Abstract
Benzo[a]pyrene (B[a]P) is a polycyclic aromatic hydrocarbon with atherogenic and carcinogenic properties. The role of B[a]P in carcinogenesis is well established, and thought to exert via enzymatic activation into reactive metabolites that are capable of binding to the DNA leading to uncontrolled proliferation. However, the mechanism underlying the atherogenic properties of B[a]P is still unclear. Therefore, the effects of chronic B[a]P exposure on atherosclerotic plaque development in apolipoprotein E knockout (apoE-KO) mice were studied. ApoE-KO mice were orally treated with 5 mg/kg/bw B[a]P once per week for 12 or 24 consecutive weeks. Levels of reactive B[a]P metabolites in the arterial tree (from the aortic arch until the iliac artery bifurcations) were high as shown by the level of B[a]P DNA-binding products measured in DNA isolated from the entire aorta (38.9 ± 4.8 adducts/108 nucleotides). Analysis of atherosclerotic lesions in the aortic arch showed no influence of B[a]P on location or number of lesions. Moreover, no increased levels of p53 nuclear protein accumulation or cell proliferation, as detected by immunohistochemistry, were seen in the plaques of the B[a]P-exposed animals. However, the effects of B[a]P on advanced lesions were obvious: advanced plaques were larger and more prone to lipid core development and plaque layering at both 12 and 24 weeks (P < 0.05). In the B[a]P-exposed animals advanced plaques contained more T-lymphocytes and macrophages than in the control animals at both end points (P < 0.05). These data suggest that B[a]P does not initiate atherosclerosis in apoE-KO mice, but accelerates the progression of atherosclerotic plaques via a local inflammatory response.
It is demonstrated that chemicals such as polycyclic aromatic hydrocarbons (PAHs) play a role in both cancer and cardiovascular diseases.1 PAHs, products of the incomplete combustion of organic materials, are a large group of structurally related lipophilic compounds with two or more condensed benzene rings. They are abundantly present in, for example, cigarette smoke and charcoal-broiled and smoked foods. The general population is exposed to PAHs on a daily basis, mainly via ingestion of contaminated foods and inhalation of polluted air.2 Benzo[a]pyrene (B[a]P), a model PAH, is metabolized via cytochrome P450s into reactive dihydrodiol epoxide derivates, eg, B[a]P-7,8-dihydrodiol-9,10-epoxide (BPDE), which are capable of binding covalently to the DNA.3 In the carcinogenic process, formation of these so-called BPDE-DNA adducts is considered to be a crucial initial step leading to mutations and subsequently to uncontrolled cell growth and tumor formation.4 Although B[a]P has been shown to influence atherosclerosis in animal models,5 the exact underlying mechanism of chemical atherogenesis is still not elucidated.
In the 1970s initial studies from Benditt and Benditt6 showed that human atherosclerotic plaques had a monoclonal origin. At the same time, the first animal experiments proved the involvement of chemical carcinogens in atherosclerotic plaque development.7 Finally, in 1986 it was shown that DNA extracted from human coronary artery plaques were capable of transforming NIH3T3 cells.8 These consecutive observations have lead to the suggestion that atherosclerotic plaques are presumably benign smooth muscle cell tumors that develop according to an initiation-promotion-progression protocol. More recently, animal studies have shown that the aorta is a target for carcinogen-induced DNA damage.9 Similarly in humans exposed to environmental carcinogens, arterial DNA damage is high and related to atherogenic risk factors.10–12 Moreover, several human studies showed that DNA damage and repair seem to be associated with atherosclerosis.13,14 However, although it is clear that carcinogens cause substantial DNA damage in the vessel wall and are able to promote atherosclerotic plaque growth, carcinogen-induced initiation of new plaques has hardly ever been observed. This suggests that the processes involved in chemical carcinogenesis (ie, DNA damage, mutagenesis, proliferation) cannot simply explain the mechanisms underlying chemical atherogenesis. Still, research into chemical atherogenesis has kept its focus mainly on arterial DNA damage as an initiating step for subsequent smooth muscle cell proliferation, akin a benign tumor.10,11
With the development of the apolipoprotein E-knockout (apoE-KO) mouse, a transgenic animal model was created in which diet-independent atherosclerotic lesions develop that have striking similarities to the human disease.15 This model proved very useful in studying biochemical and cellular events leading to several aspects of atherosclerosis such as initiation, progression, growth arrest, and regression.16 In the present study this mouse model was applied to gain more knowledge on the effects of chronic B[a]P exposure on plaque formation and differentiation. The specific aim of this study was to obtain new insights in the pathways involved in chemical atherogenesis. Therefore, rather than focusing only on DNA damage and plaque area, extensive immunohistochemistry was used to explore differences in plaque phenotype and composition more closely.
Materials and Methods
Animal Treatment
Male apoE-KO mice were purchased from IFFA CREDO S.A. (Charles River Co., Lyon, France) and fed a normal mouse chow (SRM-A; Hope Farms, Woerden, the Netherlands).
B[a]P (B1760; Sigma, St. Louis, MO, USA) was initially dissolved in acetone and added to tricaprylin (103104; ICN, Costa Mesa, CA, USA). Evaporation of acetone resulted in a homogenous solution of 0.5 mg of B[a]P/ml tricaprylin. At 5 weeks of age, animals (17.2 ± 1.8 g) were orally treated with 5 mg/kg/bw B[a]P or vehicle, after an overnight fasting period. This procedure was repeated once per week for 12 (n = 31) and 24 (n = 19) consecutive weeks. By weighing the animals weekly, growth was monitored. To confirm that B[a]P was capable of inducing vascular DNA damage before plaque formation, four wild-type C57BL/6 mice were treated once orally with 25 mg/kg/bw B[a]P. Animals were killed after 4 days and DNA adducts were measured by 32P-postlabeling.
Tissue Handling
On euthanasia, ∼0.5 ml of blood was drawn from the inferior caval vein. The arterial tree was perfused in situ for 3 minutes with 0.9% NaCl containing 20% nitroprusside (3 minutes) and subsequently with 1% phosphate-buffered paraformaldehyde (pH 7.4; 3 minutes) via a catheter in the left ventricular apex. The arterial tree was excised and fixed overnight in 1% phosphate-buffered paraformaldehyde (pH 7.4). The aortic arch (including the brachiocephalic trunk, left carotid artery, and left subclavian artery), thoracic and abdominal aorta (TA and AA, respectively) were longitudinally embedded in paraffin, and subsequently serial 4-μm sections were cut. Pieces of lung and liver tissue of all animals (n = 50) as well as the entire aorta (aortic arch and the descending aorta until the iliac artery bifurcations) of 22 animals from the 12-week group (11 B[a]P versus 11 controls) were used for DNA adduct measurement. Furthermore, >20 organs were investigated macroscopically for abnormalities. Hematoxylin and eosin (H&E)-stained sections of lung, liver, and spleen were also microscopically evaluated.
Lipid Measurement
Lipid levels were measured using standard enzymatic techniques, automated on the Cobas Fara centrifugal analyzer (Hoffmann-La Roche, Basel, Switzerland). Total plasma cholesterol and high-density lipoprotein were measured using kit no. 0736635 and no. 543004 (Hoffmann-La Roche), total glycerol using kit no. 337-40A/337-10B (Sigma) and free glycerol using kit no. 0148270 (Hoffmann-La Roche). Precipath (standardized serum) was used as an internal standard. Low-density lipoprotein was calculated using the formula: low-density lipoprotein cholesterol (mmol/L) = total cholesterol − (triglycerides/2.2) − high-density lipoprotein cholesterol.
DNA Isolation and 32P-Postlabeling
DNA was isolated from lung, liver, and aorta using a traditional phenol extraction procedure and dissolved in 2 mmol/L of Tris (2 mg/ml).17 Subsequently, 10 μg of DNA was digested into nucleotide monophosphates and treated with nuclease P1 for 40 minutes at 37°C. Then, nucleotides were 5′-labeled with 32P using T4-polynucleotide kinase (5.0 U) for 30 minutes at 37°C. Radiolabeled adduct nucleotide biphosphates were separated by chromatography on polyethyleneimine (PEI)-cellulose sheets (Macherey Nagel, Düren, Germany) using solvents as described previously.17 In each experiment, two standards of [3H]BPDE-modified DNA with known modification levels (1 per 107, 108 nucleotides) were included. Adduct quantification, by means of phosphor-imaging technology (Molecular Dynamics, Sunnyvale, CA, USA), was performed by calibrating toward the BPDE-DNA adduct standards.
Histological and Morphometrical Analysis
Of the aortic arch, the thoracic aorta, and the abdominal aorta, four H&E-stained sections, each separated by 20 μm, were used for histological analysis. Lesions were classified as initial or advanced based on the guidelines given by the American Heart Association.18 The absence or presence of a lipid core and the cell density (number of cells per μm2) of the plaques were also determined. For morphometrical analysis, four sections consecutive to the H&E-stained sections, were stained according to Lawson (a modified elastica von Giesson staining). Sirius Red staining was performed for the detection of collagen. The relative collagen content was calculated by dividing the Sirius Red-positive plaque area by the total plaque area. Morphometrical parameters such as plaque area, lipid core area, and plaque layering were measured using a computerized morphometry system (Quantimet 570; Leica).19
Immunohistochemical Analysis
Several immunohistochemical markers (cell proliferation, DNA repair, and apoptosis) generally used in cancer biology were applied on aortic sections. Cell proliferation was detected with the Zymed PCNA staining kit (Zymed Laboratories Inc., San Francisco, CA, USA) and p53 nuclear protein accumulation by using the antibody CM5 (1:500; Novocastra Laboratories Ltd., Newcastle-upon-Tyne, UK). terminal dUTP nick-end labeling staining (using the ApopTag kit; Oncor, Gaithersburg, MD, USA) for the detection of apoptotic cells was performed in the laboratory of Dr. M. Kockx (Antwerp, Belgium).
Moreover, immunohistochemical techniques were applied to classify specific cell types in the atherosclerotic plaques; smooth muscle cells were stained using an α-smooth muscle actin antibody (ASMAFITC, 1:3000, Sigma F-3777); T-cells were labeled with anti-CD3 (1:200, A0452; DAKO, Glostrup, Denmark) and macrophages with Mac 3 (1:30; Pharmingen, San Jose, CA, USA). Factor VIII (1:2000; Eurodiagnostics, Malmö, Sweden) was used for the detection of endothelial cells. Quantification of all immunohistochemical stainings was performed by counting the respective positive stained cells per plaque divided by the total number of cells in the plaque.
Statistical Analysis
Results are presented as mean ± SEM. All plaque parameters as well as DNA adduct levels and plasma lipid levels were compared by a nonparametric Mann-Whitney U-test. P < 0.05 was considered statistically significant. Two independent observers who were blinded for the exposure treatment performed the analyses. Inter- and intra-observer variation was <10%.
Results
General
Treatment with B[a]P resulted in growth retardation of the mice at both 12 and 24 weeks (P < 0.01, n = 31, and P < 0.01, n = 19, respectively; Table 1). Macroscopic and microscopic evaluations of internal organs showed no tumor development or signs of inflammation in B[a]P-exposed and control mice (data not shown).
Table 1.
Weight Gain, DNA Adducts, and Lipoprotein Levels
| 12 weeks
|
24 weeks
|
|||
|---|---|---|---|---|
| Control | B[a]P | Control | B[a]P | |
| Weight gain (g) | (n = 15) | (n = 16) | (n = 9) | (n = 10) |
| 11.3 ± 0.6 | 9.8 ± 0.6† | 13.9 ± 0.3 | 10.7 ± 0.4† | |
| DNA adducts (per 108 nucleotides) | (n = 15) | (n = 16) | (n = 9) | (n = 10) |
| Aorta | <0.01∥ | 34.6 ± 2.8‡ | <0.01 | —* |
| Lung | <0.01 | 15.8 ± 1.6‡ | <0.01 | 16.1 ± 1.0‡ |
| Liver | <0.01 | 5.2 ± 0.3‡ | <0.01 | 5.5 ± 0.5 |
| Lipoprotein levels (mmol/L) | (n = 9) | (n = 9) | (n = 9) | (n = 8) |
| Total cholesterol | 9.5 ± 0.7¶ | 11.3 ± 0.6*‡§ | 11.4 ± 0.5 | 9.5 ± 0.5†§ |
| Total glycerol | 0.8 ± 0.04¶ | 0.7 ± 0.06§ | 1.4 ± 0.1 | 1.5 ± 0.08 |
| HDL cholesterol | 0.2 ± 0.06 | 0.2 ± 0.01 | 0.3 ± 0.03 | 0.1 ± 0.03 |
| Free glycerol | 0.4 ± 0.02 | 0.3 ± 0.02 | 0.3 ± 0.06 | 0.2 ± 0.05 |
| LDL cholesterol | 9.0 ± 0.7¶ | 10.9 ± 0.6‡§ | 10.6 ± 0.5 | 8.8 ± 0.5‡ |
Values are mean ± SEM.
No arterial tissue available for DNA-adduct measurement.
P < 0.01 versus control group;
P ≤ 0.05 versus control group;
P ≤ 0.05 versus B[a]P group 24 weeks;
P ≤ 0.05 versus control 24 weeks;
n = 11.
Plasma Cholesterol and Glycerol Levels
B[a]P treatment did not affect total glycerol, high-density lipoprotein cholesterol, and free glycerol levels at both time points (Table 1). The influence of B[a]P on total cholesterol and low-density lipoprotein cholesterol was not consistent: total cholesterol and concomitant low-density lipoprotein cholesterol levels were raised after 12 weeks of B[a]P exposure (P < 0.05 versus control), but lowered after 24 weeks of B[a]P exposure (P < 0.05 versus controls). Total glycerol levels increased with age regardless of B[a]P treatment.
DNA Adducts
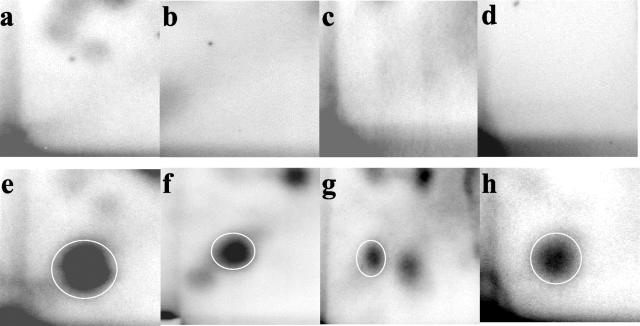
In aorta, lung, and liver of the B[a]P-exposed animals the predominant adduct spot co-migrated with the BPDE-DNA-adduct standard (Figure 1). In lung and liver minor additional adduct spots were observed. DNA adduct quantification was performed based on the major adduct spot that co-migrated with the BPDE-DNA-adduct standards. Inclusion of the minor additional adduct spots did not change the results significantly. After 12 weeks of exposure, adduct levels were ranked aorta > lung > liver (Table 1). At 24 weeks, no DNA adducts were measured in the aorta because all aortic segments were used for histological purposes. However, DNA adduct levels were determined in lung and liver and were comparable with the adduct levels found after 12 weeks of exposure. In the pilot study in which wild-type C57BL6 mice were treated acutely with B[a]P, DNA adduct distribution was comparable to the 12 and 24 weeks study in ApoE-KO mouse. High adduct levels were found in aorta (16.0 ± 1.1 adducts/108 nucleotides) and lung (14.5 ± 0.8 adducts/108 nucleotides) and lower levels were found in the liver (6.0 ± 0.6 adducts/108 nucleotides).
Figure 1.
DNA adduct profiles in apoE-knockout mice after 12 weeks of chronic B[a]P exposure. Top row: Chromatograms of aorta, lung, and liver (a, b, and c, respectively) of control animals. Bottom row: Results of aorta, lung, and liver (e, f, and g, respectively) of B[a]P-exposed animals. d and h: MQ sample and a BPDE-DNA adduct standard (1 adduct per 107 nucleotides), respectively. Differences in chromatography are because of interassay variations. In the B[a]P-treated mice a clear DNA adduct spot was found that migrated at the same position as the BPDE-DNA adduct standard (as indicated by the circles). Additional spots were not used for quantification purposes.
Plaque Characteristics
Plaque Burden
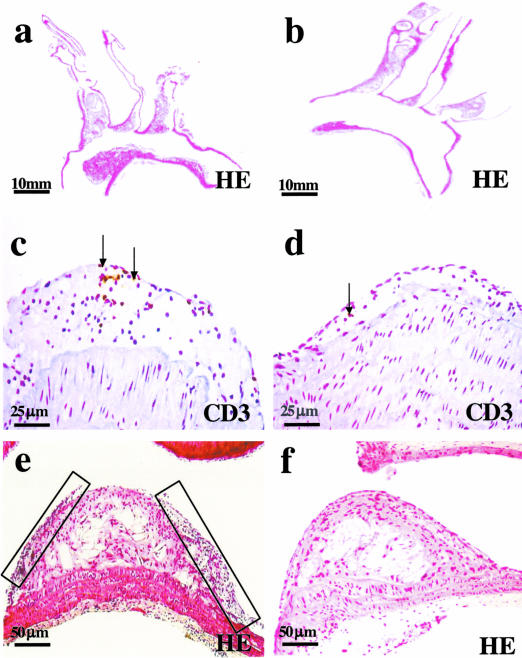
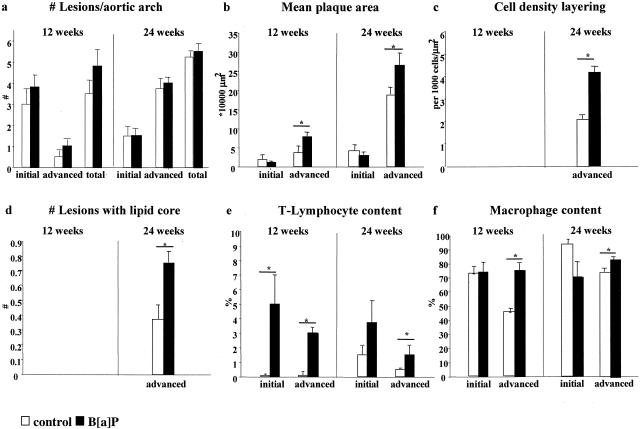
After 12 weeks, 24 lesions in the aortic arch of B[a]P mice (n = 5) and 14 lesions in controls (n = 4) were analyzed. After 24 weeks, analysis included 45 lesions in the aortic arch of B[a]P-treated mice (n = 10) and 41 lesions of the control group (n = 9). In both B[a]P-exposed and control animals lesions were mainly present in the inner curve of the aortic arch and its main branch points the brachiocephalic trunk, the left common carotid artery, and left subclavian artery (Figure 2, a and b). After 12 weeks, all mice had developed mild atherosclerosis with predominantly initial lesions, but after 24 weeks all animals exhibited severe atherosclerosis characterized by a high number of advanced lesions and large lesion areas. There were no differences in location and number of lesions per arch between groups at both time points (Figure 3a). Initial lesion area per arch was significantly smaller than the advanced lesion area within B[a]P and control animals (Figure 3b; P < 0.01). Advanced lesions occupied a significantly larger area per aortic arch in B[a]P animals than in controls at both end points (P < 0.05; Figure 3b).
Figure 2.
Representative longitudinal sections of the aortic arch of a B[a]P mouse (a) and a control apoE-KO mouse (b) after 24 weeks of exposure. Atherosclerotic plaques of the B[a]P animals contained more CD3-positive cells than plaques of the controls (c versus d). As highlighted by the boxes, advanced plaques of the B[a]P-exposed mice were more often marked by the presence of plaque layers (e) than advanced plaques of the control mice (f).
Figure 3.
Plaque characteristics of the apoE-KO mice after 12 and 24 weeks of B[a]P exposure. a: Number of lesions per aortic arch; b: mean plaque area; c: cell density of the plaque layers; d: number of lesions with a lipid core; e: T-lymphocyte content; f: macrophage content. *, P < 0.05.
Plaque Initiation
Because TA and AA are less sensitive to spontaneous plaque development, we used them to further investigate whether B[a]P treatment had any effect on plaque initiation. At 12 weeks, TA and AA showed no or few plaques and were not further used for analysis. At 24 weeks a total of 42 initial lesions in the TA of the B[a]P group and 35 initial lesions in the controls were analyzed. In the AA a total of 42 and 35 initial lesions were analyzed, respectively. Both TA and AA showed no significant differences between B[a]P and control animals in the mean number of initial lesions (4.2 ± 0.8 versus 4.8 ± 0.6 and 4.2 ± 0.8 versus 5.8 ± 0.7, respectively) or the mean initial lesion size (59,784 ± 16,711 μm2 versus 124,895 ± 49,996 μm2 and 122,368 ± 35,687 μm2 versus 152,676 ± 48,039 μm2, respectively), indicating no effects of B[a]P treatment on initiation of lesions.
P53, Proliferation, and Apoptosis
Because B[a]P is a carcinogen capable of inducing and promoting tumor formation, we were interested in its effect on the p53 protein, a very important cell-cycle regulatory protein. Very low staining of p53 nuclear protein was observed in the normal vessel wall (< 2%). In the plaques, however, percentages of cells positive for the p53 nuclear protein staining were high (69.9 ± 3.1%), but these levels were not further induced by the 24 weeks of B[a]P treatment (68.2 ± 3.2%). Moreover, B[a]P exposure did not result in enhanced proliferation as measured by the number of PCNA-positive cells in both initial and advanced plaques after 24 weeks (1.9 ± 0.8% versus 3.9 ± 2.0% and 4.0 ± 0.7% versus 2.7 ± 0.6%). Finally, the number of terminal dUTP nick-end labeling positive cells in plaques of the B[a]P-exposed animals was not significantly raised after 24 weeks (1.25 ± 0.17% versus 0.74 ± 0.12%; P = 0.13).
Plaque Phenotype
To investigate the effects of B[a]P exposure on plaque formation, we studied atherosclerotic plaque composition in more depth. After 24 weeks of exposure, advanced lesions in B[a]P-treated animals exhibited more often plaque layering than advanced lesions of the control group (Figure 2, e and f; 30 of 36 lesions versus 13 of 30 lesions, respectively). The cell density of these layers, which consisted mainly of macrophages, was significantly higher in the B[a]P-exposed animals (P < 0.05; Figure 3c). Furthermore, in the B[a]P-exposed group advanced lesions were more prone to lipid core development (P < 0.05; Figure 3d). Both lipid core area and layering area did not differ between B[a]P and control animals (lipid core, 20.0% versus 21.3%; layers, 19.3% versus 21.9%, respectively). Total cell density of initial and advanced lesions was not influenced by B[a]P exposure at 12 and 24 weeks (data not shown).
T-lymphocyte content, measured as the number of CD3-positive cells, was higher in lesions of the B[a]P-treated animals at both time points compared to the control animals (Figure 2, c and d). Analysis of lesions in TA and AA showed no difference in T-lymphocyte content between plaques of exposed and control animals (3.0 ± 0.8% versus 2.1 ± 0.5% and 1.9 ± 0.5% versus 1.7 ± 0.5%; Figure 3e). Initial atherosclerotic lesions showed no differences in macrophage content, but a significant increase in macrophages was seen in advanced atherosclerotic lesions of the B[a]P groups at both 12 and 24 weeks (P < 0.05; Figure 3f).
Levels of collagen in initial and advanced lesions were not changed on B[a]P treatment (12 weeks, 4.2 ± 2.8% versus 4.1 ± 2.1% and 29.4 ± 4.7% versus 34.5 ± 1.0%; 24 weeks, 13.3 ± 4.8% versus 16.3 ± 9.4% and 38.1 ± 2.2% versus 37.5 ± 2.3%, respectively).
Finally, no differences were observed in α-smooth muscle actin-positive SMCs in initial and advanced lesions of B[a]P and control animals after 12 (2.1 ± 1.7% versus 2.3 ± 2.3% and 7.7 ± 2.2% versus 8.8 ± 0.1%) and 24 weeks (8.2 ± 3.9% versus 2.8 ± 1.6% and 7.4 ± 1.5% versus 6.0 ± 1.0%).
Discussion
The present study has focused on atherosclerotic plaque morphology and phenotype after chronic B[a]P exposure using genetic identically apoE-KO mice. First, we confirmed that B[a]P causes very high levels of DNA damage in the vessel wall. Because these levels were even higher than in lung, which is the normal target organ, this indicates that the vessel wall is extra vulnerable for this chemical. Furthermore, we showed that the observed effects of B[a]P on the atherosclerotic plaques could not be because of the induction of plasma lipids, because there was no consistent effect of B[a]P on these levels. In B[a]P-treated ApoE−/− mice larger advanced plaque development was observed. These plaques were more prone to lipid core development and high cell-densed plaque layering. Interestingly, B[a]P treatment did not result in the induction of p53 nuclear protein or proliferation, but in a higher plaque macrophage and T-lymphocyte content, whereas no signs of systemic inflammation were present in spleen or liver. This suggests a local, plaque-specific, inflammatory response.
The effects of chemicals on atherogenesis were studied as early as the 1970s when Albert and colleagues20 demonstrated that chronic exposure to the PAH 7,12-dimethylbenz(a)anthracene (DMBA) resulted in large, focal, fibromuscular lesions in the abdominal aorta of chickens. They showed not only an increased induction, but also larger lesions in the carcinogen-treated animals as compared to the controls. Although later studies confirmed the dose-dependent increase of the plaque size, no further studies have described the induction of new plaques.21–23 Our data also show an increase in lesion size but not in number of lesions. Another observation from our study was that carcinogen exposure did not alter the location of the lesions, which is in line with observations from others.21,23 The fact that B[a]P increased lesion size, but had no influence on the number and the location of the lesions, leads to the suggestion that this environmental carcinogen accelerates the process of atherosclerosis, rather than initiating it.
The main route of PAH exposure is via dietary intake of contaminated foodstuffs. For example, the B[a]P content of charcoal-broiled and smoked foods has been reported to be as high as 50 μg/kg. Furthermore, PAHs are detected in a wide range of fresh meat, fish, vegetables, and fruits; high levels were found in leafy green vegetables as a result of the deposition from the PAH-polluted atmosphere.24 A Dutch total diet study calculated that the daily PAH intake ranged up to 1.4 μg/kg.25 Specific groups that are more frequently exposed to PAH via inhalation are cigarette smokers and workers in industries that produce as asphalt, steel, and aluminum. Epidemiological studies showed that smokers and workers in PAH-producing industries are at increased risk for cardiovascular disease.26 Actually, several cross-sectional studies have shown a relationship between both active and passive smoking and carotid artery atherosclerosis.27,28
It is suggested that B[a]P is transported through the blood via lipoproteins and may therefore preferentially be delivered to the arterial wall.29 Metabolites of carcinogens such as B[a]P are able to covalently bind to the DNA. These BPDE-DNA adducts can lead to the formation of mutations that can subsequently result in uncontrolled cell growth and possibly cancer.3 In humans, BPDE-DNA adducts have been detected in aorta by both 32P-postlabeling as well as by specific fluorimetric assays.10 Moreover, several animal studies have shown that independently of the exposure route, PAHs lead to very high adduct levels in lung and heart but also in aorta.9,30 In our study, B[a]P exposure resulted in DNA damage, which was most pronounced in the aorta. After 24 weeks of exposure, DNA-adduct levels in aorta, lung, and liver showed no increase compared to 12 weeks of exposure, which suggests that adduct levels had reached steady-state levels. Because the DNA adducts were similarly distributed in wild-type C57BL6 mouse acutely exposed to B[a]P and the chronically exposed atherosclerotic apoE-KO mouse, B[a]P-induced DNA damage proved not to be specific for the atherosclerotic vessel nor the result of metabolic changes associated with the diseased vessel wall. It can therefore be concluded that arterial DNA damage precedes plaque formation. The lung, which is considered to be a target organ for B[a]P-induced carcinogenicity, showed adduct levels 50% lower than those in the aorta. Comparable results were seen in smokers, having the highest DNA damage in organs such as heart and aorta.4
Although the pathophysiological mechanism of chemical atherogenesis is unknown, several theories have been postulated. One of the theories is the monoclonal hypothesis.6 Benditt and Benditt6 suggested that a somatic mutational event in a single smooth muscle cell gives a proliferative advantage that initiates the atherosclerotic process. Studies from Bond and colleagues21 showed that proliferation rates of medial and lesion cells were mainly because of increasing body weight because of maturing, whereas varying duration of exposure to the carcinogenic PAH DMBA in cockerels had no effect. On the other hand, Batastini and Penn23 described that DMBA enhanced proliferation of plaque cells, beyond levels normally seen in plaques. This enhanced proliferation was seen in pre-existing lesions, suggesting that chemical carcinogens act as promotors of plaque development rather than as initiators. In parallel, in our study proliferation levels in the atherosclerotic plaque were not raised on B[a]P exposure, thereby suggesting that the monoclonal theory does not explain the mechanism of chemical atherogenesis. Furthermore, we did not observe any differences in the levels of p53 nuclear protein in plaques of B[a]P-exposed and control animals. Data from a human study also suggested that p53 was not involved in atherogenesis.31 However, in this study p53 nuclear staining was not measured in the atherosclerotic plaque but in the medium layer underlying the plaque. Elevated levels of p53 lead to anti-proliferative and proapoptotic responses.32 Although it is suggested that the absence of p53 accelerates atherosclerosis by causing increased proliferation, the effect of p53 on apoptosis in the atherosclerotic plaque is less clear. For instance, van Vlijmen and colleagues32 showed that p53 deficiency resulted in a slight decrease in apoptosis, whereas Guevara and colleagues33 reported a nonsignificant increase suggesting that apoptosis could be p53-independent. In our study levels of apoptosis were slightly higher in B[a]P-exposed animals without differences in levels of p53. Although B[a]P is known to induce p53, one might speculate that the already elevated p53 levels in the atherosclerotic lesions might have masked a stimulatory effect of B[a]P.
The current view on atherosclerosis postulates that atherosclerosis is a chronic inflammatory process.34 It states that the process of atherosclerosis begins as an inflammatory response in the arterial wall as a response to the accumulation of toxic products.35 Interestingly, B[a]P is not only known because of its mutagenic and carcinogenic capacity, but also because of its immunomodulating effects.36 For example, B[a]P is capable of oxidizing low-density lipoprotein, which has proven to be a trigger for an inflammatory response.37,38 Furthermore, in rodents it is shown that B[a]P suppresses T-cell-dependent antibody formation.39 In vitro studies have suggested that BPDE might be the ultimate immunotoxic B[a]P metabolite, because inhibition of the aryl hydrocarbon (Ah)-receptor, necessary for the metabolism of B[a]P to BPDE, inhibited this suppression.36 In our study, we proved BPDE levels to be high in the arterial wall, which is accompanied by increased T-lymphocyte levels in both initial and advanced plaques of B[a]P-exposed mice. Furthermore, advanced lesions of the B[a]P animals contained a higher macrophage content and were more prone to develop lipid cores. Although detailed studies are necessary to further dissect the molecular pathway of immunomodulation by B[a]P on plaque formation, it seems that an inflammatory response plays an important role in chemical atherogenesis.
In conclusion, in the present study we showed that B[a]P was a potent promotor of the atherosclerotic process although no signs of initiation of new plaques could be observed. Although substantial aortic DNA damage was caused by B[a]P, this was not accompanied by increased levels of proliferation or p53 accumulation in the plaques. Importantly however, our data demonstrated B[a]P-related changes in plaque phenotype illustrated by an increased plaque layering and number of lipid cores, but mostly by the increased levels of inflammatory cells in the atherosclerotic plaques after B[a]P exposure. This suggests that B[a]P-related progression of atherosclerosis involves a local inflammatory response.
Acknowledgments
We thank A. Janssen, D. Pachen, and E. Moonen for their excellent analytical assistance; and Dr. A. Knaapen for critically reading the manuscript and his useful suggestions.
Footnotes
Address reprint requests to F. J. van Schooten, Department of Health Risk Analysis and Toxicology, University of Maastricht, P.O. Box 616, 6200MD Maastricht, The Netherlands. E-mail: f.vanschooten@grat.unimaas.nl.
E. L. is a postdoctoral research fellow of the Dr. E. Dekker program of the Dutch Heart Foundation (2000T041).
References
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann NY Acad Sci. 2001;947:271–292. [PubMed] [Google Scholar]
- Waldman JM, Lioy PJ, Greenberg A, Butler JP. Analysis of human exposure to benzo(a)pyrene via inhalation and food ingestion in the Total Human Environmental Exposure Study (THEES). J Expo Anal Environ Epidemiol. 1991;1:193–225. [PubMed] [Google Scholar]
- International Agency for Research on Cancer Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental and Experimental data. IARC Monogr Eval Carcinog Risk Chem Hum. 1983;32:1–453. [PubMed] [Google Scholar]
- De Flora S, Izzotti A, Randerath K, Randerath E, Bartsch H, Nair J, Balansky R, van Schooten F, Degan P, Fronza G, Walsh D, Lewtas J. DNA adducts and chronic degenerative disease. Pathogenetic relevance and implications in preventive medicine. Mutat Res. 1996;366:197–238. [PubMed] [Google Scholar]
- Wakabayashi K. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/3. Animal studies suggesting involvement of mutagen/carcinogen exposure in atherosclerosis. Mutat Res. 1990;239:181–187. doi: 10.1016/0165-1110(90)90005-v. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci USA. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A, Snyder C. Arteriosclerotic plaque development is ‘promoted’ by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988;9:2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- Penn A, Garte SJ, Warren L, Nesta D, Mindich B. Transforming gene in human atherosclerotic plaque DNA. Proc Natl Acad Sci USA. 1986;83:7951–7955. doi: 10.1073/pnas.83.20.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Camoirano A, Cariglia C, Tampa E, De Flora S. Formation of DNA adducts in the aorta of smoke-exposed rats, and modulation by chemopreventive agents. Mutat Res. 2001;494:97–106. doi: 10.1016/s1383-5718(01)00183-8. [DOI] [PubMed] [Google Scholar]
- Izzotti A, De Flora S, Petrilli GL, Gallagher J, Rojas M, Alexandrov K, Bartsch H, Lewtas J. Cancer biomarkers in human atherosclerotic lesions: detection of DNA adducts. Cancer Epidemiol Biomarkers Prev. 1995;4:105–110. [PubMed] [Google Scholar]
- Binkova B, Strejc P, Boubelik O, Stavkova Z, Chvatalova I, Sram RJ. DNA adducts and human atherosclerotic lesions. Int J Hyg Environ Health. 2001;204:49–54. doi: 10.1078/1438-4639-00072. [DOI] [PubMed] [Google Scholar]
- De Flora S, Izzotti A, Walsh D, Degan P, Petrilli GL, Lewtas J. Molecular epidemiology of atherosclerosis. EMBO J. 1997;11:1021–1031. [PubMed] [Google Scholar]
- Van Schooten FJ, Hirvonen A, Maas LM, De Mol BA, Kleinjans JC, Bell DA, Durrer JD. Putative susceptibility markers of coronary artery disease: association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. EMBO J. 1998;12:1409–1417. doi: 10.1096/fasebj.12.13.1409. [DOI] [PubMed] [Google Scholar]
- Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–D525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- Godschalk RW, Maas LM, Van Zandwijk N, van’t Veer LJ, Breedijk A, Borm PJ, Verhaert J, Kleinjans JC, van Schooten FJ. Differences in aromatic-DNA adduct levels between alveolar macrophages and subpopulations of white blood cells from smokers. Carcinogenesis. 1998;19:819–825. doi: 10.1093/carcin/19.5.819. [DOI] [PubMed] [Google Scholar]
- Stary H, Blankenhorn D, Chandler A, Glagov S, Insull W, Richardson M, Rosenfeld M, Schaffer S, Schwartz C, Wagner W, Wissler R. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1513–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- Lutgens E, de Muinck ED, Heeneman S, Daemen MJ. Compensatory enlargement and stenosis develop in apoE(−/−) and apoE*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol. 2001;21:1359–1365. doi: 10.1161/hq0801.093669. [DOI] [PubMed] [Google Scholar]
- Albert RE, Vanderlaan M, Burns FJ, Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977;37:2232–2235. [PubMed] [Google Scholar]
- Bond JA, Gown AM, Yang HL, Benditt EP, Juchau MR. Further investigations of the capacity of polynuclear aromatic hydrocarbons to elicit atherosclerotic lesions. J Toxicol Environ Health. 1981;7:327–335. doi: 10.1080/15287398109529983. [DOI] [PubMed] [Google Scholar]
- Penn A, Batastini G, Soloman J, Burns F, Albert R. Dose-dependent size increases of aortic lesions following chronic exposure to 7,12-dimethylbenz(a)anthracene. Cancer Res. 1981;41:588–592. [PubMed] [Google Scholar]
- Batastini G, Penn A. An ultrastructural comparison of carcinogen-associated and spontaneous aortic lesions in the cockerel. Am J Pathol. 1984;114:403–409. [PMC free article] [PubMed] [Google Scholar]
- Grimmer G, Stober W, Jacob J, Mohr U, Schoene K, Brune H, Misfeld J. Inventory and biological impact of polycyclic carcinogens in the environment. Exp Pathol. 1983;24:3–13. doi: 10.1016/s0232-1513(83)80002-4. [DOI] [PubMed] [Google Scholar]
- de Vos RH, van Dokkum W, Schouten A, de Jong-Berkhout P. Polycyclic aromatic hydrocarbons in Dutch total diet samples (1984–1986). Food Chem Toxicol. 1990;28:263–268. doi: 10.1016/0278-6915(90)90038-o. [DOI] [PubMed] [Google Scholar]
- Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study [see comments]. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- Tell GS, Polak JF, Ward BJ, Kittner SJ, Savage PJ, Robbins J. Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. The Cardiovascular Health Study (CHS) Collaborative Research Group. Circulation. 1994;90:2905–2908. doi: 10.1161/01.cir.90.6.2905. [DOI] [PubMed] [Google Scholar]
- Howard G, Burke GL, Szklo M, Tell GS, Eckfeldt J, Evans G, Heiss G. Active and passive smoking are associated with increased carotid wall thickness. The Atherosclerosis Risk in Communities Study. Arch Intern Med. 1994;154:1277–1282. [PubMed] [Google Scholar]
- Stavenow L, Pessah-Rasmussen H. Effects of polycyclic aromatic hydrocarbons on proliferation, collagen secretion and viability of arterial smooth muscle cells in culture. Artery. 1988;15:94–108. [PubMed] [Google Scholar]
- Godschalk RW, Vermeer IT, Kriek E, Floot B, Schilderman PA, Moonen EJ, Kleinjans JC, van Schooten FJ. Comparison of 32P-postlabeling and HPLC-FD analysis of DNA adducts in rats acutely exposed to benzo(a)pyrene. Chem Biol Interact. 1997;104:41–54. doi: 10.1016/s0009-2797(97)03765-4. [DOI] [PubMed] [Google Scholar]
- D’Agostini F, Fronza G, Campomenosi P, Izzotti A, Petrilli GL, Abbondandolo A, De Flora S. Cancer biomarkers in human atherosclerotic lesions: no evidence of p53 involvement. Cancer Epidemiol Biomarkers Prev. 1995;4:111–115. [PubMed] [Google Scholar]
- van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, Havekes LM. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ Res. 2001;88:780–786. doi: 10.1161/hh0801.089261. [DOI] [PubMed] [Google Scholar]
- Guevara NV, Kim HS, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med. 1999;5:335–339. doi: 10.1038/6585. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- White KL, Jr, Holsapple MP. Direct suppression of in vitro antibody production by mouse spleen cells by the carcinogen benzo(a)pyrene but not by the noncarcinogenic congener benzo(e)pyrene. Cancer Res. 1984;44:3388–3393. [PubMed] [Google Scholar]
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am J Pathol. 1996;149:359–366. [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Kroese ED, Vos JG, Van Loveren H. Detection of immunotoxicity of benzo[a]pyrene in a subacute toxicity study after oral exposure in rats. Toxicol Sci. 1999;50:214–220. doi: 10.1093/toxsci/50.2.214. [DOI] [PubMed] [Google Scholar]