Abstract
Here we clarified the morphology and phenotype of interleukin (IL)-17- and interferon (IFN)-γ-producing cells in both in vitro and in vivo situations. Oligoclonal activation of normal peripheral blood mononuclear cells with the superantigen Staphylococcus aureus enterotoxin B and polyclonal activation with phorbol myristate acetate/phytohemagglutinin were used as in vitro models. This study was extended to various in vivo situations such as rheumatoid arthritis, dermatomyositis, and normal activated lymph nodes. The phenotype of IL-17- and IFN-γ-producing cells was evaluated by immunohistochemistry using the CD3 and CD4 T-cell markers, the CD20, CD38, κ and λ light chain B-cell lineage markers. The expression of two chemokine receptors, CCR6 and CCR7, involved with their associated ligands CCL20 and CCL19/CCL21 in the migration of T lymphocytes, was evaluated in tissue sections. After both polyclonal and oligoclonal activation, IL-17+ and IFN-γ+ cells acquired a plasma cell-like morphology associated with a high secretory activity, the reduced expression of CD3, and no change of CD4 expression. In rheumatoid arthritis, dermatomyositis, and activated lymph nodes, both IL-17- and IFN-γ-producing cells had the same morphology. These Th1 cytokine-producing cells were CD4+-, CD3-, and B-cell lineage marker-negative. In both in vitro and in vivo situations, expression of CCR6 or CCR7 was not associated with a particular subset. In conclusion, activated T-helper CD4+ T cells, by their release of cytokines, seem to have functional similarities with plasma cells secreting immunoglobulins.
T and B cells are the two actors of the immune response, which interact with antigens and their derived peptides.1 After such activation, both T- and B-cell lineages are characterized by a differentiation process. B cells internalize specific antigens via their surface immunoglobulin receptors, process the antigen into peptides, which are presented to T cells in the context of the B-cell class II major histocompatibility complex (MHC). Terminal differentiation of B cells results in the production of soluble immunoglobulins by plasma cells, which have lost their surface immunoglobulins.1,2 T cells are also characterized by the expression of antigen-specific T-cell receptor (TCR) that is associated with the CD3 complex. As opposed to B cells, the TCR is not secreted as a soluble form after TCR activation. However, T cells have the capacity to secrete cytokines in response to antigen stimulation. The nature of secreted cytokines led to the classification of T-helper cells into Th1 proinflammatory cytokine-producing cells and Th2 anti-inflammatory cytokine-producing cells.3 A Th1/Th2 imbalance with a local predominance of Th1 cytokines can influence disease mechanisms such as rheumatoid arthritis (RA).4
During studies on the characterization of Th1-producing cytokines, we noticed the plasma cell appearance of some cytokine-producing T cells in RA synovium sections.5 To further clarify this subset, we decided to focus on its morphology and phenotype in both in vitro and in vivo situations. Activated Th CD4+ T cells were defined by the expression of two proinflammatory cytokines6 interleukin (IL)-17 and interferon (IFN)-γ using two in vitro models. The first model is the stimulation with phorbol myristate acetate (PMA) and phytohemagglutinin (PHA) for 4, 24, and 48 hours leading to a polyclonal activation of normal peripheral blood mononuclear cells (PBMCs). The second model is the oligoclonal activation with the superantigen Staphylococcus aureus enterotoxin B (SEB) of normal PBMCs during 4 hours leading to the isolation, by affinity matrix technology, of IFN-γ-secreting CD4+ cell subsets.7–9 The characterization of IL-17- and IFN-γ-producing cells was performed by immunocytochemistry, confocal microscopy, and transmission electron microscopy and extended to various normal and abnormal in vivo situations. We first looked at normal activated lymph nodes and then at inflammatory diseases such as RA and dermatomyositis (DM). DM is an idiopathic inflammatory myopathy considered as a CD4-driven disease with the involvement of Th1 proinflammatory cytokines.10,11 IL-17- and IFN-γ-producing cells were defined by immunohistochemistry using the CD3 and CD4 T-cell markers. To study the migration patterns of these cells, we looked at the expression of two chemokine receptors, CCR6 and CCR7, involved with their associated ligands CCL20 and CCL19/CCL21 in the migration of T lymphocytes.12–15 In RA synovium, we have already shown the involvement of CCL20/CCR6 and CCL19-CCL21/CCR7 in the migration of immature and mature DC subsets, respectively.16
The results indicate that IL-17- and IFN-γ-producing cells acquire a plasma cell-like morphology associated with a reduced expression of CD3 and the persistence of CD4 after in vitro polyclonal and oligoclonal activation. A similar morphology was observed in RA, DM, and activated lymph node sections. In these situations, Th1-cytokine production was associated with a similar phenotype as in plasma cells producing immunoglobulins.
Materials and Methods
Collection of Samples
PBMCs were isolated by Ficoll-Hypaque gradient centrifugation and resuspended in RPMI 1640 culture medium (Invitrogen, Cergy-Pntoise, France) supplemented with 10% fetal calf serum (Invitrogen), penicillin/streptomycin (5 μl/ml), and fungizone (2 μl/ml). Cells were cultured at a concentration of 1 × 106/ml in complete culture medium in six-well microtiter plates (Nunc, Roskilde, Denmark) and were stimulated or not for 4, 24, and 48 hours with 5 ng/ml of PMA and 1 μg/ml of PHA. Cytospin-centrifuged unstimulated and stimulated PBMCs were fixed in 2% formaldehyde solution (Merck, Darmstadt, Germany).
Synovial samples were obtained from patients with RA, defined according to the revised criteria of the American College of Rheumatology (formerly, the American Rheumatism Association).17 To assess inflammatory synovium, samples were obtained from patients undergoing knee or wrist synovectomy, and not at a late stage such as for joint replacement. Muscle samples were obtained from patients selected on the basis of the presence of an inflammatory lesions typical of DM.18,19 Activated lymph nodes were negative for lymphoma.
For detection of cytokine-producing cells, samples were fixed in 4% phosphate-buffered paraformaldehyde and then embedded in paraffin. Four-μm sections were cut and mounted on glass slides. To detect antigen expression in paraffin-embedded sections, antigen retrieval procedures were performed, including incubation in either citrate buffer (10 mmol/L, pH = 6) or ethylenediaminetetraacetic acid buffer (1 mmol/L, pH = 8) followed by a microwave oven incubation for 3 minutes for three times.
Stimulation of Normal PBMCs with SEB—Isolation of IFN-γ-Secreting Cells
Human PBMCs were isolated from fresh leukocyte filters obtained from the local blood bank by Ficoll-Paque plus (Amersham Biosciences, Sweden) density gradient centrifugation. Cells were stimulated in six-well plates at a density of 107 cells/ml with 1 μg/ml of SEB (Sigma-Aldrich, Vienna, Austria) in RPMI medium containing 10% human AB serum (PAA Laboratories, Vienna, Austria) at 37°C. For isolation of IFN-γ-secreting cells, the affinity matrix technology (Miltenyi Biotec, Cologne, Germany) was applied according to the manufacturer’s protocol. In brief, after 4 hours of culture, cells were washed, labeled with IFN-γ catch antibody, diluted with prewarmed medium to 106 cells/ml, incubated for another 45 minutes at 37°C for cytokine secretion, washed, and labeled with IFN-γ detection (PE-coupled) antibody and surface markers CD4/CD8 (Becton Dickinson, Basel, Germany). For magnetic enrichment, cells were incubated with anti-PE-microbeads; magnetically labeled cells were separated from unlabeled using LS+MACS-columns (Miltenyi Biotec). For further purification, individual cell populations were sorted by a Becton Dickinson fluorescence-activated cell sorter (FACS-DIVA). Purity was checked on a FACScalibur (Becton Dickinson). Isolated cell populations were IFN-γ+CD4+, IFN-γ−CD4+, IFN-γ+CD4−, and IFN-γ−CD4− SEB-stimulated cells and IFN-γ−CD4+, and IFN-γ−CD4− unstimulated cells. Finally, cells were washed once with cold phosphate-buffered saline (PBS) and fixed in the presence of 2% formaldehyde solution.
Detection of Cytokine-Producing Cells by Immunohistology
Paraffin-embedded sections of RA synovium, DM, and normal activated lymph nodes were treated in xylene and rehydrated in a gradient of ethanol (once in 99% ethanol, once in 95% ethanol, and once in H2O). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. The sections were then incubated with 5 μg/ml of a goat polyclonal anti-IL-17 (R&D Systems Europe, London, UK), or of a rabbit polyclonal anti-IFN-γ antibody (Genzyme, Cambridge, MA). In negative control sections, the primary antibodies were omitted or irrelevant antibody (goat or rabbit serum) was applied at the same concentration as the primary antibody. After overnight incubation at 4°C and washing, the sections were incubated with biotinylated mouse anti-goat IgG or mouse anti-rabbit IgG antibodies for 30 minutes, followed by streptavidin-peroxidase complex (DAKO, Glostrup, Denmark) for 15 minutes and 3,3′ diaminobenzidine tetrahydrochloride (DAB) (DAKO) for 20 minutes. The sections were then counterstained with Mayer’s hematoxylin.
For double staining with anti-IL-17 and anti-IFN-γ antibodies, paraffin-embedded sections were simultaneously incubated with biotinylated goat polyclonal anti-IL-17 (R&D) and rabbit polyclonal anti-IFN-γ (Genzyme) antibodies. In negative control sections, one of the two primary antibodies was omitted. After overnight incubation at 4°C and washing, the sections were incubated with streptavidin-fluorescein isothiocyanate (DAKO) and CyaninIII goat anti-rabbit Ig (DAKO) throughout 45 minutes. The samples were then examined by fluorescence microscopy.
Characterization of Cytokine-Producing Cells in RA Synovium and in PBMCs
To clarify the morphology and phenotype of IL-17- and IFN-γ-producing cells, double-labeling experiments were performed by first staining for cytokines, followed by immunophenotyping for T-cell markers with mouse monoclonal anti-CD3 (DAKO), and anti-CD4 (Novocastra, Newcastle, England), for B-cell markers with anti-CD20 (DAKO), anti-CD38 (DAKO), anti-κ (Eurodiagnostica, Arnhem, The Netherlands), and anti-λ (Eurodiagnostica), and for chemokine receptors with anti-CCR6 (R&D Systems), and anti-CCR7 (Becton-Dickinson) antibodies. After staining for IL-17 or IFN-γ as described above, incubation with one of these monoclonal antibodies was performed, then purified rabbit anti-mouse IgG (DAKO) and mouse alkaline phosphatase anti-alkaline phosphatase (DAKO) were applied. Alkaline phosphatase was revealed using Fast Blue as chromogen (blue color; Vector Laboratories, Peterborough, UK). Quantification of positive cells on cytospin slides was performed using a Lucia image analyzer (Nikon, France).
To further clarify the phenotype of cytokine-producing cells, immunofluorescence techniques were performed using PE-anti-IL-17, or PE-anti-IFN-γ (R&D Systems) and fluorescein isothiocyanate-anti-CD3, -anti-CD4, -anti-CCR6, or -anti-CCR7 antibodies (DAKO). Fluorescent images were observed under a laser-scanning confocal microscope (Leica TCS SP2).
For electron microscopic examination, the sorted cells were quickly washed in PBS, and immediately fixed with 4% paraformaldehyde and 2% glutaraldehyde. The cells were postfixed with 1% OsO4 for 2 hours, alcohol-dehydrated, and embedded in EPON. Thin sections were collected on nickel grids and stained with uranyl acetate and lead citrate. The prepared samples were observed under a transmission microscope.
Measurement of IL-17 and IFN-γ Levels on PBMC Supernatants
IL-17 and IFN-γ were measured by two-site sandwich enzyme-linked immunosorbent assay. Supernatants or serial dilutions of IFN-γ standard (Schering-Plough Research Institute) were incubated for 60 minutes at 20°C in 96-well microtiter plates (Nunc), which were coated overnight at 4°C with the mouse A35 anti-IFN-γ monoclonal antibody, and saturated for 60 minutes with PBS-0.05% Tween 20 to 5% bovine serum albumin. After washing, a biotinylated mouse B27 anti-IFN-γ monoclonal antibody (60 ng/ml) was added and cultures were incubated for 90 minutes at 20°C. After subsequent incubation with peroxidase-coupled streptavidin and visualization with orthophenylenediamine (Sigma), the plates were read at 492 nm. Levels of IL-17 in PBMC supernatants were measured by enzyme-linked immunosorbent assay (Medgenix IL-17 cytoscreen assay; BioSource Europe S.A., Nivelles, Belgium). The plates were read at 450 nm. Sensitivity of the assay was 2 pg/ml.
Results
The Th1-Producing T Cells Acquire a Plasma Cell-Like Morphology after in Vitro Activation
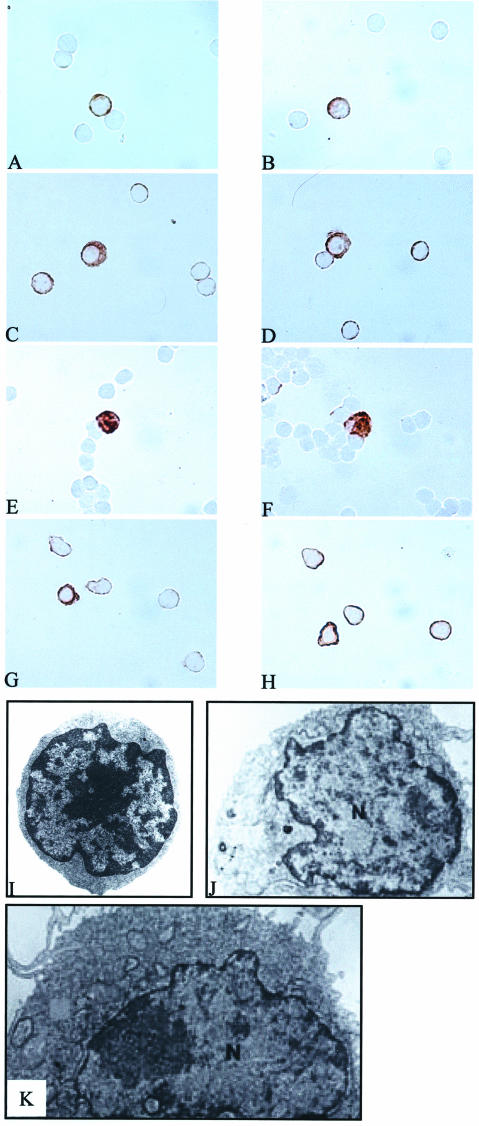
We first used a polyclonal activation with PMA and PHA. Normal PBMCs were stimulated with PMA (5 ng/ml)/PHA (1 μg/ml) for 4, 24, and 48 hours. Immunostaining using anti-IL-17 and anti-IFN-γ antibodies was performed on cytospin slides. Both IL-17- and IFN-γ-producing cells after 4 hours of stimulation had a T-cell phenotype with a central large nucleus and a reduced cytoplasm (Figure 1, A and B, respectively). After 24 hours of stimulation, some IL-17- and IFN-γ-producing cells had a plasmacytoid-like appearance with a remote nucleus in a large cytoplasm (Figure 1, C and D, respectively). After 48 hours, all Th1 cytokine-producing cells had acquired this plasma cell-like morphology (Figure 1, E and F).
Figure 1.
IL-17- and IFN-γ-producing cells acquire a plasmacytoid-like morphology after 4 hours of polyclonal activation with PMA/PHA and oligoclonal activation with SEB. Immunostaining using anti-IL-17 and anti-IFN-γ antibodies (DAB, brown) were performed on PBMCs stimulated with PMA/PHA for 4, 24, and 48 hours or with the superantigen SEB for 4 hours. After 4 hours of polyclonal activation, IL-17+ and IFN-γ+ cells had both a T-cell phenotype (A and B, respectively). After 24 hours, IL-17- and IFN-γ-producing cells had predominantly a plasmacytoid-like appearance (C and D, respectively). After 48 hours, all Th1-producing cells presented this plasma cell-like morphotype (E and F). After oligoclonal activation, IL-17+ cells and IFN-γ+ cells had a classical T-cell phenotype (G and H, respectively). Transmission electron microscopy analysis of sorted cells showed that IFN-γ−CD4+ cells (stimulated or not) had the same classical T-cell morphology (I). After 4 hours of stimulation, most of the IFN-γ+CD4+ cells showed this T-cell morphology. However, some IFN-γ+CD4+ cells stimulated with SEB for 4 hours showed a plasmacytoid-like appearance with a remote nucleus in a large cytoplasm (J and K). Original magnifications: ×1000 (A–H); ×4000 (I–K).
At the same time, we investigated the profile of secretion of both cytokines as measured by enzyme-linked immunosorbent assay in the same culture supernatants. In all supernatants of unstimulated PBMCs, no production of IL-17 or IFN-γ was detected. When PBMCs were stimulated, both cytokines were detected in culture supernatants after 4, 24, and 48 hours. The profile of secretion was quite similar for IL-17 and IFN-γ (Table 1), with a time-dependent enhanced concentration. However, the concentrations of IFN-γ were approximately threefold higher than those of IL-17 (IFN-γ, mean ± SEM, 24.3 ± 5.9 ng/ml versus IL-17, 7.4.± 1.9 ng/ml), sevenfold (IFN-γ, 70 ± 8.2 ng/ml versus IL-17, 9.8.± 0.7 ng/ml), and 10-fold (IFN-γ, 168 ± 11.4 ng/ml versus IL-17, 17.8 ± 0.3 ng/ml) after 4, 24, and 48 hours, respectively. When combined with the cell appearance described above, the time-dependent enhanced concentrations of IL-17 and IFN-γ in PBMC supernatants was linked to the acquisition of the plasma cell-like morphology.
Table 1.
Estimation of Single-Cell Production of IFN-γ and IL-17
| Duration of cultures
|
|||
|---|---|---|---|
| 4 hours | 24 hours | 48 hours | |
| Concentrations of cytokines in PBMC supernatants (1 × 106 cells/ml) (ng/ml) | |||
| IFN-γ | 24.3 ± 5.9 | 70 ± 8.2 | 168 ± 11.4 |
| IL-17 | 7.4 ± 1.9 | 9.8 ± 0.7 | 17.8 ± 0.3 |
| Number of cytokine-producing cells on cytospin slides (n = 5) (2.105 cells per slide) | |||
| IFN-γ | 4103 ± 402 | 10217 ± 421 | 11100 ± 350 |
| IL-17 | 1108 ± 116 | 1200 ± 190 | 2205 ± 180 |
| Single-cell production of cytokines (pg) | |||
| IFN-γ | 1.1 | 1.4 | 3 |
| IL-17 | 1.3 | 1.6 | 2.5 |
PBMCs were stimulated for 4, 24, or 48 hours with PMA (5 ng/ml) and PHA (1 μg/ml). Cytokine levels were measured in the supernatants by ELISA. The number of IL-17+ and IFN-γ+ cells on cytospin slides was evaluated using the Lucia image analyzer.
After taking into account the levels of IL-17 and IFN-γ in PBMC culture supernatants, the production of IL-17 and IFN-γ at a single-cell level was estimated by quantifying the number of IL-17+ and IFN-γ+ cells on cytospin slides using a Lucia image analyzer (Table 1). In the same way, a time-dependent enhanced production was observed at a single-cell level. In parallel with the acquisition of the plasma cell-like morphology, the single-cell production of IFN-γ (from 1.4 to 3 pg) and IL-17 (from 1.6 to 2.5 pg) was up-regulated (Table 1). Thus, the difference in levels in supernatants between the two cytokines was related to a difference in cell frequency, the production per cell remaining identical for each cytokine.
Next, PBMCs were exposed to an oligoclonal activation with the superantigen SEB. Normal PBMCs stimulated or not with the superantigen SEB were selected according to the expression of IFN-γ and CD4 using the affinity matrix technology and purified by fluorescence-activated cell sorting into the four subsets expressing or not IFN-γ and CD4. After such purification, staining with anti-IL-17 antibody was performed on six different cell populations: IFN-γ+CD4+, IFN-γ−CD4+, IFN-γ+CD4−, and IFN-γ−CD4− SEB-stimulated cells and IFN-γ−CD4+, IFN-γ−CD4− unstimulated cells.
As observed with polyclonal activation after 4 hours of stimulation, the predominant morphology of the IL-17+ and IFN-γ+ cells was that of a classical T-cell phenotype with a large nucleus in a reduced cytoplasm (Figure 1, G and H, respectively). IL-17 was detected in both IFN-γ+CD4+ and IFN-γ−CD4+ SEB-stimulated cells but not in IFN-γ+CD4−, and IFN-γ−CD4− SEB-stimulated cells and IFN-γ−CD4+, IFN-γ−CD4− unstimulated cells (data not shown). Transmission electron microscopy analysis of sorted cells showed that IFN-γ−CD4+ cells (stimulated or not) had the same classical T-cell morphology (Figure 1I). After 4 hours of stimulation, most of the IFN-γ+CD4+ cells showed this T-cell morphology. However, some IFN-γ+CD4+ cells stimulated with SEB for 4 hours showed a plasmacytoid-like appearance with a remote nucleus in a large cytoplasm (Figure 1, J and K).
The Th1-Producing T Cells Lose the Expression of CD3 but Not that of CD4 after Oligoclonal and Polyclonal Activation
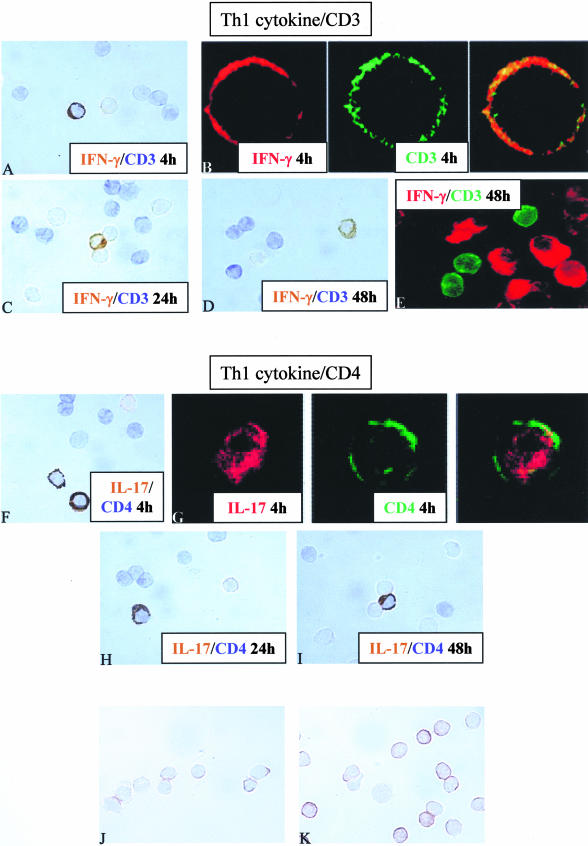
To clarify the phenotype of IL-17- or IFN-γ-producing cells, double staining with anti-IL-17 or anti-IFN-γ and anti-CD4 or anti-CD3 antibodies were performed on activated PBMCs after 4, 24, and 48 hours of stimulation with PMA/PHA. CD3 was still expressed on IL-17+ and IFN-γ+ cells after 4 hours of stimulation (Figure 2, A and B). This expression decreased after 24 hours (Figure 2C) and was not detected anymore after 48 hours of stimulation on both IL-17- and IFN-γ-producing cells (Figure 2, D and E), whereas that of CD4 did not change (Figure 2; F to I, respectively).
Figure 2.
The Th1-producing T cells lose the expression of CD3 but not that of CD4 after oligoclonal and polyclonal activation. Double staining using anti-IL-17 or anti-IFN-γ (brown) and anti-CD4 or anti-CD3 antibodies (blue) were performed in the two in vitro models. After 4 hours of stimulation with PMA/PHA, IFN-γ+ cells express the CD3 marker (A and B). After 24 hours, the lack of CD3 expression on IFN-γ-producing cells (C) was also observed after 48 hours (D and E). Double staining using anti-IL-17 (brown) and anti-CD4 (blue) shows that IL-17-producing cells express the CD4 after 4, 24, and 48 hours (F, G, H, and I, respectively). After 4 hours of stimulation with the superantigen SEB, the expression of CD3 is still present on IFN-γ+CD4+ cell subset (J) but with a lower staining intensity when compared to that of CD3 staining on IFN-γ−CD4+ cell subset (K). Original magnifications, ×1000.
Similarly, expression of CD3 on IFN-γ−CD4+ and IFN-γ+CD4+ cell subsets, after 4 hours of stimulation with the superantigen SEB, started to decrease on IFN-γ+CD4+ cell subset (Figure 2J) as indicated by the lower staining intensity when compared to that of CD3 staining on IFN-γ−CD4+ cell subset (Figure 2K).
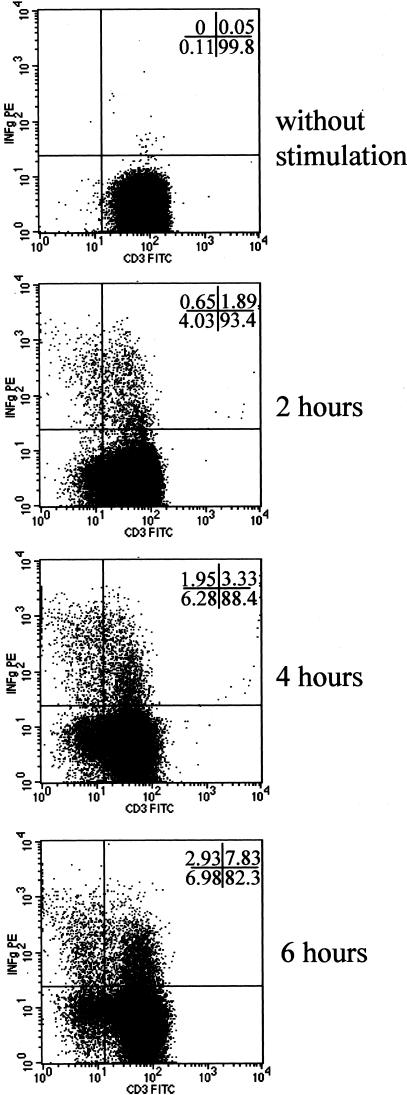
This study was further extended by a fluorescence-activated cell sorting analysis looking at the kinetics of CD3 expression from 0 to 6 hours after SEB stimulation of CD4+ T cells. The dot-plot image in Figure 3 shows CD3 expression versus IFN-γ staining gated on CD4+ cells isolated from PBMCs stimulated from 0 to 6 hours. Progressive loss of CD3 expression was observed in both IFN-γ+ and IFN-γ− subsets but was particularly clear for the IFN-γ+ subset. At 6 hours, 37% of IFN-γ+CD4+ cells (2.93 of 6.98) were CD3-negative versus 8% for the IFN-γ− cells (Figure 3).
Figure 3.
Kinetic of CD3 and IFN-γ expression on CD4+ T cells after oligoclonal activation with SEB. Using a FACS analysis, the CD3 kinetics (detected with anti-CD3-fluorescein isothiocyanate) were evaluated from 0 to 6 hours on SEB stimulation on CD4 T cells in combination with IFN-γ staining (detected with anti-IFN-γ-PE). The dot-plots display CD3 versus IFN-γ staining gated on CD4+ cells isolated from PBMCs stimulated from 0 to 6 hours. The loss of CD3 expression on CD4+ T cells is time-dependent and associated with that of IFN-γ apparition.
The Th1-Producing Cells in Activated Lymph Nodes and Inflammatory Diseases
This in vitro activation may represent an artificial situation, in particular not representing the cell-cell interactions observed in vivo, in lymphoid organs and local inflammatory lesions. Accordingly, we compared the phenotype observed after in vitro activation to that found in sections of activated reactive lymph nodes, RA synovium, and DM.
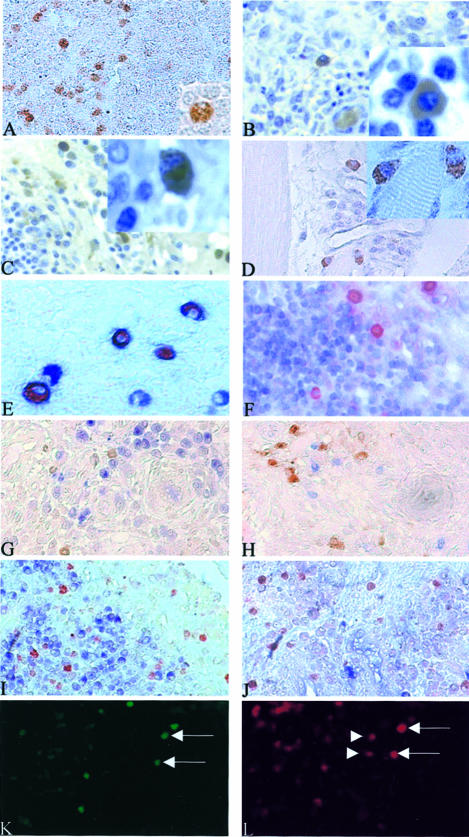
In activated lymph nodes, IL-17 or IFN-γ+ cells were detected in the T-cell zone out of germinal centers (Figure 4A). Both subsets had the plasmacytoid-like morphology described in vitro (Figure 4A, inset). In RA synovium, when studied at high magnification, the IL-17- and IFN-γ-producing cells had exactly the same plasma cell-like morphology as observed for PBMCs activated with PMA/PHA for 24 and 48 hours (insets of Figure 4, B and C, respectively). These cells were either isolated (Figure 4B) or accumulated as part of a lymphocytic infiltrate (Figure 4C).
Figure 4.
In normal activated lymph nodes, RA synovium, and DM, Th1-producing cells presented a plasma cell-like morphology. In RA synovium, these Th1 plasmacytoid-like-producing cells still express the CD4 but not the CD3 and B/plasma cell markers. In RA synovium, some of the IFN-γ+ cells can express IL-17. Immunostaining using anti-IL-17 and anti-IFN-γ antibodies (DAB, brown) were performed on normal activated lymph nodes, RA synovium, and DM paraffin-embedded sections. In activated lymph nodes, IL-17 or IFN-γ+ cells detected in the T-cell zone out of germinal center (A) have a plasma cell-like morphology (A, inset). In RA synovium, the IL-17- and IFN-γ-producing cells presented the same morphology (insets of B and C, respectively). IL-17+ cells and IFN-γ+ cells were either isolated (B) or part of a lymphocytic infiltrate (C). In DM-, IL-17-, or IFN-γ-producing cells detected in lymphocytic infiltrates in the endomysium (D) presented also a plasma cell-like morphology (D, inset). Double staining using anti-IL-17 or anti-IFN-γ (AEC, red) and anti-CD4 or anti-CD3 antibodies (Vector blue, blue) were performed on RA sections. IL-17+ cells express the CD4 marker (E) but not the CD3 (F). Double staining for IL-17 or IFN-γ (red) and CD20, CD38, κ or λ light chains (blue) showed that none of the IL-17- or IFN-γ-positive cells was expressing CD20, CD38, κ or λ light chains (G to J, respectively). Same sections of RA synovial tissue were incubated with anti-IL-17 and anti-IFN-γ and stained using the immunofluorescence technique (K and L, respectively). Some cells could be expressing at the same time both IL-17 (fluorochrome fluorescein isothiocyanate, green) and IFN-γ (fluorochrome CyaninIII, red) as indicated by the arrows in K and L. These experiments also showed that some cells secreting IFN-γ were not secreting IL-17 as indicated by the arrowheads in L. Original magnifications: ×400 (A–D, F–L); ×1000 (E and insets in A–D).
In DM sections, IL-17- or IFN-γ-producing cells were detected in lymphocytic infiltrates in the endomysium (Figure 4D) but at a lower frequency compared to RA synovium. These cells also had a plasma cell-like morphology (Figure 4D, inset).
To further characterize the Th1 cytokine-producing T cells in RA synovium, double staining was performed with anti-IL-17 or anti-IFN-γ and anti-CD3 or anti-CD4 antibodies. Here again, IL-17- and IFN-γ-producing cells expressed CD4 (Figure 4E) but not CD3 (Figure 4F), as observed with PBMCs activated for 24 hours.
As plasma cells are commonly found in these in vivo situations, double staining for cytokines and B-cell lineage markers was performed using CD20 as a B-cell marker and CD38, κ/λ light chains as plasma cell markers. Double staining with anti-IL-17 or anti-IFN-γ and anti-CD20, CD38, κ/λ light chains showed that none of the IL-17+ or IFN-γ+ cells expressed B/plasma cell markers (Figure 4; G to J, respectively). These results excluded B cells and plasma cells as IL-17- or IFN-γ-producing cells.
Co-Expression of IL-17 and IFN-γ in RA Synovium
Double staining of RA synovium sections with anti-IL-17 and anti-IFN-γ antibodies indicated that some cells could be expressing at the same time both IL-17 and IFN-γ (as indicated by the arrows in Figure 4, K and L, respectively), or IFN-γ but not IL-17 (Figure 4L, arrowheads). On the contrary, cells secreting IL-17 alone and not IFN-γ were not observed (Figure 4, K and L). However, simultaneous secretion appeared to be a rare event when compared to a single secretion of IFN-γ. Such profile of secretion was also observed in IFN-γ-producing cells isolated after stimulation with SEB. Staining with anti-IL-17 on these IFN-γ+-isolated T cells showed that some IFN-γ+CD4+ T cells can also produce IL-17 (Figure 1G).
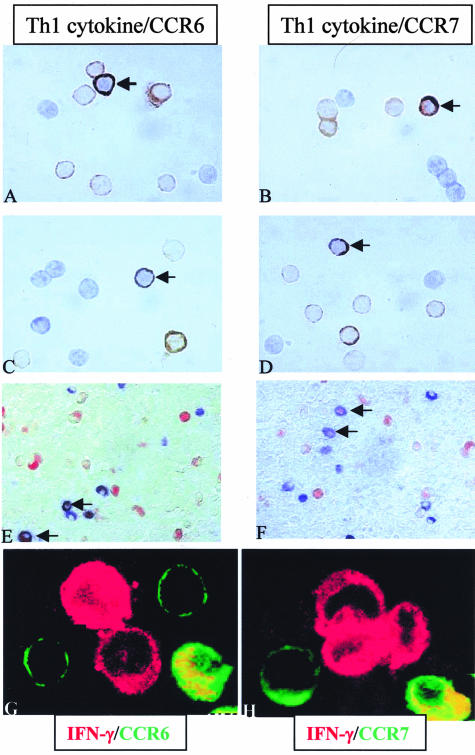
Some of the IL-17- and IFN-γ-Producing Cells Express the Chemokine Receptors CCR6 and CCR7 after Oligoclonal and Polyclonal Activation and in RA Synovium
Migration of T cells toward inflammatory sites is under the control of chemokines acting on membrane receptors. Accordingly, we focused on the expression on IL-17- and IFN-γ-producing cells of two chemokine receptors, CCR6 and CCR7, known to be involved with their associated ligands CCL20 and CCL19/CCL21 in the migration of T cells. After stimulation with PMA/PHA for 4, 24, and 48 hours and whatever the duration of stimulation, some IL-17 and IFN-γ-producing cells could express the CCR6 receptor [Figure 5, A (arrow) and G]. However, this was not a subset marker because some IL-17- and IFN-γ-producing cells did not express CCR6, and some CCR6+ cells did not produce IL-17 or IFN-γ (Figure 5, A and G). As for CCR6, double staining with anti-IL-17 or anti-IFN-γ and anti-CCR7 antibodies revealed the presence of CCR7+Th1-producing cells [Figure 5, B (arrow) and H], CCR7-Th1-producing cells and CCR7+ cells nonproducing IL-17 or IFN-γ (Figure 5, B and H). Double staining using anti-IL-17 and anti-CCR6 or anti-CCR7 antibodies of IFN-γ-producing CD4+ T cells stimulated with SEB showed that some IL-17+IFN-γ+CD4+ T cells expressed CCR6 and CCR7 [Figure 5, C and D (arrows), respectively].
Figure 5.
Some of the IL-17+ or IFN-γ+ cells can express the chemokine receptors CCR6 and CCR7 after oligoclonal and polyclonal activation and also in inflammatory diseases such as RA. Double staining using anti-IL-17 or anti-IFN-γ (DAB, brown) and anti-CCR6 or anti-CCR7 antibodies (Vector blue, blue) were performed on PBMCs stimulated with PMA/PHA for 4, 24, and 48 hours or with the superantigen SEB for 4 hours. After polyclonal activation and whatever duration of stimulation, some of the IL-17+ cells express the CCR6 receptor as indicated by the arrows in A and G. However, two other subsets were detected: IL-17+CCR6− and IL-17-CCR6+ (A and G). As for CCR6, double staining using anti-IL-17 and anti-CCR7 antibodies revealed the presence of IL-17+CCR7+ (arrow), IL-17+CCR7− and IL-17-CCR7+ subsets (B and H). Staining on IFN-γ+CD4+ isolated cells showed the same pattern of CCR6 and CCR7 expression (C and D, respectively). Double staining using anti-IL-17 or anti-IFN-γ (AEC, red) and anti-CCR6 or anti-CCR7 antibodies (blue) were performed in RA synovium sections. IL-17+ cells have the same pattern of CCR6 and CCR7 expression (E and F, respectively). Original magnifications: ×1000 (A–D, G, H); ×400 (E, F).
A similar observation was made with RA synovium. CCR6 was expressed by IL-17+ and IFN-γ+ cells (Figure 5E, arrow) such as IL-17− and IFN-γ− cells (Figure 5E). Similarly, CCR7 was observed in Th1 cytokine-producing cells (Figure 5F, arrow) such as IL-17− and IFN-γ− cells (Figure 5F), indicating that the chemokine receptors do not delineate Th1-secreting cell subsets.
Discussion
This study was initiated after our initial observation of the contribution of IL-17 to the pathogenesis of RA.5 We were impressed by the plasma cell-like appearance of IL-17-producing cells. Using clones of T cells obtained from the synovium, we were able to link IL-17 production to the Th1 profile,5,6 with a particular proinflammatory and destructive pattern associated with IL-17 secretion.20 Here we show that this phenotype can be induced in vitro and is indeed associated with cytokine production.
First, we focused on the phenotype and morphology of IL-17- and IFN-γ-producing cells in normal PBMCs after polyclonal and oligoclonal activation. This study was extended to normal activated lymph nodes and to inflammatory diseases such as RA and DM. We looked at the expression by immunohistochemistry of the CD3 and CD4 T-cell markers, the CD20, CD38, κ and λ B-cell lineage markers, and the chemokine receptors CCR6 and CCR7.
A classical T-cell morphology was observed in IL-17- and IFN-γ-producing cells when stimulated for a short-time period (4 hours) with PMA/PHA or with the superantigen SEB. Later, when studied at high magnification, IL-17- and IFN-γ-producing cells showed a plasma cell-like morphology, with a remote nucleus in a large cytoplasm, in activated PBMCs stimulated with PMA/PHA for 24 and 48 hours and also in normal activated lymph nodes, RA, and DM. These IL-17- or IFN-γ-producing cells looked like but were not plasma cells, as indicated by the lack of expression of the CD20 B-cell marker, and the CD38, κ and λ light chain plasma cell markers. For B cells, the acquisition of the plasma cell morphology has been associated with the start of immunoglobulin production. In the same way, the time-dependent enhanced concentrations of IL-17 and IFN-γ in PBMC supernatants measured by enzyme-linked immunosorbent assay was linked to the acquisition of the plasma cell morphology. As for B cells, the plasmacytoid appearance of Th cells was associated with a secretory activity of cytokines in this case. When expressed at a single cell level, production of IFN-γ or IL-17 was identical, and differences in supernatant levels were explained by a higher frequency of IFN-γ- or IL-17-producing cells.
Regarding the expression of the CD3 T-cell marker, IL-17- and IFN-γ-producing cells still express CD3 until 4 hours of polyclonal or oligoclonal activation. But after 24 and 48 hours of stimulation with PMA/PHA, IL-17- or IFN-γ-producing cells lose the CD3 as also observed in normal activated lymph nodes, RA synovium, and DM. There was a link between the reduced expression of CD3 and the plasma cell morphology of Th1+ cells. At 4 hours of stimulation, the classical T-cell morphology was associated with the expression of CD3, which disappeared with the appearance of the plasmacytoid-like morphology after 24 hours. As for the acquisition of the plasma cell morphology, a parallel can be drawn between B- and T-cell lineages regarding the expression of molecules on their membrane. When B cells become plasma cells, they lose their membrane immunoglobulin and start to secrete. Here for Th1 cells, the plasmacytoid-like appearance was associated with the loss of CD3 expression. As opposed to B cells, the TCR is not secreted as a soluble form after TCR activation. The TCR loss would imply a loss of interactions with antigen-presenting cells, after TCR activation. This lack of interaction could result in the persistence of the release of Th1 cytokines perpetuating then the inflammatory and destructive processes in RA or DM.
When we compared the in vitro models with the in vivo situations, we observed the same pattern in RA and on PBMCs stimulated with PMA/PHA for 24 and 48 hours. The level of activation in RA appears to be equivalent to a strong stimulation with PMA (5 ng/ml)/PHA (1 μg/ml) during 24 hours. In normal activated lymph nodes and inflammatory diseases such as RA and DM, the cytokine microenvironment and cell interactions leading to Th cell activation are potent contributors to chronicity.
Migration of T cells is critical in the pathogenesis of RA and DM. Thus we looked at the expression of two chemokine receptors CCR6 and CCR7 on IL-17- or IFN-γ-producing cells in both in vitro and in vivo conditions. In both situations, only some IL-17+ cells express the CCR6 or CCR7 receptors indicating that these receptors are not subset markers of T helper cells. This reflects also the heterogeneity of chemokine receptor expression on effector T cells, as observed in another in vitro study looking at such expression on PBMCs stimulated with PMA and ionomycin for 4 hours.21 It has been shown also that the in vitro migration of human and murine CD4+- IFN-γ-, IL-4-, and IL-10-producing cells were comparable to that of naïve CD4+ cells in response to CCL19 and CCL21.22 This indicates that CCR7 is also expressed on activated CD4+ T cells.22 In RA synovium, the close association between CCL21 and CCL20 with IL-17+CCR7+ and IL-17+CCR6+ cells, respectively, suggests the contribution of CCL21 and CCL20 to the migration of CCR7+ and CCR6+ Th1-producing cells into RA synovium.
In conclusion, Th1-producing cells are rather rarely found in normal activated and inflammatory conditions. As for plasma cells compared to B cells, their differentiation into producing cells implies an important secretory activity at a single level.23,24 Despite such low distribution, IL-17- and IFN-γ-producing cells, with this plasma cell-like morphology can still represent major actors of the inflammatory and destructive processes in inflammatory diseases. This makes proinflammatory cytokine-producing T cells a potential therapeutic target.
Footnotes
Address reprint requests to Pierre Miossec, M.D., Ph.D., Clinical Immunology Unit, Departments of Immunology and Rheumatology, Hôpital Edouard Herriot, 69437 Lyon Cedex 03, France. E-mail: miossec@univ-lyon1.fr.
Supported by a grant from la Fondation pour la Recherche Médicale (to G. P.).
References
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- Assenmacher M, Manz R, Miltenyi S, Scheffold A, Radbruch A. Fluorescence-activated cytometry cell sorting based on immunological recognition. Clin Biochem. 1995;28:39–40. doi: 10.1016/0009-9120(94)00063-2. [DOI] [PubMed] [Google Scholar]
- Assenmacher M, Scheffold A, Schmitz J, Segura Checa JA, Miltenyi S, Radbruch A. Specific expression of surface interferon-gamma on interferon-gamma producing T cells from mouse and man. Eur J Immunol. 1996;26:263–267. doi: 10.1002/eji.1830260141. [DOI] [PubMed] [Google Scholar]
- Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG, Goebels N, Behrens L. Cellular immune mechanisms in inflammatory myopathies. Curr Opin Rheumatol. 1997;9:520–526. doi: 10.1097/00002281-199711000-00007. [DOI] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagira M, Imai T, Yoshida R, Takagi S, Iwasaki M, Baba M, Tabira Y, Akagi J, Nomiyama H, Yoshie O. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur J Immunol. 1998;28:1516–1523. doi: 10.1002/(SICI)1521-4141(199805)28:05<1516::AID-IMMU1516>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kim CH, Pelus LM, White JR, Applebaum E, Johanson K, Broxmeyer HE. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J Immunol. 1998;160:2418–2424. [PubMed] [Google Scholar]
- Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Nenstadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GC. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bohan A, Peter JB. Polymyositis and dermatomyositis. Part 2. N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- Bohan A, Peter JB. Polymyositis and dermatomyositis Part 1. N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, Hopken UE, Hamann A. In vivo differentiated cytokine-producing CD4(+) T cells express functional CCR7. J Immunol. 2002;168:5441–5447. doi: 10.4049/jimmunol.168.11.5441. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Durand I, Banchereau J, Miossec P. The ability of synoviocytes to support terminal differentiation of activated B cells may explain plasma cell accumulation in rheumatoid synovium. J Clin Invest. 1995;95:456–463. doi: 10.1172/JCI117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Rissoan MC, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993;177:523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]