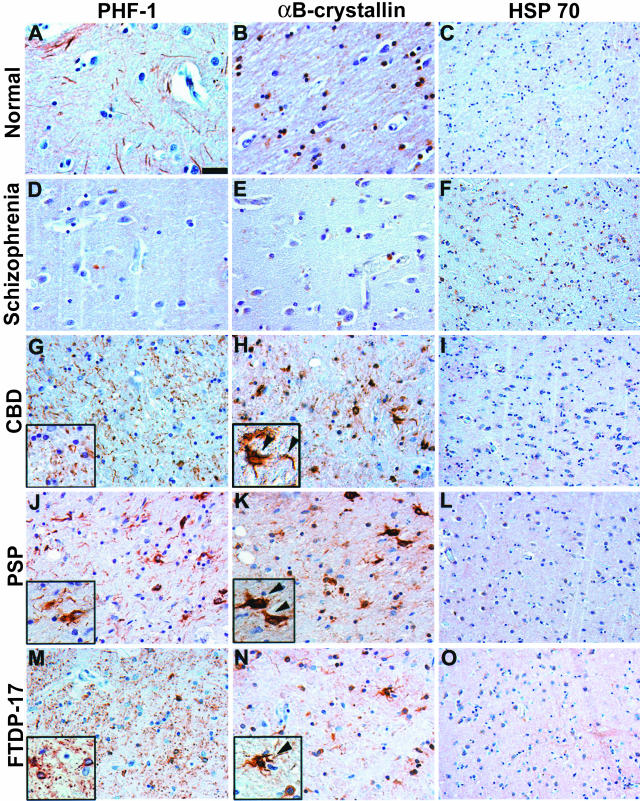
Figure 1.
Expression of αBC in subcortical white matter of patients with tauopathies. Adjacent sections of mid-frontal [normal, schizophrenia (patient 2), PSP (patient 2), and FTDP-17 (patient 1)] or temporal cortex [CBD (patient 5)] were immunostained with PHF1 (A, D, G, J, M), αBC (B, E, H, K, N), or HSP70 (C, F, I, L, O). A, D, G, J, and M: Robust tau pathology including threads and coiled bodies was observed in the subcortical white matter of CBD and FTDP-17 brains. The PSP brain showed mild tau pathology whereas no pathology was detected in schizophrenia or normal control brains. Insets in G and M show high-power magnification of characteristic glial pathology in CBD and FTDP-17 including coiled bodies and neuropil threads. B, E, H, K, and N: Adjacent sections show robust αBC staining in regions with abundant tau pathology of CBD and FTDP-17 brains. Insets in H and N show αBC staining in CBD and FTDP-17 that resembles coiled bodies (arrowheads). PSP, which showed mild tau pathology, also showed mild αBC immunoreactivity (arrowheads). In contrast, the control and schizophrenia brains show normal levels of αBC staining. C, F, I, L, and O: No increase in the iHSP70 immunoreactivity was observed in disease relative to control brains. Scale bar, 100 μm (A, B, D, E, G, H, J, K, M, N); 200 μm (C, F, I, L, O).
Figure 2.
Expression of αBC in the basal ganglia of patients with tauopathies. Adjacent sections of basal ganglia were immunostained with PHF1 (A, D, G, J, M), αBC (B, E, H, K, N), and HSP70 (C, F, I, L, O). A, D, G, J, and M: Robust tau pathology including threads and coiled bodies was observed in the globus pallidus and to a lesser extent, striatum in CBD (patient 2), PSP (patient 6), and FTDP-17 (patient 1) brains. No tau pathology was detected in the brains from schizophrenia (patient 2) or normal controls. Insets in G, J, and M show high-power magnifications of characteristic glial pathology in CBD, PSP, and FTDP-17 including coiled bodies and neuropil threads. B, E, H, K, and N: Similar to the neocortex, adjacent sections show robust αBC staining in sections with abundant tau pathology (H, K, N). Insets show αBC staining that resembles coiled bodies (arrowheads, H and K) in CBD and PSP or astrocytic inclusions (N) in FTDP-17. In contrast, the normal and schizophrenia brains show baseline levels of αBC staining. C, F, I, L, and O: No increase in iHSP70 immunoreactivity was detected in the basal ganglia of tauopathies. Scale bar, 100 μm (A, B, D, E, G, H, J, K, M, N); 200 μm (C, F, I, L, O).
Figure 3.
αBC expression is increased in affected brain regions of patients with tauopathies. The mid-frontal and occipital cortex (gray and white matter) and basal ganglia (globus pallidus and caudate/putamen) from five CBD, six PSP, two FTDP-17, and six schizophrenia (SCH) were analyzed by IHC for tau and αBC. Immunoreactivity was graded semiquantitatively as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe). Bars in the graph represent mean tau pathology (open bars) and αBC immunoreactivity (solid bars). Error bars indicate SD of the mean. *, Denotes regions with a mean value of zero.
The small HSP, HSP27, and the mitochondrial chaperone HSP60 also showed increased immunostaining in disorders with tau pathology. Similar to αBC, HSP27 reactivity was detected in glia in CBD, PSP, and FTDP-17 patients albeit to a lesser extent than that observed for αBC (Figure 4). In contrast to the small HSPs, HSP60 was detected predominantly in cells with the morphology of reactive astrocytes throughout the subcortical white matter and basal ganglia of CBD, PSP, and FTDP-17 patients relative to the corresponding brain regions of control schizophrenia and normal brains (Figure 4). Furthermore, the increase in HSP60 levels was not limited to regions with tau pathology consistent with HSP60 expression in reactive astrocytes. In contrast, we detected no increase in HSP40, HSP90 (data not shown), or the inducible Hsp70 (iHSP70) in both affected and unaffected brain regions (Figures 1 and 2). This latter finding was unexpected because increased iHSP70 expression was described in cultured astrocytes, oligodendrocytes, and microglia subjected to a variety of physiological stressors.37–41
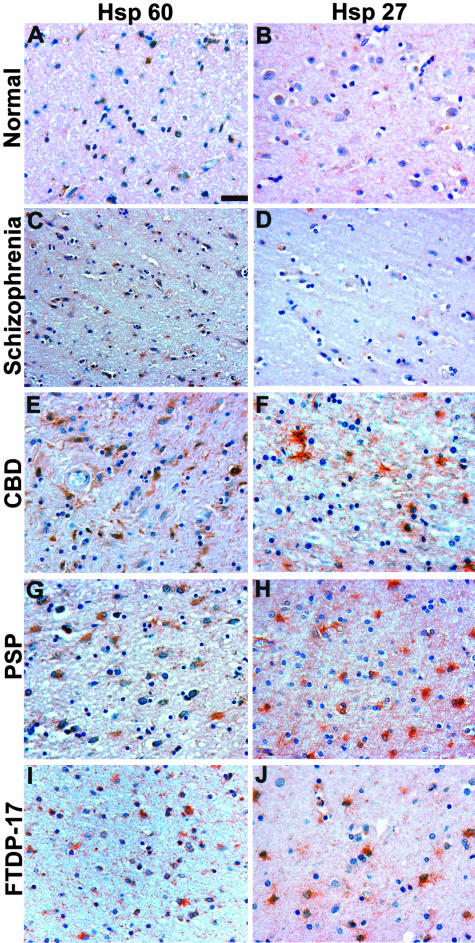
Figure 4.
HSP60 and HSP27 immunoreactivity in tauopathies. Adjacent sections of neocortex as shown in Figure 1 were immunostained with HSP60 (A, C, E, G, I) and HSP27 (B, D, F, H, J). A, C, E, G, and I: HSP60 immunoreactivity was detected in reactive astrocytes in the neocortex of CBD (patient 5), PSP (patient 6), and FTDP-17 (patient 1) patients. Similar HSP60 staining was also observed in the basal ganglia (data not shown). B, D, F, H, and J: HSP27 staining was increased in CBD, PSP, and FTDP-17 brains predominantly in glial cells in the neocortex. The normal and schizophrenia brains show baseline levels of HSP60 (A, C) and HSP27 (B, D) staining. Scale bar, 100 μm.
Expression and Co-Localization of αBC with Tau Pathology in Glia
The increase in αBC immunostaining was detected in tauopathies with prominent glial pathology including CBD, PSP, and FTDP-17.1,3 The αBC immunostaining exhibited a variety of morphologies including cytoplasmic staining in astrocytes and oligodendrocytes, often resembling coiled bodies (Figures 1 and 2), as well as the well-documented ballooned neurons (data not shown) that are only variably tau-positive.18,42 There was also prominent staining in cellular processes morphologically resembling the neuritic or thread pathology observed in these tauopathies (Figure 2H, inset). In contrast, there was only a modest increase in αBC immunostaining in the AD neocortex and entorhinal cortex wherein neuronal tau pathology predominates (Figure 5). In addition, immunostaining for GFAP, a marker for reactive astrocytes, revealed no qualitative differences between tauopathies with prominent glial pathology and AD (data not shown), suggesting that this increase in αBC expression is not simply a manifestation of the astrogliosis that accompanies neurodegeneration.
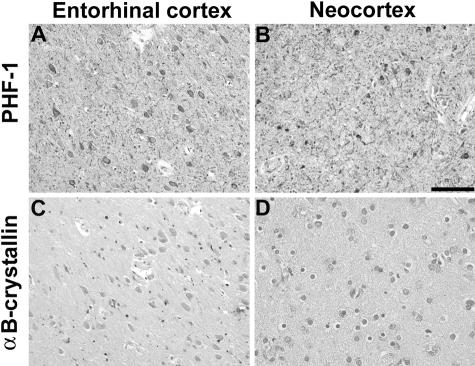
Figure 5.
The increase in αBC expression is relatively specific to glial pathology. Adjacent sections from AD entorhinal (A, C) and frontal cortex (B, D) were immunostained with PHF1 (A, B) and αBC (C, D). A and B: The AD brain sections show robust tau pathology in entorhinal cortex and neocortex. C and D: In contrast, immunostaining for αBC is similar to that observed in control and schizophrenia brains. Similar results were detected in the brains of six patients pathologically diagnosed as AD. In contrast, reactive astrocytes in the subcortical white matter were focally positive for αBC, as described previously (data not shown).16,17 Scale bar, 100 μm.
To determine whether the αBC expression was specifically induced in glia with tau pathology, we analyzed brain sections by two-color immunofluorescence. A subset of tau-positive oligodendrocytes (coiled bodies), astrocytes, and neuropil threads co-localized with αBC in the neocortex and globus pallidus of tauopathy brains (Figure 6). However, although the majority of the αBC staining was detected in cells with the morphology of glia, only a subset of the αBC expression co-localized with glial tau pathology. These findings suggest that expression of αBC may be a protective response before and/or during the accumulation of tau aggregates in glia.
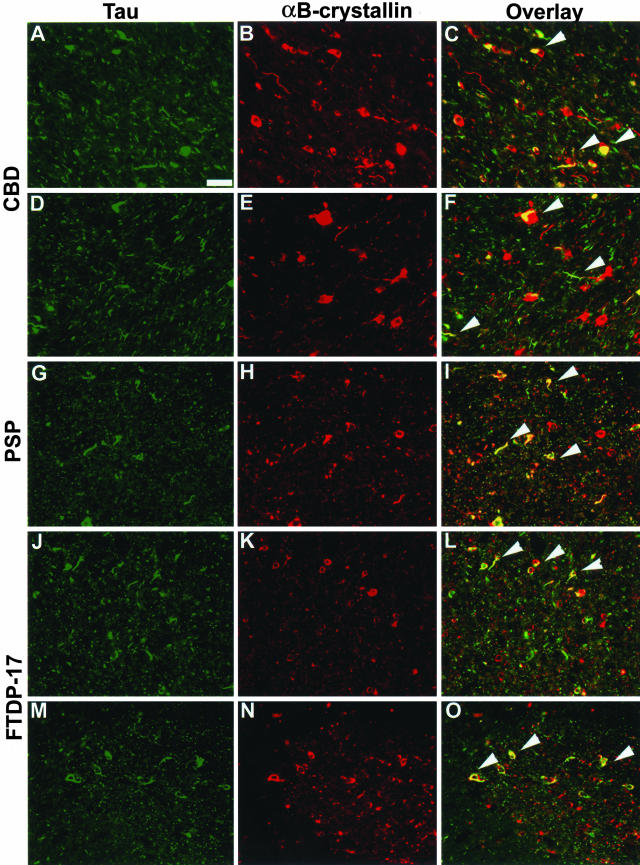
Figure 6.
Co-localization of αBC with tau-immunoreactive inclusions. Adjacent sections from affected regions were double stained for tau (green; A, D, G, J, M) and αBC (red; B, E, H, K, N). Merged images are depicted in C, F, I, L, and O. αBC co-localized with a subset of glial tau pathology in the neocortex of CBD (A–C, patient 5) and FTDP-17 (J–L, patient 1). Similar co-localization was also observed in the basal ganglia of CBD (D–F, patient 2), PSP (G–I, patient 2) and FTDP-17 (M–O, patient 1). Arrowheads indicate some of the inclusions in which co-localization was observed. Scale bar, 100 μm.
Increased Expression of αBC in Tauopathies with Prominent Glial Pathology
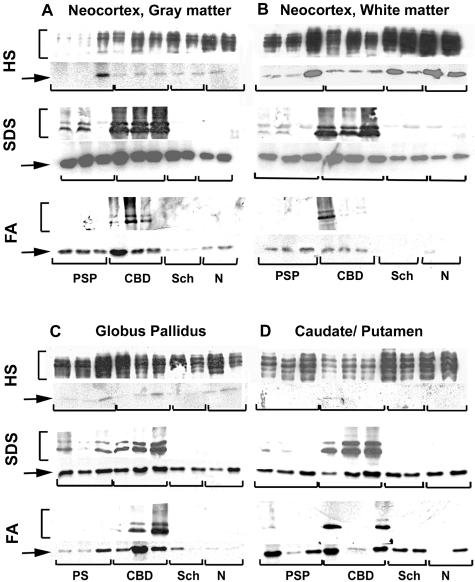
To further characterize the relationship between the αBC expression and tau pathology, we analyzed the expression and solubility of αBC in affected regions of tauopathy patients. We performed serial protein extractions under conditions of increasing ability to solubilize proteins followed by quantitative Western blotting similar to that described previously to characterize tau pathology.35 Although the majority of tau in both normal and affected brains is extracted in the soluble fractions (high-salt TBS and 1% Triton X-100), the amount of soluble αBC was low and variable in both control and tauopathy brains (Figure 7). Instead, the majority of αBC was detected in the fractions extracted with 2% SDS or 70% FA. Similar relocalizations from the detergent soluble to the insoluble cytoskeletal fractions were described in rat astrocytoma cells43 and NIH 3T3 cells.44 Consistent with the above, in affected neocortex and basal ganglia of both CBD and PSP brains, there was an increase in the amount of insoluble αBC in both the 2% SDS and 70% FA extracts, compared to the corresponding regions of schizophrenia and normal control brains, and this correlated with the accumulation of insoluble, hyperphosphorylated tau in CBD and PSP (Figure 7). In addition, there were modest increases in insoluble αBC detected in PSP brains despite the absence of αBC detected by immunohistochemistry. Interestingly, this correlates with the accumulation of insoluble tau detected in subcortical white matter without significant tau pathology detected by immunohistochemistry.45
Figure 7.
Biochemical analysis of tauopathy brains demonstrate increased insoluble αBC in affected brain regions. Sequential tau extractions from frontal gray (A) and white (B) matter, globus pallidus (C), and caudate/putamen (D) from PSP, CBD, schizophrenia (Sch), and normal (N) brains were performed as described.35 Soluble (HS-TBS) and insoluble (2% SDS and 70% FA) fractions were generated and analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting for tau using a cocktail of Tau14 and Tau 46 (brackets, top) and mAbs specific for αBC (arrow, bottom). The soluble extracts showed robust tau expression and variable levels of αBC in both affected and control brains. In contrast, there were increased amounts of insoluble αBC (FA > SDS) in brains from tauopathy patients that paralleled the elevated levels of insoluble tau (see Figure 8).
Quantitative analyses performed on αBC extracted in 2% SDS and 70% FA fractions demonstrated marked increases in insoluble protein levels as compared to normal brains (Figure 8). We compared levels of insoluble αBC from CBD, PSP, and schizophrenia patients with that from normal controls. Although only small increases in insoluble αBC were detected in the 2% SDS fractions, there was a marked increase (10- to 40-fold) in the αBC detected in the 70% FA fraction from affected regions of tauopathy patients compared to normal controls. In general, CBD patients demonstrated larger increases in insoluble αBC that correlated with the amount of insoluble (FA-extractable) tau. In contrast, there was no significant increase in insoluble αBC in schizophrenia brains.
Figure 8.
Quantitative Western blot analyses of insoluble tau shows a marked increase in insoluble αBC in affected brain regions of some tauopathies. Quantitative Western blots of insoluble SDS (open bars) and FA (solid bars) αBC fractions from frontal gray and white matter, globus pallidus, and caudate/putamen were performed using I125-labeled secondary antibodies. SDS- and FA-insoluble fractions from schizophrenia (SCH), CBD, and PSP cases were individually compared with the corresponding fractions from normal (N) cases. Graph represents average fold increase in αBC expression compared to normal brains. There is a marked increase in insoluble αBC detected in FA, and to a lesser extent in SDS extracts. Error bars represent SD of the mean.
Discussion
In several neurodegenerative disorders, molecular chaperones co-localize with inclusion pathology and they may provide neuroprotection to vulnerable neurons and glia.9,11,46 In this study, we examined the role of several molecular chaperones in disorders with tau pathology. Although the majority of HSPs showed little or no reactivity in tauopathies, the expression of the small HSP αBC and to a lesser extent HSP27 was specifically increased in tauopathies with abundant glial pathology. Similar changes in expression were not observed in AD, a neurodegenerative disorder with predominantly neuronal tau pathology.47 Furthermore, a subset of the αBC expression co-localized in oligodendrocytes and astrocytes with tau inclusions. The increased αBC immunostaining correlated with large increases in insoluble, FA extractable αBC in affected brain region with glial tau pathology.
Several previous studies suggested a role for αBC in both brain injury and neurodegeneration.48 Although in the normal adult human brain, low levels of αBC are expressed in oligodendroglia and astrocytes,14,15 there is increased expression of αBC in reactive astrocytes in various neurodegenerative diseases.15,16,49 In AD, αBC expression is increased primarily in reactive astrocytes, but also in microglia and oligodendrocytes.16 Furthermore, in several neurodegenerative disorders, ballooned neurons express the small HSPs αBC and HSP27.50,51 However, although ballooned neurons are often detected in tauopathies such as CBD, these cells are only variably immunoreactive for the tau protein.18
In glial cell lines, αBC is predominantly a soluble protein that can be extracted with 0.2 to 0.5% Triton X-100. In contrast, under conditions of serum starvation, most of the αBC was sequestered in the detergent-insoluble fraction.43 Similarly, in response to physiological stressors such as heat shock, there is an increased partitioning of αBC into the detergent insoluble cytoskeletal fraction.52–54 In our study, in both normal and affected brain tissue only a subset of αBC is extracted with high-salt buffers or Triton X-100, whereas the majority of αBC is extractable only with detergents that disrupt the cytoskeleton, such as 2% SDS. However, in affected regions of brains with glial tau pathology, there is a dramatic increase in the SDS-insoluble/FA-soluble αBC that corresponds to the presence of insoluble, hyperphosphorylated tau. Furthermore, this increase in detergent-insoluble αBC was more dramatic in the cortical white matter relative to gray matter, which correlates with the robust glial tau pathology in the white matter of these disorders. In contrast, the tau pathology in the gray matter involves both neurons and glia.47
The role of increased expression of the low-molecular weight HSPs in tauopathies and neurodegeneration is primarily unknown. One possibility is that αBC expression reflects reactive astrogliosis in the brain. However, in our study, there was no significant difference in reactive changes as assessed by GFAP expression between tauopathies with robust glial pathology and AD. This finding suggests that induction of αBC was specifically linked to the glial tau pathology observed in these disorders. The expression of molecular chaperones has also been suggested to be neuroprotective.9,11,41 This hypothesis is supported by studies in Drosophila models of Parkinson’s disease and Huntington’s disease, wherein HSP70 has shown to be a potent modulator of the toxicity associated with aggregated polyglutamine and α-synuclein toxicity.9,12,13 Furthermore, up-regulation of HSP70 in transgenic mice expressing Ataxin-1 implicated in spinocerebellar ataxia type 1 (SCA1) disease prevented deterioration of Purkinje cells and the consequent motor impairments.55
HSP70 has also been implicated in the modulation of neuronal tau pathology observed in AD.41 In this recent study, Dou and colleagues41 demonstrated that HSP70 and HSP90 prevent tau aggregation and promote its partitioning into microtubules. Furthermore, HSP70 predominantly stained neurons devoid of neurofibrillary tangles (NFTs) suggesting that HSP70 might be protective and antagonize NFT formation. In our study, occasional neurons with the morphology of NFTs were stained for iHSP70 in AD brains (data not shown). In contrast, we did not detect an increase in iHSP70 expression in both sporadic and familial tauopathies other than AD using two different mAbs. This difference in HSP expression is probably because of biochemical differences in composition of protein aggregates and the cell types affected in sporadic and familial tauopathies in contrast to AD, further suggesting distinct pathogenetic mechanisms in neurons and glia.
The detection of αBC and HSP27 in glial tau inclusions may be an attempt by the cell to prevent tau aggregation and/or reduce its cytotoxicity. These small HSPs have been implicated in stress-induced cell death. Specifically, αBC confers resistance to apoptosis induced by a wide range of stimuli such as oxidative stress and heat shock.56 Moreover, αBC can negatively regulate tumor necrosis factor-α and inhibit the activation of caspase-3, a key proapoptotic protease.56 However, although glial cells undergoing apoptosis are associated with development of NFTs in affected areas of AD brains,57 there is no evidence of apoptosis in glial cells with tau inclusions in sporadic or familial tauopathies. In our study, although a subset of the αBC expression co-localized in cells and processes with tau inclusions, the majority of αBC expression was detected in glia that lacked tau pathology. This finding is consistent with the hypothesis that αBC is neuroprotective by preventing the formation of tau aggregates. We speculate that accumulation of tau aggregates in glia is cytotoxic but that the up-regulation of small HSPs might delay and/or prevent this aggregation and consequent cell death. However, an alternative hypothesis is that the altered αBC expression contributes to the pathogenesis of glial tau aggregates. Richter-Landsberg and Goldbaum58 demonstrated that glia respond to cellular stress by up-regulating the expression of HSPs. However, when a certain critical threshold is passed, these stress responses can cause cellular dysfunction, thus contributing to the degenerative processes.58
In our study, αBC immunoreactivity was specific to diseases with prominent glial pathology, implying distinct pathogenic mechanisms in neuronal and glial tau pathology. Previous studies reported αBC expression in AD, primarily in reactive astrocytes, microglia, and oligodendrocytes.16 Similarly, we observed αBC-positive reactive astrocytes and rare αBC-positive tangle-bearing neurons. However, our data are more consistent with observations made by Mao and colleagues59 who showed no correlation between αBC-positive neurons and tau-positive tangles. The biochemical composition of aggregates formed in CBD and PSP as well as the two FTDP-17 cases are intrinsically different from that of AD. The neurofibrillary tau pathology in AD is composed of all six central nervous system tau isoforms containing both three and four microtubule-binding repeats.1,3 In contrast, both the neuronal and glial tau pathology in CBD and PSP as well as the two FTDP-17 patients (N279K and intron 10 + 16 mutations) are composed predominantly of only those tau isoforms containing four microtubule-binding repeats.1,3 Ultrastructurally, the tau pathology in AD is characterized primarily by paired helical filaments that are 8 to 20 nm in width with a periodicity of 80 nm,60 in contrast to 15 to 18 nm straight filaments and twisted ribbons with a long periodicity detected in CBD and PSP, respectively.4 Furthermore, in CBD, there may be distinct patterns of tau phosphorylation in gray matter versus white matter pathology.8 Thus, the observed differences in αBC immunoreactivity between tauopathies with prominent glial pathology and AD lead us to speculate that different classes of HSPs are differentially up-regulated in specific cell types. Alternatively, neurons and glial cells might have different thresholds for stress response and consequently induction of αBC. Nonetheless, αBC expression may be a cell-specific response to tau aggregates in glia, and it will be important to elucidate the roles that HSPs and glial tau pathologies play in neurodegenerative diseases.
Acknowledgments
We thank the family members of the patients studied for making the research described here possible and Kangning Liu for critical reading of the manuscript.
Footnotes
Address reprint requests to Mark S. Forman, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 36th and Spruce Sts., Maloney Building, 3rd Fl., Philadelphia, PA 19104-4283. E-mail: formanm@mail.med.upenn.edu.
Supported by the National Institute on Aging (mentored Clinical Scientist Development Award to M. S. F.).
V. M. Y. L. is the John H. Ware Third Chair of Alzheimer’s disease research; and J. Q. T. is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology.
References
- Lee VM-Y, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Ann Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977;116:207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000;33:1–36. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Komori T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999;9:663–679. doi: 10.1111/j.1750-3639.1999.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin RW, Iwaki T, Kitamoto T, Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab Invest. 1991;64:693–702. [PubMed] [Google Scholar]
- LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Ksiezak-Reding H, Liu WK, Vincent I, Yen SH, Dickson DW. Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol (Berl) 1995;90:37–43. doi: 10.1007/BF00294457. [DOI] [PubMed] [Google Scholar]
- Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, Clark C, Lee VM, Trojanowski JQ. Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol. 2002;160:2045–2053. doi: 10.1016/S0002-9440(10)61154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini NM. Chaperoning brain degeneration. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16407–16411. doi: 10.1073/pnas.152330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–236. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Warrick JM, Chan HY, Gray-Board Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Wisniewski T, Iwaki A, Corbin E, Tomokane N, Tateishi J, Goldman JE. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am J Pathol. 1992;140:345–356. [PMC free article] [PubMed] [Google Scholar]
- Renkawek K, de Jong WW, Merck KB, Frenken CW, van Workum FP, Bosman GJ. Alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 1992;83:324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol (Berl) 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. Alpha B crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer’s disease. J Neurol Sci. 1993;119:203–208. doi: 10.1016/0022-510x(93)90135-l. [DOI] [PubMed] [Google Scholar]
- Smith TW, Lippa CF, de Girolami U. Immunocytochemical study of ballooned neurons in cortical degeneration with neuronal achromasia. Clin Neuropathol. 1992;11:28–35. [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Ann Neurol. 1997;41:706–715. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Kondo T, Matsuda T, Tashima M, Umehara H, Domae N, Yokoyama K, Uchiyama T, Okazaki T. Suppression of heat shock protein-70 by ceramide in heat shock-induced HL-60 cell apoptosis. J Biol Chem. 2000;275:8872–8879. doi: 10.1074/jbc.275.12.8872. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Meacham GC, Browne BL, Zhang W, Kellermayer R, Bedwell DM, Cyr DM. Mutations in the yeast Hsp40 chaperone protein Ydj1 cause defects in Axl1 biogenesis and pro-a-factor processing. J Biol Chem. 1999;274:34396–34402. doi: 10.1074/jbc.274.48.34396. [DOI] [PubMed] [Google Scholar]
- Harris N, MacLean M, Hatzianthis K, Panaretou B, Piper PW. Increasing Saccharomyces cerevisiae stress resistance, through the overactivation of the heat shock response resulting from defects in the Hsp90 chaperone, does not extend replicative life span but can be associated with slower chronological ageing of nondividing cells. Mol Genet Genomics. 2001;265:258–263. doi: 10.1007/s004380000409. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Satoh J. Heat shock proteins and neurological disorders. Nippon Rinsho. 1994;52:2861–2867. [PubMed] [Google Scholar]
- Mitra DK, Mehra NK, Maiti TK, Banerjee A, Taneja V, Rajalingam R, Ahuja RK, Bhattacharya BC. CD4+ T-cell responses to recombinant hsp65 and hsp18 of M. leprae and their trypsin-digested fragments in leprosy: diversity in HLA-DR restriction. Int J Lepr Other Mycobact Dis. 1995;63:518–528. [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Mawal-Dewan M, Schmidt ML, Balin B, Perl DP, Lee VM-Y, Trojanowski JQ. Identification of phosphorylation sites in PHF-TAU from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J Neuropathol Exp Neurol. 1996;55:1051–1059. [PubMed] [Google Scholar]
- Zhukareva V, Shah K, Uryu K, Braak H, Del Tredici K, Sundarraj S, Clark C, Trojanowski JQ, Lee VM. Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer’s disease, and Pick’s disease: a comparative study. Am J Pathol. 2002;161:1135–1141. doi: 10.1016/s0002-9440(10)64390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Satoh J, Yamamura T, Kunishita T, Tabira T. Heterogeneous induction of 72-kDa heat shock protein (HSP72) in cultured mouse oligodendrocytes and astrocytes. Brain Res. 1992;573:37–43. doi: 10.1016/0006-8993(92)90111-l. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN, De Vellis J, Clegg KB. Regulation of heat shock protein synthesis in rat astrocytes. J Neurosci Res. 1991;28:352–358. doi: 10.1002/jnr.490280306. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kim SU. Constitutive and inducible expression of heat shock protein HSP72 in oligodendrocytes in culture. Neuroreport. 1995;6:1081–1084. doi: 10.1097/00001756-199505300-00002. [DOI] [PubMed] [Google Scholar]
- Goldbaum D, Richter-Landsberg C. Stress proteins in oligodendrocytes; differential effects of heat shock and oxidative stress. J Neurochem. 2001;78:1233–1242. doi: 10.1046/j.1471-4159.2001.00507.x. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci USA. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Yen SH, Suzuki KI, Davies P, Garcia JH, Hirano A. Ballooned neurons in select neurodegenerative diseases contain phosphorylated neurofilament epitopes. Acta Neuropathol. 1986;71:216–223. doi: 10.1007/BF00688042. [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Shinohara H, Goto S, Kato K. Translocation and induction of alpha B crystallin by heat shock in rat glioma (GA-1) cells. Biochem Biophys Res Commun. 1992;182:844–850. doi: 10.1016/0006-291x(92)91809-5. [DOI] [PubMed] [Google Scholar]
- Aoyama A, Frohli E, Schafer R, Klemenz R. Alpha B-crystallin expression in mouse NIH 3T3 fibroblasts: glucocorticoid responsiveness and involvement in thermal protection. Mol Cell Biol. 1993;13:1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkins AR, Forman MS, Uryu K, Hinkle DA, Asbury AK, Lee VM, Trojanowski JQ. Co-occurrence of Parkinson’s disease with progressive supranuclear palsy. Acta Neuropathol (Berl) 2002;103:526–530. doi: 10.1007/s00401-001-0483-7. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70). Mol Med Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- Head MW, Goldman JE. Small heat shock proteins, the cytoskeleton, and inclusion body formation. Neuropathol Appl Neurobiol. 2000;26:304–312. doi: 10.1046/j.1365-2990.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- Lowe J, Errington DR, Lennox G, Pike I, Spendlove I, Landon M, Mayer RJ. Ballooned neurons in several neurodegenerative diseases and stroke contain alpha B crystallin. Neuropathol Appl Neurobiol. 1992;18:341–350. doi: 10.1111/j.1365-2990.1992.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Kato S, Hirano A, Umahara T, Llena JF, Herz F, Ohama E. Ultrastructural and immunohistochemical studies on ballooned cortical neurons in Creutzfeldt-Jakob disease: expression of alpha B-crystallin, ubiquitin and stress-response protein 27. Acta Neuropathol (Berl) 1992;84:443–448. doi: 10.1007/BF00227673. [DOI] [PubMed] [Google Scholar]
- Kato S, Hirano A, Umahara T, Kato M, Herz F, Ohama E. Comparative immunohistochemical study on the expression of alpha B crystallin, ubiquitin and stress-response protein 27 in ballooned neurons in various disorders. Neuropathol Appl Neurobiol. 1992;18:335–340. doi: 10.1111/j.1365-2990.1992.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Voorter CE, Wintjes L, Bloemendal H, de Jong WW. Relocalization of alpha B-crystallin by heat shock in ovarian carcinoma cells. FEBS Lett. 1992;309:111–114. doi: 10.1016/0014-5793(92)81075-w. [DOI] [PubMed] [Google Scholar]
- Djabali K, de Nechaud B, Landon F, Portier MM. AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci. 1997;110(Pt 21):2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IJ, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2002;28:238–251. doi: 10.1046/j.1365-2990.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Goldbaum O. Stress proteins in neural cells: functional roles in health and disease. Cell Mol Life Sci. 2003;60:337–349. doi: 10.1007/s000180300028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Katayama S, Watanabe C, Harada Y, Noda K, Yamamura Y, Nakamura S. The relationship between alphaB-crystallin and neurofibrillary tangles in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2001;27:180–188. doi: 10.1046/j.1365-2990.2001.00310.x. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Wischik CM. Image reconstruction of the Alzheimer paired helical filament. EMBO J. 1985;4:3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]