Abstract
Central nervous system (CNS) disease is a frequent complication of human immunodeficiency virus (HIV)-1 infection. Identification of cellular mechanisms that control virus replication and that mediate development of HIV-associated neuropathology will provide novel strategies for therapeutic intervention. The milieu of the CNS during HIV infection is extraordinarily complex because of infiltration of inflammatory cells and production of chemokines, cytokines, and neurotoxic molecules. Cells in the CNS must integrate signaling pathways activated simultaneously by products of virus replication and infiltrating immune cells. In this study, we examined activation of mitogen-activated protein kinases (MAPKs) in the CNS of simian immunodeficiency virus-infected macaques during acute, asymptomatic, and terminal infection. We demonstrate that significantly increased (P < 0.02) activation of ERK MAPK, typically associated with anti-apoptotic and neuroprotective pathways, occurs predominantly in astrocytes and immediately precedes suppression of virus replication and macrophage activation that occur after acute infection. In contrast, significantly increased activation of proapoptotic, neurodegenerative MAPKs JNK (P = 0.03; predominantly in macrophages/microglia), and p38 (P = 0.03; predominantly in neurons and astrocytes) after acute infection correlates with subsequent resurgent virus replication and development of neurological lesions. This shift from classically neuroprotective to neurodegenerative MAPK pathways suggests that agents that inhibit activation of JNK/p38 may be protective against HIV-associated CNS disease.
Although highly active anti-retroviral therapy has reduced the incidence of clinical signs of neurological disease in human immunodeficiency virus (HIV)-infected individuals, autopsy studies suggest that there has been no corresponding decline in the incidence of inflammatory lesions in the central nervous system (CNS).1–3 In addition, there is evidence that highly active anti-retroviral therapy has been less effective in lowering virus replication in the CNS than in the blood, and highly active anti-retroviral therapy resistant viruses have been identified in the CNS.4–6 Further, anti-HIV drugs have widely differing abilities to cross the blood-brain barrier and have different bioavailabilities in the brain.7–9 Finally, there is substantial evidence that the CNS constitutes a significant reservoir for virus in HIV individuals despite anti-retroviral therapy.10,11 These observations emphasize the importance of developing novel therapeutic strategies to suppress HIV replication in the CNS and prevent the development of neuropathological changes.
Simian immunodeficiency virus (SIV) infection of macaques provides an excellent model to investigate the cellular mechanisms that control acute virus replication in the CNS and lead to neurological disease in HIV-infected people. SIV-infected macaques develop acquired immune deficiency syndrome and neuropathological changes similar to those of HIV-infected individuals, including motor and cognitive deficits as well as multifocal and perivascular aggregates of brain macrophages and multinucleated giant cells, which serve as the major hosts for productive replication of virus in the CNS.12–17
To identify events during acute and asymptomatic infection that lead to CNS disease an SIV/macaque model was needed in which the vast majority of infected animals develop CNS disease. We developed such a model by inoculating pigtailed macaques with a neurovirulent molecularly cloned virus, SIV/17E-Fr, and a virus swarm, SIV/DeltaB670.18–20 This model exhibits the classical stages (acute, asymptomatic, and terminal) of HIV/SIV infection on an accelerated time schedule, with more than 90% of macaques developing inflammation in the CNS by 84 days after inoculation. Analysis of early events in the CNS indicated that acute (10 days after inoculation) virus replication was accompanied by activation of macrophages and microglia.11 Virus replication and macrophage activation markers in the CNS declined in all macaques by 21 days after inoculation, despite high-level virus replication in the peripheral blood at this time. These findings suggest the presence of mechanisms in the CNS that quell SIV replication and macrophage activation early after infection. Importantly, levels of viral DNA in the CNS were unchanged between 10 and 21 days after inoculation, indicating that virus-infected cells were not eliminated from the brain during this transition from acute to asymptomatic infection. During late infection (56 to 84 days after inoculation) increased expression of macrophage and astrocyte activation markers and increased infiltration of macrophages and cytotoxic lymphocytes accompanied resurgent virus replication in the brain and the development of encephalitis.11,20,21 Macaques with the most severe neurological lesions exhibited the most precipitous declines in CD4+ cell counts, the highest cerebrospinal fluid viral load and the greatest expression of viral RNA and protein in the brain.
Identification of viral and cellular mechanisms in the CNS that control virus replication after acute infection, maintain latency during the asymptomatic stage and mediate resurgence of virus replication and development of HIV-associated encephalitis during late infection is critical. The dynamic signaling pathways that are activated by virus replication and activated/infiltrating macrophages and lymphocytes and the cytokines, chemokines, and neurotoxic products they elaborate may interfere with the homeostatic signaling pathways that maintain normal quiescent brain function. It is essential to understand how specific CNS cells respond to the inflammatory environment because activation of specific signaling pathways in the CNS may help control local virus replication and/or mediate the development of neurological lesions. To date, although a single in vivo study reported dysregulation of protein kinases in CD4+ T cells derived from peripheral blood during SIV infection (after achieving the viral load set point),22 no studies have examined dysregulation of intracellular signaling pathways during HIV or SIV infection of the CNS.
Using our accelerated, consistent model of HIV-associated CNS disease, we quantitated activation of three different mitogen-activated protein kinases (MAPKs), ERK, JNK, and p38, in the CNS during acute, asymptomatic, and terminal infection. MAPK signal transduction cascades are commonly activated in response to diverse stimuli including cytokines, chemokines, cell-cell contact, matrix proteins, stress, growth factors, and viral proteins.23,24 Activation of ERK MAPK is typically associated with events promoting cell growth and differentiation, whereas activation of JNK and p38 MAPK are associated with growth arrest, apoptosis, and oncogenic transformation.25–27 Our results indicate that increased activation of ERK occurs predominantly in astrocytes and is present as early as 10 days after inoculation. Because no significant changes in JNK or p38 activation are observed at this time, the homeostatic balance between ERK and JNK/p38 activation in the CNS favors activation of ERK pathways. This response immediately precedes control of acute SIV replication and down-regulation of proinflammatory responses, which occur between 10 and 21 days after inoculation. During terminal infection (56 to 84 days after inoculation), a period characterized by resurgent virus replication and the development of neurological lesions, the balance of MAPK activation is markedly shifted in favor of JNK/p38 pathways, reflecting a failure to sustain critical signaling mechanisms required to maintain homeostasis in the CNS.
Materials and Methods
Animals
Twenty-eight male, 2- to 3 year-old pigtailed macaques (Macaca nemestrina) were intravenously inoculated with a virus swarm, SIV/DeltaB670 (50 AID50), and a cloned, neurovirulent virus, SIV/17Efr (10,000 AID50), as described.20 Samples of brain tissue (basal ganglia and adjacent subcortical white matter) from macaques euthanized at 10 (n = 6), 21 (n = 5), 56 (n = 5), and 84 (n = 12) days after inoculation, and from age- and gender-matched uninfected control macaques (n = 3 to 8) were fixed and embedded and 5-μm sections were cut for immunohistochemical analysis of MAPKs as described below. Of the macaques euthanized at 84 days after inoculation, one had no morphological signs of encephalitis, two had mild encephalitis, five had moderate encephalitis, and four had severe encephalitis based on morphological criteria described previously.21 Behavioral tests were performed on six macaques euthanized at each of 56 and 84 days after inoculation (terminal stage of infection). These animals demonstrated motor and cognitive changes as described elsewhere.17
Quantitative Immunohistochemical Analysis
Monoclonal anti-active ERK antiserum (recognizes only dual-phosphorylated ERK-1/2 isoforms) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal anti-active JNK antibody (recognizes only phosphorylated JNK and is reactive against the pTPpY motif present in all activated isoforms of JNK) was obtained from Promega (Madison, WI). Monoclonal anti-active p38 antiserum that recognizes only dual-phosphorylated p38 (all isoforms) was obtained from Sigma (St. Louis, MO). The specificity of each antiserum for activated MAPKs in pigtailed macaques was confirmed by Western blot analysis of primary macaque macrophages that had been treated with lipopolysaccharide for 15 minutes as described previously28 because lipopolysaccharide activates ERK, JNK, and p38 MAPK.29 Brain sections from uninfected macaques and SIV-infected macaques euthanized at 10, 21, 56, and 84 days after inoculation were stained immunohistochemically with each of the above antibodies, and staining was quantitated in a 6-mm2 section of brain from the same location in each macaque by digital image analysis as described.20,30 Briefly, quantitation of immunohistochemical staining on tissues was performed on 40 adjacent fields of tissue examined at ×200 magnification encompassing a 6-mm2 area of subcortical white matter adjacent to the cingulate gyrus. Images were captured using a Sensys 2 digital camera (Photometrics, Tucson, AZ) and analyzed using IP Lab imaging software (Scanalytics, Vienna, VA). Images were binarized (each pixel converted to a value of 1 for positive or 0 for negative) and the total percent area occupied by positive pixels was calculated. This provides a quantitative measure of the total area occupied by positively stained cells or portions of cells in the area evaluated.
To identify the cells that expressed activated ERK, JNK, and p38, immunohistochemically stained tissues were double-labeled with KP-1 (DAKO, Carpinteria, CA), which recognizes CD68 to identify macrophages/microglia, anti-glial fibrillary acidic protein (GFAP) to identify astrocytes, and anti-neuron-specific enolase (UBI, Lake Placid, NY) to identify neurons.30 Activation of MAPK in endothelium was determined morphologically. Viral antigen was identified with an anti-gp41 monoclonal antibody (kk41) obtained from the AIDS Reagent Program (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Statistical comparisons between two groups were made using the Student’s two-sample t-test. This test compares the means from each group while taking into consideration the respective levels of variation (ie, the standard deviations). Small P values (eg, P < 0.05) indicate that the data in the two samples were not likely to have been drawn from the same population; that is, the two groups are not similar. To quantify the changes in marker value, compared to the control group, percent change (%Δ) was calculated, eg, [(day 90 − control)/control]. These changes were designated positive or negative percent change for each marker. The net effect of changes in all markers was then calculated as: net response = [%Δ (ERK)] − {[%Δ (JNK)] + [%Δ (p38)]}. Representing the protective effect of either positive percent change in pERK or negative percent change for pJNK and p-p38. The change in net response was presented as protective being greater than zero and detrimental being less than zero.
Results
Increased Activation of ERK during Acute and Asymptomatic Infection
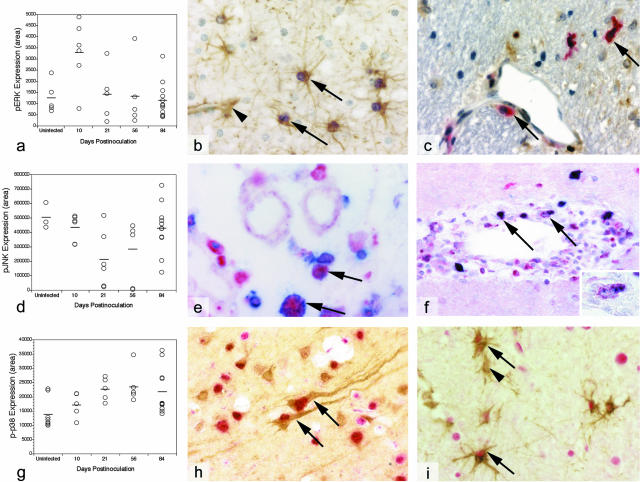
Expression of pERK in the brains of uninfected and SIV-infected macaques euthanized at various stages of infection was quantitated by digital analysis of brain sections stained immunohistochemically with antibodies specific for activated (phosphorylated) ERK (pERK; Figure 1a). pERK expression in the brain increased significantly (P = 0.019) during acute infection, peaking at 10 days after inoculation. Levels of pERK declined quickly from 10 to 21 days (P = 0.05), then maintained a relatively constant level during asymptomatic infection. At terminal infection, expression of activated ERK declined further, reaching levels that were somewhat less than those in control (uninfected) macaques (P = 0.01).
Figure 1.
Immunohistochemical analysis of brain sections derived from control and SIV-infected macaques. a: Quantitative immunohistochemical analysis of activated ERK (pERK); horizontal bars represent the means of each group. pERK expression was statistically different between control and 10-day groups (P = 0.019), 10-day and 21-day groups (P = 0.05), and control and 84-day groups (P = 0.01) b: Co-localization of pERK (purple) and GFAP (brown) in astrocytes (arrows) next to a capillary (arrowhead) in brain from a representative SIV-infected macaque at 10 days after inoculation. c: Co-localization of pERK (brown) and SIV gp41 (red) in macrophages (arrows) in the brain of a representative SIV-infected macaque at 10 days after inoculation. d: Quantitative immunohistochemical analysis of activated JNK (pJNK); horizontal bars represent the means of each group. pJNK expression was statistically different between 10-day and 21-day groups (P = 0.04) and 21-day and 84-day groups (P = 0.048). e: Co-localization of pJNK (blue) and CD68 (red) in macrophages (arrows) in brain from a representative SIV-infected macaque at 84 days after inoculation. f: Co-localization of pJNK (red) and SIV gp41 (blue) in perivascular macrophages (arrows and inset) in brain from a representative SIV-infected macaque at 84 days after inoculation. g: Quantitative immunohistochemical analysis of activated p38 (p-p38); horizontal bars represent the means of each group. p-p38 expression was statistically different between control and 21-day groups (P = 0.008) and control and 84-day groups (P = 0.022) h: Co-localization of p-p38 (red) and neuron-specific enolase (brown) in neurons (arrows) in brain from a representative SIV-infected macaque at 84 days after inoculation. i: Co-localization of p-p38 (red) and GFAP (brown) in astrocytes (arrows) in brain from a representative SIV-infected macaque at 84 days after inoculation. Arrowhead indicates an astrocyte that is not expressing p-p38.
Astrocytes were the predominant cells expressing activated ERK at all time points, and astrocytes near capillaries in white matter (proximal to newly trafficking cells) were most intensely stained for pERK at 10 days after inoculation (Figure 1b). Occasional perivascular macrophages and multinucleated giant cells also stained for pERK. When co-stained with GFAP, which is a cytoskeletal protein in the cytoplasm, pERK appears to be present only in the nucleus. However, higher magnification reveals the presence of both nuclear and cytoplasmic pERK, which is characteristic of activated MAPKs.31 There was no correlation between activation of ERK and virus infection as both infected and uninfected astrocytes and perivascular macrophages were positive for pERK (Figure 1c), and some SIV-infected astrocytes did not stain positive for pERK.
Suppressed JNK Activation during Acute Infection and Rebound during Terminal Infection
Quantitative immunohistochemical analysis of activated (phosphorylated) JNK (pJNK) in brain sections prepared from uninfected and SIV-infected macaques indicated that JNK activation declined significantly between 10 and 21 days after inoculation (P = 0.04), then gradually rebounded between 21 and 84 days after inoculation (P = 0.048; Figure 1d). Infected macaques exhibiting moderate or severe encephalitis had significantly higher levels of JNK activation than infected macaques with no or mild encephalitis (P = 0.03). Lesion severity has been previously correlated with increasing viral load in this model.11,20,21
Activated JNK was expressed in neurons, macrophages, and endothelium at each time point. At 56 and 84 days after inoculation, neuronal expression of pJNK was particularly intense. In addition, at 84 days after inoculation, pJNK was very prominent in cells of macrophage lineage with most perivascular macrophages expressing activated JNK (Figure 1e). Activated pJNK was detectable in the nucleus and cytoplasm. Macrophages that expressed activated JNK were not necessarily infected with SIV (Figure 1f).
Increased Activation of p38 after Acute Infection
Expression of activated (phosphorylated) p38 (p-p38) was significantly elevated at 21 days after inoculation (P = 0.008) and remained significantly elevated throughout the remainder of the infection period (compared to uninfected controls; P < 0.02; Figure 1g). Infected macaques exhibiting moderate or severe encephalitis (and high viral load) had significantly higher levels of p38 activation than infected macaques with no or mild encephalitis at 84 days after inoculation (P = 0.03).
Activated p38 was observed predominantly in neurons and astrocytes, although some staining was observed in endothelium at each time point (Figure 1, h and i). At 84 days after inoculation, the majority of neurons and astrocytes expressed activated p38, and only rare inflammatory macrophages were positive for p-p38. p-p38 was present in both the nucleus and the cytoplasm, although immunohistochemical staining was more prominent in the nucleus because of the co-localization of neuron-specific enolase in the cytoplasm. No correlation was observed between SIV infection of astrocytes and activation of p38.
Comparison between Protective (ERK) and Degenerative (JNK and p38) MAPK Activation in the CNS from Acute through Terminal SIV Infection
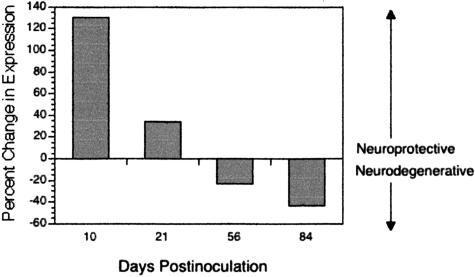
Activation of ERK is typically associated with cell survival and neuroprotective events, whereas activation (greater than basal levels) of JNK and p38 is typically associated with apoptosis and neurodegeneration.25–27,32–34 To determine the net phenotype with regard to neuroprotective or neurodegenerative MAPK potential at each time after inoculation, levels of pERK, pJNK, and p-p38 in SIV-infected macaques during acute (10 days after inoculation), asymptomatic (21 and 56 days after inoculation), and terminal (84 days after inoculation) infection were expressed as percent change from levels in uninfected macaques (n = 3). We compared the change in activation of the survival/neuroprotective MAPK, ERK, with the change in activation of the proapoptotic/neurodegenerative MAPKs JNK and p38 (Figure 2). During acute infection (10 days after inoculation), when there is active virus replication and increased expression of macrophage activation markers in the brain, protective pERK expression predominated. During the asymptomatic stage of infection (21 and 56 days after inoculation), pERK still predominated although less so and the overall change in activation of protective to degenerative MAPKs more closely resembled that observed in uninfected macaques. However, at terminal infection (84 days after inoculation), sustained activation of p38, rebounded activation of JNK and a marked decline in activation of ERK resulted in a net response in which the neurodegenerative MAPKs predominated. Collectively, these data suggest that early in infection the balance of survival and proapoptotic signals in the brain favors activation of neuroprotective ERK pathways. By terminal infection there is dysregulation of these effector kinases with a net increase in activation of the proapoptotic/neurodegenerative MAPKs, JNK, and p38.
Figure 2.
Comparative expression of pERK (neuroprotective) versus pJNK and p-p38 (neurodegenerative) in brains of macaques during acute (10 days after inoculation), asymptomatic (21 and 56 days after inoculation), and terminal (84 days after inoculation) infection. Bars represent the net effect of neuroprotective and neurodegenerative influences based on percent change in expression of these signaling molecules, assuming that pERK, pJNK, and p-p38 have equal and independent influences. During acute infection, there was a net increase in expression of pERK, whereas during terminal infection neurodegenerative pathways, particularly p-p38, predominated.
Discussion
In HIV/SIV infection, the CNS is a complex and dynamic environment in which cells are exposed to inflammatory and neurotoxic viral and cellular proteins. MAPK signal transduction cascades are commonly activated in response to such diverse stimuli and because MAPKs control many important cellular functions, these kinases have been under intense scrutiny to define their roles in human brain physiology and disease.23,26,27,35–37 In this study, we examined cell-specific activation of MAPKs in this complex and dynamic environment. These findings represent the first quantitative comparison of ERK, JNK, and p38 activation in virus-induced neurological disease. Our results revealed that distinct patterns of MAPK activation occur in distinct CNS cell types in response to SIV infection. Astrocytes were the first (as early as 10 days after inoculation) cells to demonstrate a MAPK response to acute SIV infection in the CNS as evidenced by an almost threefold increase in ERK activation. Activation of ERK early in infection likely represents a compensatory, neuroprotective response to SIV infection of the CNS and may in fact initiate cellular mechanisms mediating the down-regulation of virus replication and macrophage activation markers in the CNS observed between 10 and 21 days after inoculation.11 In contrast to the early ERK response, during late infection, activation of JNK and p38 was observed in neurons, macrophages/microglia expressed increased activated JNK and astrocytes expressed increased activated p38.
It is important to define the cell-specific signaling responses in the complex environment of the CNS during SIV infection. Concomitant activation of multiple intracellular signaling pathways can be subject to cross-regulation and/or interference, such that the expected response to a single stimulus may not be observed in the presence of other stimuli.38,39 For example nuclear factor-κB negatively regulates activation of the JNK pathway induced by tumor necrosis factor-α but not interleukin-1.35,40 Further, viral proteins can modulate the expression of specific cell-surface receptors and regulate activation of various intracellular signaling pathways.24,41 As a result, analysis of intracellular signaling pathways, such as MAPKs, will provide important information regarding cell-specific responses in these complex environments and may reveal important pathogenic mechanisms that lead to the development of neurodegeneration.
Acute SIV infection of the CNS is characterized by enhanced expression of chemokines and cytokines, including tumor necrosis factor-α and interleukin-1β,30,42,43 yet there is little evidence of neuropathological damage at this stage. Importantly, although many of these factors stimulate the activation of ERK, JNK, and p38,23,44 we only observed significant activation of ERK during acute infection, and this occurred predominantly in astrocytes. Activation of ERK is characteristic of reactive astrocytes that are beneficial for neuronal survival, re-establishment of the blood-brain barrier, and regulation of leukocyte infiltration.45,46 In our SIV model, ERK activation in astrocytes temporally correlates with the onset of down-regulated virus replication and suppressed macrophage activation in the CNS. It is likely that other cell types also responded to expression of chemokines and cytokines in the CNS during acute infection, but in a typical manner (rapid and transient) such that the increase was not detectable in vivo in which it is unlikely that every cell is exposed to a given stimulus at the same. It is our opinion that activation of ERK in astrocytes at 10 days after inoculation reflects a sustained rather than transient cellular response to the environment of the CNS during acute SIV infection. Sustained activation of ERK has been previously linked to reactive astrocytosis.45 Because up-regulation of GFAP, a classical characteristic of astrogliosis, occurs in HIV infection of the CNS and in our SIV model between 21 and 56 days after inoculation,30,47 it seems likely that ERK activation plays an important role in that phenotypic transition after SIV infection of the CNS. ERK activation and up-regulation of GFAP have been linked in vitro in astrocytes exposed to tumor necrosis factor-α and in vivo in a rat model of traumatic brain injury.48,49
During terminal infection (from 56 to 84 days after inoculation), when there is resurgent virus replication and the development of encephalitic lesions, the balance of MAPKs favors activation of JNK and p38. Essentially every inflammatory macrophage and numerous resident microglia expressed activated JNK, whereas the majority of neurons expressed both activated JNK and p38 and astrocytes expressed mainly activated p38. Increased activation of JNK and p38 during terminal infection in macaques with moderate to severe encephalitis likely reflects the considerable activation of macrophages/microglia and neurodegenerative pathways in neurons and astrocytes during SIV/HIV-associated CNS disease.50,51 Significantly, overactivation of microglia results in apoptosis, which is also characteristic of SIV/HIV-associated CNS disease and has been widely linked to activation of JNK and p38 pathways.25,26,32,52–54 Further, and in keeping with significant experimental evidence in the literature,35 our data suggest that the increased activation of JNK observed in neurons that also exhibit increased activation of p38, may well promote an apoptotic fate for these cells, especially in macaques with severe encephalitis. Further co-localization experiments are necessary to identify direct relationships between activation of JNK and p38 and apoptosis.
In vivo, enhanced activation of JNK and p38 has been observed in a number of animal models of neuronal death and in a variety of neurodegenerative diseases in humans, including Alzheimer’s disease, Pick disease, progressive supranuclear palsy, corticobasal degeneration, Gerstmann-Straussler-Scheinker disease–Indiana kindred, Huntington’s disease, and frontotemporal dementia with parkinsonism linked to chromosome 17.55–58 Thus, it is not surprising that JNK and p38 have been referred to as degenerative effectors of signal transduction cascades in the CNS.26,27 Activated ERK has been observed in some tauopathies, but as an early event preceding neurofibrillary degeneration.58–60 In this study, we clearly demonstrate elevated activation of collective JNK and p38 pathways in macaques with SIV encephalitis. These results indicate that SIV- and HIV-associated CNS disease should be added to the list of neurological conditions exhibiting dysregulated activation of JNK/p38. Therefore, development of pharmacological agents that inhibit pathological activation of JNK/p38 may provide a beneficial supplement to highly active anti-retroviral therapy in the treatment of HIV-associated CNS disease. One initially promising compound, CPI-1189, demonstrated no improvement in cognitive or functional assessments in one short clinical trial.61–64 However, the ability of CPI-1189 to inhibit JNK and p38 and/or activate ERK is inconsistent in the literature.63,65 Several potent inhibitors of JNK and p38 pathways are currently available (and in some cases in clinical trials).26,66,67
Footnotes
Address reprint requests to Sheila A. Barber, Department of Comparative Medicine, The Johns Hopkins University School of Medicine, 600 N. Wolfe St., Jefferson St. Bldg. 3-120, Baltimore, MD 21287. E-mail: sabarber@jhmi.edu.
Supported by the National Institutes of Health (grants MH61189, NS36911, NS44815, and DA12829).
References
- Jellinger KA, Setinek U, Drlicek M, Bohm G, Steurer A, Lintner F. Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol (Berl) 2000;100:213–220. doi: 10.1007/s004010000245. [DOI] [PubMed] [Google Scholar]
- Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 2003;13:195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Bestetti A, Morelli P, Presi S. Molecular analysis of cerebrospinal fluid: potential for the study of HIV-1 infection of the central nervous system. J Neurovirol. 2000;6(Suppl 1):S95–S102. [PubMed] [Google Scholar]
- Cinque P, Presi S, Bestetti A, Pierotti C, Racca S, Boeri E, Morelli P, Carrera P, Ferrari M, Lazzarin A. Effect of genotypic resistance on the virological response to highly active antiretroviral therapy in cerebrospinal fluid. AIDS Res Hum Retroviruses. 2001;17:377–383. doi: 10.1089/088922201750102409. [DOI] [PubMed] [Google Scholar]
- Gisolf EH, Enting RH, Jurriaans S, de Wolf F, van der Ende ME, Hoetelmans RM, Portegies P, Danner SA. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–1589. doi: 10.1097/00002030-200007280-00014. [DOI] [PubMed] [Google Scholar]
- Acosta EP, Page LM, Fletcher CV. Clinical pharmacokinetics of zidovudine. An update. Clin Pharmacokinet. 1996;30:251–262. doi: 10.2165/00003088-199630040-00001. [DOI] [PubMed] [Google Scholar]
- Kravcik S, Gallicano K, Roth V, Cassol S, Hawley-Foss N, Badley A, Cameron DW. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–375. [PubMed] [Google Scholar]
- Aweeka F, Jayewardene A, Staprans S, Bellibas SE, Kearney B, Lizak P, Novakovic-Agopian T, Price RW. Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1-infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:39–43. doi: 10.1097/00042560-199901010-00006. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2003;13:95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Sharer LR, Baskin GB, Cho ES, Murphey-Corb M, Blumberg BM, Epstein LG. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23:S108–S112. doi: 10.1002/ana.410230727. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B, Chakrabarti L, Hurtrel M, Maire MA, Dormont D, Montagnier L. Early SIV encephalopathy. J Med Primatol. 1991;20:159–166. [PubMed] [Google Scholar]
- Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Gold LH, Fox HS, Henriksen SJ, Buchmeier MJ, Weed MR, Taffe MA, Huitron-Resendiz S, Horn TF, Bloom FE. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Weed MR, Hienz RD, Brady JV, Adams RJ, Mankowski JL, Clements JE, Zink MC. Central nervous system correlates of behavioral deficits following simian immunodeficiency virus infection. J Neurovirol. 2002;9:452–464. doi: 10.1080/13550280390218751. [DOI] [PubMed] [Google Scholar]
- Flaherty MT, Hauer DA, Mankowski JL, Zink MC, Clements JE. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Flaherty MT, Spelman JP, Hauer DA, Didier PJ, Amedee AM, Murphey-Corb M, Kirstein LM, Munoz A, Clements JE, Zink MC. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- Bostik P, Wu P, Dodd GL, Villinger F, Mayne AE, Bostik V, Grimm BD, Robinson D, Kung HJ, Ansari AA. Identification of protein kinases dysregulated in CD4(+) T cells in pathogenic versus apathogenic simian immunodeficiency virus infection. J Virol. 2001;75:11298–11306. doi: 10.1128/JVI.75.23.11298-11306.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Popik W, Pitha PM. Exploitation of cellular signaling by HIV-1: unwelcome guests with master keys that signal their entry. Virology. 2000;276:1–6. doi: 10.1006/viro.2000.0581. [DOI] [PubMed] [Google Scholar]
- Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases—degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Barber SA, Perera PY, McNally R, Vogel SN. The serine/threonine phosphatase inhibitor, calyculin A, inhibits and dissociates macrophage responses to lipopolysaccharide. J Immunol. 1995;155:1404–1410. [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Franklin RA, McCubrey JA. Kinases: positive and negative regulators of apoptosis. Leukemia. 2000;14:2019–2034. doi: 10.1038/sj.leu.2401967. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zhang Y, Chen X, Lam PY, Yang H, Xu Q, Yu AC. Activation of Erk1/2 and Akt in astrocytes under ischemia. Biochem Biophys Res Commun. 2002;294:726–733. doi: 10.1016/S0006-291X(02)00540-5. [DOI] [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MJ, Lin YG, Ciallella JR, Flood DG, Scott RW. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci. 2002;22:3376–3385. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, Jones DR, Toran JL, Martinez AC. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deon D, Ahmed S, Tai K, Scaletta N, Herrero C, Lee IH, Krause A, Ivashkiv LB. Cross-talk between IL-1 and IL-6 signaling pathways in rheumatoid arthritis synovial fibroblasts. J Immunol. 2001;167:5395–5403. doi: 10.4049/jimmunol.167.9.5395. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Renkema GH, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–D283. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76:5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Khatissian E, Gray F, Falanga P, Montagnier L, Hurtrel B. Viral load and neuropathology in the SIV model. J Neurovirol. 1999;5:232–240. doi: 10.3109/13550289909015809. [DOI] [PubMed] [Google Scholar]
- Saklatvala J, Davis W, Guesdon F. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond B Biol Sci. 1996;351:151–157. doi: 10.1098/rstb.1996.0011. [DOI] [PubMed] [Google Scholar]
- Mandell JW, VandenBerg SR. ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport. 1999;10:3567–3572. doi: 10.1097/00001756-199911260-00019. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Sopper S, Koutsilieri E, Scheller C, Czub S, Riederer P, ter Meulen V. Macaque animal model for HIV-induced neurological disease. J Neural Transm. 2002;109:747–766. doi: 10.1007/s007020200062. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao W, Li B, Alkon DL, Barker JL, Chang YH, Wu M, Rubinow DR. TNF-alpha induced over-expression of GFAP is associated with MAPKs. Neuroreport. 2000;11:409–412. doi: 10.1097/00001756-200002070-00037. [DOI] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Nomura N, Shima K. Temporal and spatial profile of phosphorylated mitogen-activated protein kinase pathways after lateral fluid percussion injury in the cortex of the rat brain. J Neurotrauma. 2002;19:1587–1596. doi: 10.1089/089771502762300247. [DOI] [PubMed] [Google Scholar]
- Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- Atzori C, Ghetti B, Piva R, Srinivasan AN, Zolo P, Delisle MB, Mirra SS, Migheli A. Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J Neuropathol Exp Neurol. 2001;60:1190–1197. doi: 10.1093/jnen/60.12.1190. [DOI] [PubMed] [Google Scholar]
- Hull M, Lieb K, Fiebich BL. Pathways of inflammatory activation in Alzheimer’s disease: potential targets for disease modifying drugs. Curr Med Chem. 2002;9:83–88. doi: 10.2174/0929867023371292. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Pastor P, Rey MJ, Munoz E, Puig B, Pastor E, Oliva R, Tolosa E. Tau phosphorylation and kinase activation in familial tauopathy linked to deln296 mutation. Neuropathol Appl Neurobiol. 2003;29:23–34. doi: 10.1046/j.1365-2990.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Puig B. Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm. 2001;108:1397–1415. doi: 10.1007/s007020100016. [DOI] [PubMed] [Google Scholar]
- Gartner U, Holzer M, Arendt T. Elevated expression of p21ras is an early event in Alzheimer’s disease and precedes neurofibrillary degeneration. Neuroscience. 1999;91:1–5. doi: 10.1016/s0306-4522(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Vinals F, Ribalta T. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 2001;11:144–158. doi: 10.1111/j.1750-3639.2001.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjugstad KB, Flitter WD, Garland WA, Su GC, Arendash GW. Preventive actions of a synthetic antioxidant in a novel animal model of AIDS dementia. Brain Res. 1998;795:349–357. doi: 10.1016/s0006-8993(98)00351-5. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Flitter WD, Garland WA, Philpot RM, Kirstein CL, Arendash GW. CPI-1189 prevents apoptosis and reduces glial fibrillary acidic protein immunostaining in a TNF-alpha infusion model for AIDS dementia complex. J Neurovirol. 2000;6:478–491. doi: 10.3109/13550280009091948. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Irwin I, Kusdra L, Rempel H, Flitter WD, Garland WA. CPI-1189 attenuates effects of suspected neurotoxins associated with AIDS dementia: a possible role for ERK activation. Brain Res. 2001;893:95–103. doi: 10.1016/s0006-8993(00)03293-5. [DOI] [PubMed] [Google Scholar]
- Clifford DB, McArthur JC, Schifitto G, Kieburtz K, McDermott MP, Letendre S, Cohen BA, Marder K, Ellis RJ, Marra CM. A randomized clinical trial of CPI-1189 for HIV-associated cognitive-motor impairment. Neurology. 2002;59:1568–1573. doi: 10.1212/01.wnl.0000034177.47015.da. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Pye QN, Floyd RA, Cheng I, Garland WA, Irwin I. CPI-1189 inhibits interleukin 1beta-induced p38-mitogen-activated protein kinase phosphorylation: an explanation for its neuroprotective properties? Neurosci Lett. 2000;281:179–182. doi: 10.1016/s0304-3940(00)00861-2. [DOI] [PubMed] [Google Scholar]
- Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61–64. doi: 10.1016/s0304-3940(01)02324-2. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, Saporito MS, Hudkins RL. Targeting the JNK pathway for therapeutic benefit in CNS disease. Curr Drug Target CNS Neurol Disord. 2002;1:31–49. doi: 10.2174/1568007023339472. [DOI] [PubMed] [Google Scholar]