Abstract
Arachidonic acid metabolism through cyclooxygenase, lipoxygenase, or P-450 epoxygenase pathways can generate a variety of eicosanoids. Thromboxane synthase (TxS) metabolizes the cyclooxygenase product, prostanglandin H2, into thromboxane A2 (TXA2), which can cause vessel constriction, platelet activation, and aggregation. Here we demonstrate that human prostate cancer (PCa) cells express enzymatically active TxS and that this enzyme is involved in cell motility. In human PCa cell lines, PC-3, PC-3M, and ML-2 cells expressed higher levels of TxS than normal prostate epithelial cells or other established PCa cell lines such as DU145, LNCaP, or PPC-1. We cloned and sequenced the full-length TxS cDNA from PC-3 cells and found two changes in the amino acid residues. Immunohistochemical analysis of tumor specimens revealed that expression of TxS is weak or absent in normal differentiated luminal, or secretory cells, significantly elevated in less differentiated or advanced prostate tumors, and markedly increased in tumors with perineural invasion. TxS expressed in PC-3 cells was enzymatically active and susceptible to carboxyheptal imidazole, an inhibitor of TxS. The biosynthesis of TXA2 in PC-3 cells was dependent on COX-2, and to a lesser extent, COX-1. Treatment of PC-3 cells with a COX-1 selective inhibitor, piroxicam, reduced TXA2 synthesis by approximately 40%, while the COX-2 specific inhibitor NS398 reduced TXA2 production by ∼80%. Inhibition of TxS activity or blockade of TXA2 function reduced PC-3 cell migration on fibronectin, while having minimal effects on cell cycle progression or survival. Finally, increased expression of TxS in DU145 cells increased cell motility. Our data suggest that human PCa cells express TxS and that this enzyme may contribute to PCa progression through modulating cell motility.
Prostate cancer (PCa) is one of most common cancers of males in the United States. With an aging population, new cases of PCa have risen steadily in the past two decades. High consumption of fat, especially red meat, is a risk factor for prostate cancer.1 Arachidonic acid and its precursor, linoleic acid, are major ingredients in animal fats and many vegetable oils. Arachidonic acid can be converted to various eicosanoids by enzymes such as cyclooxygenase (COX), lipoxygenase (LOX), or P450 epoxygenase. Eicosanoids possess potent and diverse biological activities and have been implicated in a variety of human diseases such as inflammation, fever, arthritis, and recently, cancer.2 For example, COX-2 expression has been found to be up-regulated in a variety of cancers when compared to their normal counterparts.3–7 It has been reported that in prostate cancer, COX-2 expression is increased in tumor tissues and that inhibitors of COX-2 such as celecoxib and NS398 induce prostate cancer cell apoptosis.8,9 Indomethacin, an inhibitor of COX, was shown to reduce pulmonary metastasis in NB rats bearing subcutaneous implants of an androgen-insensitive prostate adenocarcinoma.10 These studies suggest an important role for the COX pathway of arachidonic acid metabolism in the progression of human prostate cancer.
Downstream of the COX pathway, the COX product PGH2 can be converted to thromboxane A2 (TXA2) by TxS.11,12 Known activities of TXA2 include stimulation of platelet activation, aggregation, and thrombosis.13 TXA2 also causes contraction of vascular smooth muscle cells14 or release of prostacyclin from endothelial cells.15 In cancer, there is little study so far regarding TxS or TXA2, although platelet abnormality and thromboembolic disorders affect 15 to 20% of all cancer patients and platelet activation and aggregation have been known to facilitate tumor angiogenesis and metastasis.16,17
In the present study, the expression of TxS is examined in human PCa cells as well as in normal prostate epithelial cells by Western blot as well as reverse transcriptase-polymerase chain reaction. TxS protein was minimally expressed in normal prostate epithelial cells but remarkably increased in some prostate carcinoma cells. The enzyme is active and the activity of TxS is dependent on the activity of COX. Cancer profiling array analysis found an increase in TxS mRNA level in prostate carcinoma tissues when compared to the matched normal tissue samples. Immunohistochemistry revealed that TxS was weakly expressed in basal cells of the normal gland but essentially absent in luminal differentiated cells. TxS expression was increased as prostate carcinoma progresses to advanced stage, especially at regions of perineural invasion. Finally, we demonstrate a potential role for TxS or TXA2 in cell motility. This is the first report on the expression of TxS in human prostate carcinoma and its potential involvement in tumor progression and metastasis.
Materials and Methods
Materials
Arachidonic acid, U46619, SQ29548, TxS polyclonal antibody and its blocking peptide were purchased from Cayman Chemical Co. (Ann Arbor, MI). U46619, carboxyheptal imidazole (CI), and furegrelate sodium were purchased from Biomol (Plymouth Meeting, PA). Monoclonal antibody (JL-8) against green fluorescent protein (GFP) was purchased from Clontech (Palo Alto, CA). Pre-made RPMI 1640 medium, fetal bovine serum, penicillin-streptomycin solution, trypsin-ethylenediaminetetraacetic acid solution, and other regular cell culture reagents were purchased from Gibco BRL. The horseradish peroxidase-conjugated secondary antibody and Luminol reagents were from Amersham. The TOPO PCR cloning vector and DNA ligation kit were obtained from Invitrogen (Carlsbad, CA). The normal goat serum, the streptavidin-peroxidase, the streptavidin-phosphatase, and the diaminobenzidine reagent set were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD).
Cell Lines and Cell Culture
Prostate cancer PC-3, DU145, LnCaP, and T3 cell lines were originally obtained from ATCC and cultured in RPMI 1640 with 10% FBS. PPC-1, ML-2, and PC-3M cells were obtained from Dr. Avraham Raz (Karmanos Cancer Institute, Wayne State University, Detroit, MI). Human normal epithelial cells were purchased from Clonetics (San Diego, CA) and cultured according to the manufacturer’s recommendation.
Immunoblot Analysis of Thromboxane Synthase Expression
Semiconfluent confluent (70 to 80%) cultured cells were rinsed with ice-cold PBS, scraped into lysis buffer containing 20 mmol/L Tris-HCl, pH 7.5, 2 mmol/L EDTA, 0.5 mmol/L EGTA, 0.5 mmol/L PMSF, 0.5 mmol/L leupeptin, 0.15 mmol/L pepstatin A, 1 mmol/L dithiothreitol, and 1% NP-40. Protein concentration was measured using BCA protein assay kit (Pierce, Rockford, IL). Approximately 30 μg of protein from each sample was loaded into a minigel for electrophoresis separation. The proteins in the gel were then transferred onto a PVDF membrane and processed for immunodetection using a TxS polyclonal antibody (Cayman Chemical Co.) using an enhanced chemiluminescent (ECL) method.
RT-PCR Analysis of TxS Expression
Total RNA was isolated from semiconfluent PC-3 and DU145 cells as described previously.18 Total RNA (4 μg) was reverse-transcribed with oligo(dT) using MMLV reverse transcriptase (Life Technologies, Inc.). For PCR, the upper primer is AACCGAGACGAACTGAAT located between bp 913 and 930 of human TxS cDNA.19 The lower primer is TTCTCTTGGCAGTCAGGG complementary to the region between 1254 and 1271 of human TxS cDNA.19 The expected size of PCR product is about 341 bp. For nested PCR, another set of primers, the lower primer being TAGGTGGCAAAAGAAAGT complementary to the region between 1221 and 1204 and the upper primer ACTGTGGATGAGATTGTG located between 1153 and 1170 of human TxS cDNA. The PCR profile is as follows: 94°C for 5 minutes for denaturation, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 45 seconds, and placed under 72°C for 5 minutes for extension. The PCR products were resolved with 2% agarose gel and stained with ethidium bromide.
Cloning and Characterization of TxS in PC-3 Cells
Total RNA was isolated from PC-3 cells using TRI reagent from Molecular Research Center Inc. cDNA was produced using the 3′-RACE system from Gibco BRL according to manufacturer’s instructions. The coding region of TxS was amplified from cDNA using Advantage 2 polymerase with primers TXS59U/TXS1822L, followed by a nested PCR with primers TXS59U/TXS1818L. The sequence of primer TXS59U is TGTTTGCTTGGTTGCCTGTT. The sequence for TXS 1822L is TCCACACTTAGGGTTTT. The sequence for TXS1818L is CCACACTTAGGGTTTTCTTT. The PCR profile for amplifying TxS cDNA is as follows: 94°C for 5 minutes for denaturation, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 2 minutes, and held under 72°C for 5 minutes for extension. The PCR product was run in a 1.2% agarose gel and the DNA fragment was cut out and purified using QIAquick Gel Extraction Kit from Qiagen. The resulting DNA was cloned using the pUni/V5-His-TOPO Echo Cloning system from Invitrogen. Plasmids from the resulting clones were isolated using the Quantum Prep Plasmid Miniprep Kit from Bio-Rad. The sequence and orientation of inserts into plasmid were confirmed by sequencing using the forward and reverse primers provided in pUni/V5-His-TOPO Echo Cloning System kit. The full-length sequence of TxS cDNA cloned was determined by sequencing with various PCR primers described above. Once a plasmid containing the insert in the correct orientation was identified, we fused it with an acceptor vector using pcDNA3.1-E Echo Cloning System from Invitrogen and confirmed by sequencing using the T7 primer. Echo constructs (Echo 6 or Echo 14) with correct TxS cDNA orientation were used to express TxS, while echo construct with TxS in antisense orientation was used as a control.
Northern Blot Analysis of TxS mRNA Expression
Total RNA samples were isolated using Tri-reagent and aliquots of RNA samples (10 μg) were subjected to electrophoresis on a 1% agarose formaldehyde gel and transfer to Duralon-UV membranes (Stratagene, La Jolla, CA). The membranes were subjected to UV cross-linking in a Stratagene Crosslinker (Stratagene). Membranes containing the transferred RNAs were prehybridized with QuikHyb solution (Stratagene) at 65°C for 1 hour and then hybridized with denatured 32Pi-labeled TxS cDNA and salmon sperm DNA at 65°C for 2 hours. Membranes were washed with 2X standard saline citrate (SSC)/0.1% SDS for 15 minutes at room temperature twice and then rinsed with 0.1X SSC/0.1% SDS for 5 minutes at 65°C. The membranes were monitored with a Geiger counter until the background was low, and the wet membranes were wrapped with plastic wrap and exposed to a Kodak XAR-5 film for 24 hours at −80°C.
Dot Blot Analysis of TxS mRNA Expression in Prostatic Tumor Versus Normal Tissues
A cancer profiling membrane arrayed with cDNA derived from prostate tumor tissues and matched normal tissues was purchased from Clontech (Palo Alto, CA). The profiling membrane also contained paired samples from other cancers including renal and breast carcinoma. The level of TxS expression was analyzed by probing the membrane with denatured 32Pi-labeled TxS cDNA, the same probe used in Northern blot, according to the manufacturer’s protocol.
Immunohistochemical Evaluation of TxS Expression in Prostatic Tissues
Forty radical prostatectomy specimens containing prostatic carcinoma were retrieved from the Department of Pathology at Wayne State University. The median age of the patients was 61 years, with a range of 47 to 73 years. Of the 40 patients, 19 had organ-confined and 21 had advanced disease, which included extraprostatic extension and/or seminal vesicle involvement and/or lymph node metastasis. The Gleason score of each cancer foci, which combined the most and second most prominent Gleason patters, and pathological stage of each specimen were reviewed on H&E-stained sections. One representative tissue block was selected from each specimen for immunohistochemistry study.
Immunohistochemistry assays were performed on 5-μm thick, formalin-fixed, paraffin-embedded tissue sections using avidin-biotin complex immunostaining method according to manufacture protocol (Dako Corporation). Briefly, the paraffin sections were dewaxed and hydrated in a graded series of alcohol, followed by blockage in 3% H2O2 for 20 minutes at room temperature. The slides were incubated with TxS polyclonal antibody (Cayman Chemical Co.) overnight at 4°C. Then the slides were rinsed in PBS (pH 7.4) and incubated with a second biotinylated antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 minutes at room temperature. Detection was performed with the avidin-biotin complex (ABC kit; Dako Corporation). The peroxidase-catalyzed product was visualized using AEC substrate. Sections were finally counterstained with Mayer’s hematoxylin blue and mounted in GVA mounting solution (Zymed, South San Francisco, CA). The sections of negative controls were stained following the same procedure except substitution of primary antibody with antibody dilution buffer. Tissue sections were scored in a semiquantitative fashion combining both the intensity and percentage of cells stained according to the method described.20 Intensities were classified into 0 (no staining), 1 (weak), 2 (moderate), and 3 (strong) and 10% groupings were used for the percentage of cells that stained positive. For each slide, a value-designated H score was derived from the following formula:
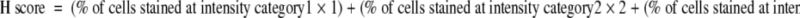 |
This formula produces a H score in the range of 0 to 300, where 0 = none of the tumor cells stained and 300 = 100% of tumor cells stained strongly.
For data analysis, the median values of patients’ ages and H scores were used as cut-off points in grouping patients. Comparisons of patient and tumor characteristics were performed using χ2 statistics. P < 0.05 was considered statistically significant.
Measuement of Thromboxane A2 Biosynthesis
Enzyme immunoassay (EIA) kits for measuring TXB2, the stable product of TXA2 in aqueous solutions, were purchased from Cayman Chemical Co. or Assay Designs Inc. (Ann Arbor, MI) and used according to manufacturer’s instructions. PC-3, DU145, or HEL cells (positive control) were seeded at the same density into culture plates, treated with carboxyheptal imidazole,21 and culture supernatants were harvested after 24 hours for determination of TXB2 levels. The data are presented as the concentration of TXB2 in culture media.
In an alternative approach, we determined the biosynthesis of TXA2 by measuring TXB2 levels in lipid extracts from cell lysates. PC-3 or DU145 cells (1 ∼ 2 × 107) were plated at same density (about 80 to 90% confluence), serum starved overnight, and then treated with 1 μmol/L arachidonic acid, along with various TxS inhibitors, CI and furegelate sodium,22 or COX inhibitors, piroxicam (50 μmol/L) or NS398 (10 μmol/L). Piroxicam is a selective inhibitor for COX-1 with an IC50 of 17.7 μmol/L, while the IC50 of piroxicam to inhibit COX-2 is over 500 μmol/L.23 NS398 is highly selective for COX-2 with IC50 about 1 μmol/L. No inhibition of COX-1 by NS398 is noticed at concentrations up to 100 μmol/L.23–25 After overnight treatment, cells were washed in PBS once and harvested using cell scrapers. After centrifugation, the cell pellets were resuspended in ice-cold 100% ethanol and sonicated. The samples were purified using Bakerbond SPE Octadecyl (C18) columns (J.T. Baker, Phillipsburg, NJ), eluted by ethyl acetate, dried under N2 air flow, and resuspended in 100 μl of assay buffer provided in the EIA kit for determination of the concentration of TXB2.
Flow Cytometric Analysis of Cell Cycle Progression and Apoptosis
1 × 106 PC-3 cells were plated out in 35-mm culture plates in RPMI-10% FBS. After overnight culture, cells were treated with graded levels of CI as indicated or 10 μmol/L SQ29548, a TXA2 receptor antagonist,26 in serum-free medium. Phase contrast micrographs were taken 24 hours after treatment. Cells were then harvested, fixed, and processed for propidium iodide (PI) staining for cell cycle analysis and TUNEL staining for apoptosis using a commercial kit (APOP-Direct, PharMingen, Palo Alto, CA), according to the manufacturer’s instructions.
Cell Migration Assay
Cell migration assay was conducted using 96-well Chemo TX invasion plates (Neuroprobe, Gaithersburg, MD). The plates were coated with 20 μg/ml of fibronectin in PBS on both sides of the membrane. To study the involvement of TxS in cell migration, PC-3 cells were harvested and resuspended in RPMI 1640 with 0.1% BSA at density of 5 × 105 cells per ml. RPMI 1640 (30 μl) medium with different treatments, as described in text, was added in the bottom chamber. Migration was initiated by adding cells (20 μl) in the upper chamber. After 12 to 18 hours, the cells on the upper side of the membrane were removed by Kimwipes and the plate was fixed and stained. Cells migrated were enumerated in a double blind approach. For each treatment, at least six chambers were used unless otherwise indicated.
To study the effect of enhanced TxS expression on DU145 cell migration, DU145 cells were transfected with pEGFP, along with the same amount of a TxS expression construct or its vector control, using GenePorter reagent according to manufacturer’s instruction. Twenty-four hours after transfection, cells were harvested and subjected to FAC sorting to select transfectants (GFP). The transfectants were then cultured in G418 containing media and the expression of TxS confirmed by Western blot. The transfectants were then used in a migration assay essentially as described above, with or without TXA2 functional antagonist, SQ29548 (10 μmol/L).
Results
Expression of TxS in Human Prostate Cancer Cell Lines
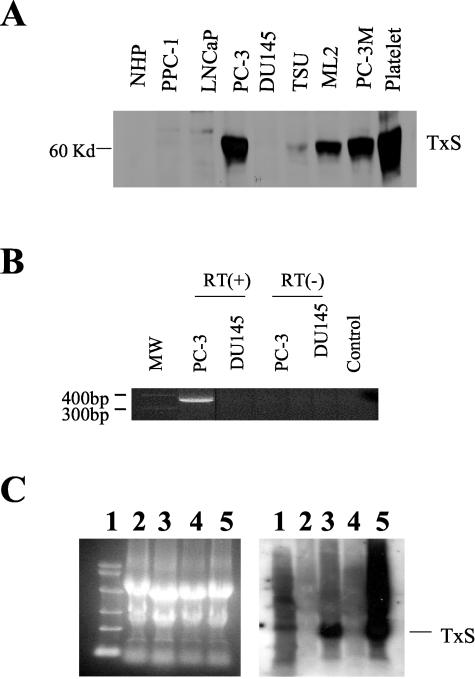
The expression of TxS was examined in various established prostate cancer cell lines as well as in normal prostate epithelial cells using Western blot with a polyclonal antibody raised against the synthetic peptide encompassing amino acid residues between 359 and 377. As shown in Figure 1A, TxS was expressed in various established PCa cell lines such as PC-3, PC-3M, and ML-2. The band, located at 60 kd, could be blocked by the synthetic peptide against which the antibody was developed (data not shown), confirming the authenticity of the band. While PC-3 cells had strong expression of TxS, normal prostate epithelial cells had a minimal level of TxS expression (Figure 1A). Other prostate cancer cell lines such as TSU, DU145, PPC-1, and LNCaP cells had much lower or minimal TxS expression. The data suggest a differential expression of TxS in prostate cancer cells.
Figure 1.
Expression of TxS in established prostate cancer cells. A: Western blot analysis of TxS expression in different PCa cell lines. TS denotes TxS. NHP, normal prostate epithelial cells. Platelet lysates as positive control. B: RT-PCR analysis of TxS mRNA expression. Shown is one round PCR product using first set of TxS specific primers as described in Materials and Methods. RT(-), RNA samples without reverse transcriptase were used as negative control to eliminate possible false positive results from genomic DNA contamination. C: Northern blot analysis of TxS expression. Left panel, ethidium bromide staining of RNA in agarose gels indicating equal loading of RNA samples. Right panel, autoradiograph of membrane probed with TxS cDNA probe. Lane 1, RNA molecular weight markers; lane 2, LNCaP; lane 3, PC-3; lane 4, DU145; lane 5, HEL cells as positive control. The approximate size for TxS mRNA in PC-3 cells is 1.8 ∼1.9 kb.
Next we analyzed TxS expression in mRNA levels by RT-PCR in PC-3 cells, which have a relatively high level of TxS protein expression, and in DU145 cells, which have low TxS expression at the protein level. As shown in Figure 1B, PC-3 cells had higher level of TxS mRNA than did DU145 cells in one round of PCR. However, it should be noted that DU145 cells did express TxS at the mRNA level, albeit at a much lower level that required nested PCR to detect the expression of TxS expression at mRNA level in DU145 cells (data not shown). To characterize TxS expressed in prostate cancer cells, the RT-PCR product was cloned into TOPO PCR vector and sequenced. The sequence was found essentially identical to the published TxS cDNA sequence originally identified in platelets.19,27 Next we used the cloned TxS cDNA as a probe for Northern blot analysis of TxS mRNA expression. As shown in the figure, prostate carcinoma cells PC-3 cells express TxS mRNA at much higher levels of TxS mRNA than DU145 cells, consonant with the results from Western blot and RT-PCR analysis (Figure 1C).
Cloning and Characterization of TxS from PC-3 Cells
To further characterize TxS expressed in prostate cancer cells, we amplified the coding region of TxS cDNA and cloned the cDNA into pUni/V5-His-TOPO vector. The TxS cDNA insert was sequenced and 5 variations were revealed when comparing the PCa TxS cDNA sequence with those published19,27 (Figure 2). As shown in the figure, the G at 382 replaced T in the published sequence, leading to a change of amino acid residue from V90 to G90. The G of 698 was replaced with C, leading to a change of amino acid residue from P195 to A195. Other variations include the change of C with T at 503, the C at 696 with G, and the G at 1652 with A, when compared to the published sequence.19,27
Figure 2.
Sequence of TxS cDNA from PC-3 cells and deduced amino acid sequence. Mutations in nucleotide sequence were shaded and indicated by the nucleotides published. The resulting changes in amino acid residues were indicated by the parentheses.
Increased Expression of TxS at mRNA Levels in Human Tumor Tissues
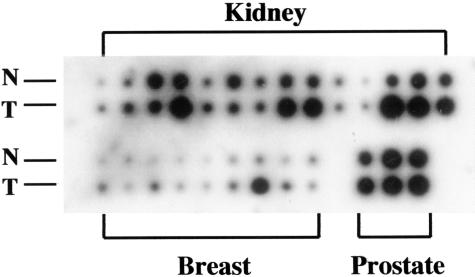
To determine the expression of TxS in prostate tumor tissues, we conducted a dot blot analysis of TxS expression using a commercial cancer profiling membrane arrayed with cDNA derived from tumor tissues and matched normal tissues. As shown in Figure 3, in two of three prostatic carcinoma cases examined, there was an increase in TxS expression at mRNA level in tumor tissues when compared to their normal counterparts. In the third case, there was no change in the level of TxS between normal and tumor tissues. The presence of TxS mRNA expression in normal tissues observed here seems contradictory to our results in western blot (Figure 1A). This may be due to a possible contamination in tissue samples by endothelial cells, smooth muscle cells, or stroma cells which are expressing TxS.28 Another possibility is an up-regulation of TxS expression in normal tissues by its proximity to tumor cells via a paracrine loop. Nevertheless, the results from these three limited cases suggest an increase in TxS mRNA in prostate tumor tissues when compared to their matched normal tissues. Also as shown in Figure 3, increased TxS expression was found in 11 of 14 cases of renal carcinoma and 7 of 9 cases of breast carcinoma.
Figure 3.
Increased level of TxS mRNA levels in tumor tissues in comparison to their matched normal tissue. Commercial cancer profile array membrane (Clontech) was probed with 32P-labeled TxS cDNA. Note the increased expression of TxS in tumor tissues in comparison with their respective matched normal tissues in 2 of 3 cases of prostate carcinoma, 11 of 14 cases of renal carcinoma, and 7 of 9 cases of breast carcinoma examined. N, normal tissue; T, tumor tissue.
Immunohistochemical Analysis of TxS Expression in Human Prostatic Carcinoma Tissues
The expression of TxS in prostate tumor tissues was further evaluated using immunohistochemistry. In 40 human radical prostatectomy specimens evaluated, the staining pattern for TxS was associated with staining intensity. When the cells were weakly or moderately stained, immunoreactivity was mainly confined to the supranuclear region. When the cells were strongly stained, immunoreactivity became diffusely cytoplasmic (Figure 4). In normal prostatic glands, TxS immunoreactivity was either weak or not detected in the secretory cells and was weak to moderate in the basal cells. Increased TxS expression (with intensity 2 or 3) was mainly observed in the areas of inflammation and atrophy in the benign glands. In the glands with high-grade prostatic intraepithelial neoplasia (HGPIN), TxS expression was uniformly increased with intensity ranging between 2 and 3. A total of 38 of 40 prostatic carcinomas (95%) were moderately and strongly stained for TxS focally or diffusely. The H score of TxS expression in prostatic carcinoma ranged from 100 to 280 (median 200). The H score was significantly associated with tumor differentiation and tumor extent (Table 1). High expression (H score ≥200) was observed in 11 of 23 well to moderately differentiated tumors (Gleason score ≤3 + 4) (48%) and 14 of 17 moderately to poorly differentiated tumors (Gleason score ≥4 + 3) (82%) correspondingly (P = 0.026). In the organ-confined or advanced tumors, a high H score (≥ 200) was found in 7 of 19 (37%) or 18 of 21 (86%) cases, respectively (P = 0.001). There was no significant difference in H scores between two age groups. In addition, a few poorly to undifferentiated tumor foci (Gleason pattern 5) showed reduced TxS expression. No immunoreactivity was observed in the sections where the primary antibody was omitted (negative controls).
Figure 4.
Immunohistochemical analysis of TxS protein expression in prostatic carcinoma. The immunoreactivity for TxS is indicated by the brown to red color. A: In the benign prostatic glands, TxS was weakly expressed in basal cells but not in normal luminal or secretory cells. B: TxS was markedly increased in high-grade prostatic intraepithelial neoplasia. A gland showing transition from benign (upper left) to high-grade prostatic intraepithelial neoplasia (mid- and lower right) is shown. C: A moderately well differentiated prostatic carcinoma (Gleason pattern 3) shows mildly to moderately increased TxS expression (mid- and upper left). Benign glands are present in the same micrograph (lower right). D: A poorly differentiated prostatic carcinoma (Gleason pattern 4) with a complex cribriform pattern shows markedly increased TxS expression. E: A poorly to undifferentiated prostatic carcinoma shows intermediate TxS expression in the ill-formed neoplastic glands (Gleason pattern 4) (top) and reduced expression in the infiltrating single cells (Gleason pattern 5) (bottom). F: A focus of perineural invasion within extraprostatic tissue showed high TxS expression in the neoplastic glands surrounding a nerve (arrow).
Table 1.
Distribution of Patient and Tumor Characteristics by Thromboxane Synthase H Score
| Characteristics | N | H score
|
P | |
|---|---|---|---|---|
| <200 | ≥200 | |||
| Total tumors | 40 | 15 | 25 | |
| Age | ||||
| <61 | 18 | 7 | 11 | 0.87 |
| ≥61 | 22 | 8 | 14 | |
| Gleason score | ||||
| ≤3 + 4 | 23 | 12 | 11 | 0.026 |
| ≥4 + 3 | 17 | 3 | 14 | |
| Tumor extent | ||||
| Organ confined | 19 | 12 | 7 | 0.001 |
| Advanced | 21 | 3 | 18 | |
Perineural invasion (or growth of tumor cells along nerve sheaths) is a common finding in prostatic carcinoma. Extensive perineural invasions are often associated with tumor extension into extraprostatic soft tissue.29 In this study, 24 of 40 (60%) cases showed evident perineural invasions. Remarkably, in over 90% of the perineural invasions, neoplastic glands were diffusely and intensely stained for TxS.
Biosynthesis of TXA2 in Prostate Cancer Cells
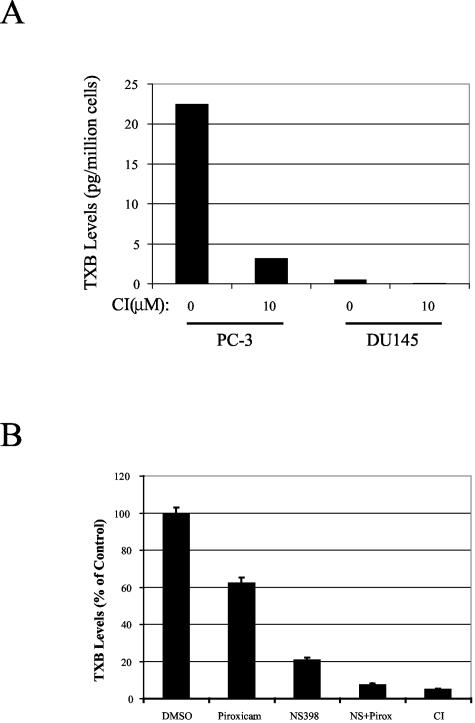
TxS utilizes intermediate prostanoid product of COX to form TXA2, a bioactive eicosanoid involved in platelet activation and aggregation, vessel constriction, and proliferation of smooth muscle cells. We hypothesize that TxS may contribute to PCa progression through the activities of TXA2. We evaluated whether TXA2 is produced in PCa cells, especially when we found that there were two variations in the amino acid sequence of TxS expressed in PC-3 cells. First we compared the biosynthesis of TXA2 in PC-3 cells with that in HEL cells, an erythroleukemia cell line,24 by measuring the accumulation of TXB2, the stable product of TXA2, in culture supernatants. The concentration of TXB2 in culture supernatants from PC-3 cells was 1295 pg/ml, compared to 580 pg/ml in culture supernatants from a same number of HEL cells. Treatment with CI (10 μmol/L), a TxS inhibitor, reduced the production of TXA2 from 1295 pg/ml to 685 pg/ml in PC-3 cells. At the same concentration, CI also reduced the production of TXA2 from 580 pg/ml to 292 pg/ml in HEL cells. The results suggest that TxS expressed in PC-3 cells were active enzymatically, even though it had two variations in amino acid sequence (Figure 2). The results further suggest that PC-3 cells produced more TXB2 than HEL cells. More extensive studies are needed to fully evaluate whether the higher level of TXB2 biosynthesis in PC-3 cells, compared to HEL cells, is due to the two mutations in TxS or a dysregulation of TxS activity in PC-3 cells.
Since TxS expression varies among different prostate cancer cell lines, we measured the concentration of TXB2 in culture supernatants from DU145 cells, which had much lower expression of TxS than PC-3 cells (Figure 1, A and B). The concentration of TXB2 in culture supernatants from DU145 cells was 30 pg/ml. For comparison, the culture supernatants from PC-3 cells contained 1295 pg/ml of TXB2. We also determined the level of TXB2 in PC-3 and DU145 cell lysates. As shown in Figure 5A, PC-3 cells produced 22.5 pg of TxB2 per million cells. Treatment with 10 μmol/L of CI, a TxS inhibitor, reduced TXB2 level or TXA2 production in PC-3 cells by 86%. DU145 cells produced only 0.475 pg of TxB2 per million cells, approximately 50-fold less than PC-3 cells, consistent with the low level of TxS expression in DU145 cells.
Figure 5.
Biosynthesis of TXA2 in human PCa cells. The TxS activity was assessed by measuring the levels of TXB2, a stable inactive metabolite of TXA2, using EIA as described in Materials and Methods. A: TxS activities in PC-3 and DU145 cells and their sensitivity to the TxS inhibitor CI. Shown is a representative result from three independent experiments. B: Inhibition of TXB2 biosynthesis in PC-3 cells by COX inhibitors. Cells were treated with piroxicam (50 μmol/L), or NS398 (20 μmol/L), or both (NS + Pirox), or TxS inhibitor CI (10 μmol/L). Columns, the average amount of TXB2 from duplicate samples expressed as the percentage of control group; bars, standard deviation. Note the reduction of TXB2 levels by COX inhibitors in comparison to the CI-treated group.
As an enzyme downstream of COX, TxS converts PGH2 to TXB2. Expression of COX-2 and COX-1 in prostate cancer has been extensively reported.3–7 Through Western blot, we also detected the expression of both COX-1 and COX-2 in PC-3 cells (data not shown). To study whether TXA2 production is dependent on COX activities, PC-3 cells were treated with either 50 μmol/L piroxicam, a COX-1 selective inhibitor (IC50 for COX-1 is 17.7 μmol/L, for COX-2, over 500 μmol/L),23 or 10 μmol/L NS398, a highly selective inhibitor for COX-2 with IC50 of 1 μmol/L,23–25 or a combination of the two inhibitors. As shown in Figure 5B, piroxicam alone reduced TXA2 synthesis by approximately 40%. Treatment of PC-3 cells with NS398 reduced TXA2 production by ∼80%. Treatment of PC-3 cells with both piroxicam and NS398 reduced TXA2 production by 95%, a level comparable to that achieved by the TxS inhibitor, CI. The data suggest that both COX-1 and COX-2 can provide TxS with the substrate, PGH2, for TXA2 synthesis.
Role of TxS in Cell Proliferation, Survival, and Migration
The pattern of TxS expression in prostate tumor regions, especially in areas of perineural invasion, led us to examine whether TxS plays a role in tumor progression by modulating cell proliferation, survival, or invasion. First we examined the effect of inhibitors of TxS on cell proliferation and apoptosis in PC-3 cells. CI, a TxS inhibitor, did not exert significant cytotoxic effects on PC-3 cells morphologically (Figure 6A). Flow cytometric analysis of cell cycle (PI staining) and TUNEL assay for apoptosis did not reveal notable effect of CI (50 μmol/L) on cell cycle progression or survival in PC-3 cells (Figure 6B). Neither did SQ29548 (10 μmol/L), a TXA2 receptor antagonist, have noticeable effect on cell cycle progression or survival in PC-3 cells (Figure 6B).
Figure 6.
Involvement of TxS in cell migration but not in cell proliferation or survival. A: Lack of noticeable effect of TxS inhibitor CI on PC-3 cell survival. PC-3 cells were treated in graded levels of CI (0, 1, 10, 100 μmol/L) in serum-free RPMI medium. Phase contrast micrograph of cells were taken 24 hours after initiation of treatment. B: Effects of TxS inhibitor CI and TXA2 antagonist, SQ29548 on cell cycle progression and survival in PC-3 cells. PC-3 cells were treated with 50 μmol/L CI or 10 μmol/L SQ29548 for 24 hours in serum-free RPMI medium and then harvested for flow cytometric analysis of cell cycle progression (PI staining) and apoptosis (TUNEL staining). Note the absence of significant changes in cell cycle or apoptosis as result of treatments. C: Inhibition of PC-3 cell migration by TxS inhibitor CI. The cell migration assay is detailed in Materials and Methods. Each data point is the average of six repeats. **, P < 0.01 when compared to the vehicle control. D: Inhibition of PC-3 migration by a TXA2 receptor antagonist, SQ29548. Columns, the average of six repeats; bars, SD **, P < 0.01 when compared to the vehicle control. E: Increased expression of TxS in DU145 cells by transfection with a TxS expression construct, echo14. Control, DU145 cells transfected with Echo construct of TxS in antisense orientation. Echo14, DU145 cells transfected with a TxS expression construct. Upper panel, Western blot with TxS antibody; lower panel, immunoblot for expression of GFP. F: Increased migration for DU145 cells transfected with a TxS expression vector. The cell migration assay for DU145 cells transfected with control construct (Control) or a TxS expression construct (Echo14) was detailed in Materials and Methods. Columns, the average of 12 repeats; bars, standard deviation. a, P < 0.05 when compared to the ethanol treated control cells. b, P < 0.01 when compared to ethanol treated Echo14.
In contrast to its lack of substantial effect on cell progression or cell survival, TxS inhibitor CI reduced PC-3 cell migration on fibronectin (Figure 6C). Another TxS inhibitor, fureglerate sodium, had a similar inhibitory effect on PC-3 cell motility (data not shown). Interestingly, blockade of TXA2 function with antagonist SQ29548 (10 μmol/L) significantly reduced PC-3 migration (Figure 6D). The data suggest an involvement of TxS activity or TXA2 in tumor cell motility.
Compared to PC-3 cells, DU145 cells express TxS and produce TXA2 at much lower levels. U46619 (30 nmol/L), a TXA2 receptor agonist, slightly but consistently stimulated DU145 cells migration (an average of 137 ± 10 cells per field for ethanol control versus 152 ± 11 for 30 nmol/L U46619, P < 0.05). Next we examined whether an increased expression of TxS is consequential in DU145 cell migration. We transiently co-transfected DU145 cells with an expression construct of TxS cDNA in sense direction (Echo14) or its control (TxS cDNA insert in antisense direction), in the presence of a green fluorescent protein construct. The transfectants were selected via FACS for green fluorescence and maintained in G418 containing media. The transfectants with the TxS expression construct had an increased expression of TxS in DU145 cells (Figure 6E) and an increased synthesis of TXB2 (data not shown). As shown in Figure 6F, DU145 cells transfected with the TxS expression construct (Echo 14) had a significantly higher motility than the control cells (Control EtOH versus Echo14 EtOH, P < 0.01), suggesting that an increase in TxS expression enhances PCa cell migration. The increased motility in TxS transfected cells was abolished by SQ29548 (Echo14 EtOH versus Echo14 SQ29548, P < 0.001, Figure 6F), further confirming a role for TXA2 in modulating prostate cancer cell motility.
Discussion
In the present study, we found that the expression of TxS is elevated in human prostatic adenocarcinoma tissues, especially in less differentiated tumors and at the sites of perineural invasion. In established prostate cancer cell lines, PC-3 cells notably express high levels of TxS and despite the fact that there are two variations in the deduced amino acid sequence, the TxS expressed in PC-3 cells is enzymatically active. As an enzyme downstream of cyclooxygenase, the biosynthesis of TXA2 by TxS was found to depend on COX-1 and COX-2 activities. Inhibition of endogenous TXA2 synthesis by CI significantly reduced PC-3 migration on fibronectin. Finally increased expression of TxS in DU145 cells enhanced cell motility. This is the first report detailing the endogenous expression of TxS in human prostate carcinoma and its potential involvement in tumor cell motility and invasion.
One striking finding in the expression profile of TxS in human prostate carcinoma as revealed by immunohistochemistry is its persistent expression in tumors with perineural invasion. Perineural invasion is a known mechanism by which prostate cancer cells penetrate the prostatic capsule and spread.29 The increased expression of TxS in prostate tumor cells with perineural invasion indicates a potential involvement of TxS in tumor cell invasion and metastasis. This notion is further supported by the following in vitro studies. First, inhibitors of TxS such as CI or furegrelate sodium significantly decreased, but did not abolish, PC-3 migration on fibronectin, suggesting a potential modulating role, but not a requisite role, for TxS activity in tumor cell motility. Second, SQ29548, a TXA2 function antagonist, was able to reduce PC-3 migration, suggesting that the product of TxS, TXA2, is involved in PC-3 migration. Finally, when expressed in DU145 cells, it was found that TxS increased DU145 cell migration. These studies further suggest a modulating role for TxS or TXA2 in PCa cell motility and invasiveness.
Interestingly, TxS was also implicated in the migration of human astrocytoma cells. McDonough et al30 selected a population of cells from a long-term human astrocytoma cell line for their ability to migrate on a glioma-derived extracellular matrix. The migration-selected strain showed a genetically stable, enhanced migration rate compared with the parental cells. Using differential display, they found that a 300-bp sequence homologous to TxS was up-regulated in the migration-selected cells relative to the parental cells, which was further confirmed by RNase-protection assay and flow cytometry analysis,30 suggesting that an increase in TxS expression is associated with enhanced tumor cell migration.
TxS uses the product of COX-1 and COX-2, ie, PGH2, as a substrate to synthesize TXA2. Several studies have implicated a facilitative role of COX in the progression of colon cancer by up-regulating tumor angiogenesis and metastatic potential. It has been reported that COX-2 is overexpressed in human prostate adenocarcinoma.3–7 A number of COX-2 inhibitors such as celecoxib and NS398 were found to induce apoptosis in human PCa cells.8,9 In the present study, we found that TXA2 production in PC-3 cells can be inhibited by COX-2 selective inhibitor, NS398, and to lesser extent, COX-1 selective inhibitor, piroxicam. Combination of piroxicam and NS398 further reduced TXA2 production, to a level comparable to TxS inhibitor fureglerate sodium or carboxyheptal imidazole. The results suggest that in PC-3 cells, both COX-1 and COX-2 can provide TxS with PGH2 and in so doing, may contribute to an increased motility or invasiveness of PCa cells.
Sequencing of the full length TxS cDNA cloned from PC-3 cells revealed two alterations in the deduced amino acid sequence (V90 to G90 and P195 to A195). The changes in these two amino acid residues did not disrupt the enzymatic activity of TxS because PC-3 cells produced a large amount of TXA2 or TXB2. In fact, PC-3 cells produced more TXA2 than HEL cells, when compared on the basis of same cell number. This may be due to a dysregulation of TxS activity in PC-3 cells while TxS activity in HEL cells is tightly regulated. Another possibility is a possible stimulation of TxS activity by the variations in TxS in PC-3 cells. Further studies are needed to elucidate how TxS activity is regulated in PCa cells.
A well-established activity of TXA2 is its stimulation of platelet activation and aggregation. Platelets have long been implicated in tumor angiogenesis and metastasis.31 The aggregation of platelets can release VEGF and other angiogenic factors to facilitate tumor angiogenesis.32 Clinically, 15 to 20% of advanced cancer patients possess platelet abnormalities, such as thrombocytosis and many other thromboembolic disorders. Also, activated platelets have been frequently associated with many malignant tumors33 and may facilitate hematogous metastasis of tumor cells. Inhibition of TxS by CI has been demonstrated to reduce experimental metastasis of B16a cells.28 In prostate cancer, PC-3 cells have been shown to induce platelet aggregation.34 It is unknown, however, when and how PCa cells acquire the ability to induce platelet aggregation and whether an increase in TxS expression and activity in PCa cells is part of this acquisition.
In summary, the present study is the first report detailing the endogenous expression of TxS in human prostate cancer cells and its potential involvement in PCa cell motility and perineural invasion. Further elucidation of the role of TxS and its product, TXA2, in tumor cell motility and metastasis will provide insights into how prostate cancer cells progress to a less differentiated and more invasive stage.
Footnotes
Address reprint requests to Dr. Kenneth V. Honn, Department of Radiation Oncology, 431 Chemistry Bldg., Wayne State University, Detroit, MI 48202. E-mail: k.v.honn@wayne.edu.
Supported by National Institutes of Health grant CA-29997 and United States Army Research Program grant DAMD 17–98-1–8502 (to K.V.H.).
References
- Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci. 2002;59:799–807. doi: 10.1007/s00018-002-8468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Lee LM, Pan CC, Cheng CJ, Chi CW, Liu TY. Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer Res. 2001;21:1291–1294. [PubMed] [Google Scholar]
- Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–596. [PubMed] [Google Scholar]
- Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res. 2001;29:23–28. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, Levine AC. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–676. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- Drago JR, Al-Mondhiry HA. The effect of prostaglandin modulators on prostate tumor growth and metastasis. Anticancer Res. 1984;4:391–394. [PubMed] [Google Scholar]
- Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Needleman P, Minkes M, Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976;193:163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Oelz O, Roberts LJ, 2nd, Payne NA, Sweetman BJ, Nies AS, Oates JA. Coronary arterial smooth muscle contraction by a substance released from platelets: evidence that it is thromboxane A2. Science. 1976;193:1135–1137. doi: 10.1126/science.959827. [DOI] [PubMed] [Google Scholar]
- Hunt JA, Merritt JE, MacDermot J, Keen M. Characterization of the thromboxane receptor mediating prostacyclin release from cultured endothelial cells. Biochem Pharmacol. 1992;43:1747–1752. doi: 10.1016/0006-2952(92)90705-n. [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- Honn KV, Sloane BF. Prostacyclin, thromboxanes, and hematogenous metastasis. Adv Prostaglandin Thromboxane Leukotriene Res. 1983;12:313–318. [PubMed] [Google Scholar]
- Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95:2304–2311. [PubMed] [Google Scholar]
- Ohashi K, Ruan KH, Kulmacz RJ, Wu KK, Wang LH. Primary structure of human thromboxane synthase determined from the cDNA sequence. J Biol Chem. 1992;267:789–793. [PubMed] [Google Scholar]
- McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, Nicholson RI. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50:3545–3550. [PubMed] [Google Scholar]
- Grimm LJ, Knapp DR, Senator D, Halushka PV. Inhibition of platelet thromboxane synthesis by 7-(1-imidazolyl) heptanoic acid: dissociation from inhibition of aggregation. Thromb Res. 1981;24:307–317. doi: 10.1016/0049-3848(81)90004-9. [DOI] [PubMed] [Google Scholar]
- Gresele P, Deckmyn H, Nenci GG, Vermylen J. Thromboxane synthase inhibitors, thromboxane receptor antagonists and dual blockers in thrombotic disorders. Trends Pharmacol Sci. 1991;12:158–163. doi: 10.1016/0165-6147(91)90533-x. [DOI] [PubMed] [Google Scholar]
- Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- Martin P, Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982;216:1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Tobias LD, Hamilton JG. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids. 1979;14:181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Miyata A, Ihara H, Ullrich V, Tanabe T. Molecular cloning of human platelet thromboxane A synthase. Biochem Biophys Res Commun. 1991;178:1479–1484. doi: 10.1016/0006-291x(91)91060-p. [DOI] [PubMed] [Google Scholar]
- Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, Chen Y, Honn KV. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun. 2000;267:245–251. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- McDonough W, Tran N, Giese A, Norman SA, Berens ME. Altered gene expression in human astrocytoma cells selected for migration: i. Thromboxane synthase. J Neuropathol Exp Neurol. 1998;57:449–455. doi: 10.1097/00005072-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Honn KV, Steinert BW, Moin K, Onoda JM, Taylor JD, Sloane BF. The role of platelet cyclooxygenase and lipoxygenase pathways in tumor cell induced platelet aggregation. Biochem Biophys Res Commun. 1987;145:384–389. doi: 10.1016/0006-291x(87)91333-7. [DOI] [PubMed] [Google Scholar]
- Maloney JP, Silliman CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol. 1998;275:H1054–1061. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- Swaim MW, Chiang HS, Huang TF. Characterisation of platelet aggregation induced by PC-3 human prostate adenocarcinoma cells and inhibited by venom peptides, trigramin and rhodostomin. Eur J Cancer. 1996;32A:715–721. doi: 10.1016/0959-8049(95)00648-6. [DOI] [PubMed] [Google Scholar]