Abstract
Male gender is associated with a more rapid progression of renal disease independent of blood pressure, dietary protein intake, or serum lipid levels. Recently, we reported a key role for the intrarenal vasculature in progressive renal disease (Kang D-H, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of endothelium in progressive renal disease. J Am Soc Nephrol 2002, 13:806–816). We hypothesized that estrogen-mediated preservation of the renal vasculature could account for the better renal outcome in female rats. We analyzed micro- and macrovascular changes in the 5/6 remnant kidney (RK) models both in male (n = 24) and female (n = 24) Sprague-Dawley rats up to 12 weeks after renal mass reduction. At 12 weeks, male and female RK rats had equivalent blood pressure, glomerular tuft area, and RK/body weight, but male rats showed worse renal function, proteinuria, glomerulosclerosis (%), and tubulointerstitial fibrosis. At 12 weeks peritubular capillary (PTC) EC proliferation and PTC density were higher in female RK rats whereas macrovascular changes in preglomerular vessels (smooth muscle cell proliferation, medial wall thickening, and adventitial fibrosis) were less prominent. The expression of vascular endothelial growth factor (VEGF) and VEGF type 2 receptor (flk-1) in renal cortex assessed by immunostaining were higher in female RK rats. To dissect the mechanism of sex hormone-induced vascular remodeling and VEGF regulation, we investigated the in vitro effect of 17β-estradiol (17βE, 10 nmol/L) on proliferation and VEGF expression of renal tubular cells (rat proximal tubular cells), vascular smooth muscle cells (VSMCs), and human umbilical vein endothelial cells (HUVECs). 17βE directly stimulated the proliferation of HUVECs, whereas it inhibited serum-induced proliferation of VSMCs. 17βE stimulated VEGF mRNA expression both in renal tubular cells and VSMCs. However, when cells were pretreated with a nitric oxide donor to simulate the in vivo condition, 17βE inhibited VEGF mRNA expression and protein release in VSMCs. In conclusion, female RK rats developed less glomerulosclerosis and renal failure compared to male RK rats in association with greater preservation of PTC and less preglomerular arteriopathy. Estrogen stimulated basal VEGF expression in renal tubular cells. We propose that estrogen may protect female rats in progressive renal disease by stimulating VEGF expression and maintaining a healthy intrarenal vasculature.
Male gender is associated with a more rapid progression in most experimental models of renal injury and in human kidney disease.1,2 Although the protective effect of female gender on renal disease has been the subject of investigation, the exact cellular and molecular mechanisms remain unclear. Potential mechanisms for gender-related protection include differences in renal structure including glomerular number and size, renal hemodynamics, and other effects of estrogen or androgen on the synthesis and release of vasoactive substances, growth factors, and cytokines.1,3 Recently, we have found that changes in the intrarenal vasculature are closely related to the progression of renal disease in various animal models.4–6 Vascular remodeling (vessel wall thickening with smooth muscle cell proliferation) in renal arterioles and arteries as well as a progressive loss of renal capillaries [both glomerular and peritubular capillary (PTC)] are characteristic findings in progressive renal disease.6,7
Because estrogen has vasoprotective effects including vasodilatation, inhibition of pathological vascular remodeling, and induction of endothelial cell (EC) survival and growth,3 we hypothesized that the gender difference in progressive renal disease may relate to the effects of estrogen on the vasculature. The present study compared the pattern and the mechanisms of vascular changes in animal models of progressive renal disease in male and female rats. We also performed in vitro studies to examine the potential mechanisms for the differential vascular changes in cultured renal tubular cells and vascular cells. Our data suggest a new mechanism of gender-related renal protection in which estrogen-mediated regulation of vascular endothelial growth factor (VEGF) expression in the kidney may play an important role in the prevention of microvascular endothelial loss and hypoxic damage.
Materials and Methods
Experimental Protocol
To produce a classic animal model of progressive renal disease, age-matched male (n = 24) and female (n = 24) Sprague-Dawley rats (mean body weight, 203.9 ± 6.7 g and 176.9 ± 3.9 g, respectively) underwent the remnant kidney (RK) surgery. After measurement of baseline blood pressure and renal function, a right subcapsular nephrectomy followed by surgical resection of the upper and lower thirds of the left kidney was performed. Right kidney and resected left kidneys were weighed and immediately fixed for baseline histological evaluation. Strict hemostasis and aseptic techniques were used during the surgical procedure. In the control group (n = 18, male and female, respectively), a sham operation was performed, consisting of laparotomy and manipulation of renal pedicles but without the destruction of renal tissue. All animals were fed a standard laboratory diet and water ad libitum. After 1, 2, and 12 weeks of renal mass reduction, animals were anesthetized with xylazine and ketamine, a blood sample was obtained, and the RK was collected for histological evaluation.
Twenty-four-hour urinary protein excretion was measured using the sulfosalicylic acid method, and blood urea nitrogen and creatinine were determined colorimetrically using a commercial kit (Sigma Diagnostics, St. Louis, MO). Systolic arterial blood pressure was monitored by a tail cuff sphygmomanometer using an automated system with a photoelectric sensor (IITC, Life Science, Woodland Hills, CA) that has been shown to closely correlate with intra-arterial measurement of blood pressures.8
Renal Morphology and Immunohistochemistry
Tissue for light microscopy and immunoperoxidase staining was fixed in methyl Carnoy’s solution and embedded in paraffin. Four-μm sections were stained with the periodic acid-Schiff (PAS) reagent and counterstained with hematoxylin. Indirect immunoperoxidase staining of 4-μm sections was performed as previously described9 with specific monoclonal and polyclonal antibodies directed to the following antigens: ECs with the mouse monoclonal antibody JG-12 directed against a 70-kd cell membrane antigen present on rat ECs9 and monoclonal anti-EC antibody, RECA-1 (Serotec, Indianapolis, IN); vascular endothelial growth factor (VEGF) with rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA); VEGF type-2 receptor (VEGFR-2) with rabbit polyclonal antibody (gift of RA Brekken, The Hope Heart Institute, Seattle, WA), and monocytes-macrophages with mouse monoclonal antibody ED-1 (Serotec). Controls included omitting the primary antibody and substitution of the primary antibody with preimmune rabbit or mouse serum.
To examine whether there is any evidence of EC proliferation, double immunostaining was performed with an EC antibody (RECA-1) and an antibody to the proliferating cell nuclear antigen (PCNA) (PC 10; Cappel, Aurora, OH). Tissue sections were first incubated with PCNA antibody overnight at 4°C, followed sequentially by biotinylated horse anti-mouse IgG serum, peroxidase-conjugated avidin D, and color development with diaminobenzidine with nickel chloride. After incubation in 3% H2O2 for 8 minutes to eliminate any remaining peroxidase activity, sections were incubated with primary antibody for α-smooth muscle cell actin for 3 hours at room temperature, followed by biotinylated horse anti-mouse IgG for 30 minutes at room temperature. After incubation to alkaline phosphatase-streptavidin (Vector, Burlingame, CA), color was developed using the AP-RED substrate kit (Zymed, San Francisco, CA). Proliferation of vascular smooth muscle cells (VSMCs) was assessed by double staining of α-smooth muscle cell actin and PCNA.
Quantification of Morphological Data
Changes in endothelial density and morphology were evaluated by immunostaining with JG-12 and RECA-1 as previously reported.5,10 In brief, the number of glomerular capillary loops per glomerular cross-section, was counted in all glomeruli in the tissue section at ×400 magnification. Glomerular capillary density was defined as the number of capillaries per glomerular cross-sectional area (/0.01 mm2), the latter determined by computer image analysis. PTC density was quantified and expressed as the percentage of the area identified by positive staining for JG-12 containing 100 cortical tubules to account for changes in tubular size. A PTC rarefaction index was also measured as described.5 The mean number of proliferating ECs (RECA- and PCNA-positive cells) in each glomerular cross-section and in 0.25-mm2 grids of tubulointerstitial area at ×200 magnification was measured using the entire kidney section.
The percent area of the cortex and outer medulla that was positive for VEGF was measured by computer image analysis (Optimas 6.2; Media Cybernetics; Silver Spring, MD).9 In each biopsy, the background negative staining was calibrated to zero, and the area in each field of positive staining above the background level was measured. The measurement was derived from computer analysis of the integrated logarithm of the inverse gray value, which is proportional to the total amount of absorbing material in the light path. This system enables the percentage area of positive staining in each biopsy to be accurately quantified.
The mean number of macrophages (ED-1-positive cells) in glomeruli and interstitial area of each biopsy was calculated in a blinded manner by averaging the total number of positive cells in each glomeruli or 30 sequentially selected 0.25-mm2 grids at ×200 magnification.
The percentage of glomeruli exhibiting focal or global glomerulosclerosis was determined using all glomeruli present in the biopsy section (range, 108 to 144 glomeruli/each animal). Glomerulosclerosis was defined as segmental increases in glomerular matrix, segmental collapse, and obliteration of capillary lumina and accumulation of hyaline, often with synechial attachment to Bowman’s capsule. Tubulointerstitial injury was defined as inflammatory cell infiltrates, tubular dilatation and/or atrophy, or interstitial fibrosis. Injury was graded semiquantitatively by a blinded observer who examined at least 40 cortical fields (×100 magnification) of PAS-stained biopsies. Only cortical tubules were included in the following scoring systems9: score 0, normal; 1, involvement of less than 10% of the cortex; 2, involvement of 10 to 25% of the cortex; 3, involvement of 26 to 50% of the cortex; 4, involvement of 51 to 75% of the cortex; 5, extensive damage involving more than 75% of the cortex. All measurements were performed blinded.
Western Blot Analysis
Isolation of whole protein from cortex of the RK was performed by homogenization of tissue with Tris-glycine buffer and proteinase inhibitor (Complete; Roche, Indianapolis, IN). After determination of protein concentration (Bio-Rad, Hercules, CA), protein samples (30 μg) were mixed in reducing buffer, boiled, resolved on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and transferred to a nitrocellulose membrane by electroblotting. Membranes were blocked in 5% w/v nonfat milk powder in Tris-buffered saline for 30 minutes in room temperature. An affinity-purified rabbit polyclonal antibody to human VEGF was used (VEGF; Santa Cruz Biotechnology, Santa Cruz, CA) that recognizes all four isoforms of human VEGF and is reported to cross-react with rat VEGF. This was followed by wash and incubation with alkaline phosphatase-conjugated mouse anti-rabbit antibody (Santa Cruz) and enhanced chemiluminescence detection (ECL; Amersham Life Science, Little Chalfont, UK). Positive immunoreactive bands were quantified densitometrically and compared to the controls.
In Vitro Effect of Estradiol on VEGF Expression
Rat proximal tubular cells (NRK-52E cells, CRL-1571; American Type Culture Collection, Manassas, VA) and rat VSMCs (CRL-2018, American Type Culture Collection) were cultured in phenol red-free Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, G418 (50 mg/ml) and l-glutamine. After cells were grown to 70% confluency in multiwell plates (Beckton Dickinson, Franklin Lakes, NJ), the culture medium was changed into serum-free media for 24 hours before each experiment. After synchronization of cell growth, cells were washed three times with Hanks’ balanced salt solution and exposed to 17βE (10 nmol/L; Sigma, St. Louis, MO) for 6 and 24 hours.
To quantify the level of VEGF protein secretion under the different conditions, VEGF protein was measured in cell-culture supernatants using a commercial murine enzyme-linked immunosorbent assay kit for VEGF (R&D Systems, Minneapolis, MN) which is sensitive to 3 pg/ml. Interassay coefficient of variation is <7% and the intra-assay coefficient of variation is <5% among the standards. All data for VEGF production detected by enzyme-linked immunosorbent assay are expressed as pg/μg of protein and percent of control.
Effect of Nitric Oxide (NO) on Estrogen-Induced VEGF Expression
To simulate the in vivo condition in which VEGF expression of SMC is controlled by NO produced by ECs,7,11 NO donors, sodium-nitroprusside (SNP), or 3-morpholinosydnonimine (SIN-1), a molsidomine metabolite, were added to cell-free culture media for 10 minutes to initiate NO generation. NRK-52E cells or VSMCs were then added with estradiol. In a preliminary experiment, after 10 minutes of incubation with cell-free culture media, SNP (100 μmol/L) or SIN-1 (100 μmol/L) led to a significant increase in nitrite that was measured using Griess reagent (data not shown). On the basis of these data, a 10-minute preincubation with SNP or SIN-1 was considered sufficient to initiate NO generation and was used in subsequent experiments.
Generation of Rat VEGF Riboprobes and RNase Protection Assay
The rat VEGF probe (1-327 of an EST clone, AA850734) was generated by PCR and subcloned into pGEM4 Z with 3′end toward T7 promoter. After linearization with an appropriate restriction enzyme, an anti-sense riboprobe was synthesized with the T7 polymerase in the presence of 32P-labeled UTP.7
After incubation with 17βE with or without NO precursor pretreatment, total RNA was prepared from the NRK-52E cell and VSMC monolayers using the RNeasy96 total RNA isolation protocol manufactured by Qiagen (Valencia, CA). After determination of RNA purity and concentration by spectrophotometry, 2 μg of RNA samples were hybridized for 30 minutes at 90°C with a mixture of 32P-UTP labeled riboprobe and the housekeeping gene (L32) (1 × 105 cpm for each probe), and RNase protection assay was performed as described previously using a RNase protection assay kit7 (Torrey Pines Biolabs, Houston, TX) according to the manufacturer’s instruction. The protected hybridized RNA was denatured at 85°C and electrophoresed on 10% polyacrylamide gels. The gels were transferred to 3-mm Whatman filter paper, dried, and exposed to Kodak X-Omat film overnight at −70°C.
In Vitro Effect of Estradiol on the Proliferation of ECs and VSMCs
We also investigated the direct effect of 17βE on proliferation of vascular ECs as well as VSMCs. Human umbilical vein endothelial cells (HUVECs, CRL-1730; American Type Culture Collection) were cultured in phenol red-free Ham’s F12K medium containing 0.1 mg/ml heparin and 0.03 mg/ml EC growth supplement (American Type Culture Collection) and 10% fetal bovine serum.
After HUVECs and VSMCs were grown to 70% confluency in multiwell plates (Beckton Dickinson), the culture medium was changed into serum-free media for 24 hours before each experiment. After synchronization of cell growth, cells were washed three times with Hanks’ balanced salt solution and exposed to 17βE (10 nmol/L) and 5% fetal bovine serum for 24 and 48 hours. At each time point, cell proliferation was assessed by counting cells with a hemocytometer and by measuring 3H-thymidine uptake. For measurement of 3H-thymidine uptake, 3H-thymidine (2 μCi/well; NEN Life Science Products, Boston, MA) was added to each well and incubated for the last 4 hours of each experiment. Cells were washed three times with cold PBS and then incubated in ice-cold 10% trichloroacetic acid for 25 minutes. The cells were solubilized with 0.2 mol/L NaOH, neutralized with 0.4 mol/L glacial acetic acid, and 3H-thymidine was counted on a Beckman β-scintillation counter.
Statistical Analysis
All data are presented as mean ± SD. Differences in the various parameters between groups were evaluated by a two-way analysis of variance to assess whether there was a significant overall effect of gender and time. Where significant effects were found, individual analyses were performed by multiple comparison test. Differences in parameters at each time point after RK surgery and in gender were compared by paired and unpaired t-test. The relation between variables was assessed by Pearson correlation analysis. Significance was defined as a P value <0.05.
Results
Body Weight, Blood Pressure, and Renal Function
Initial body weight (BW) was higher in male rats than in female rats because we used rats of the same age based on the findings that the number of glomeruli per kidney do not differ between male and female rats of the same age.12 Lombet and colleagues13 also reported mean glomerular volume was comparable for age-matched male and female rats despite different kidney weight between the sexes suggesting age-matched rats were suitable controls. BW gain for 12 weeks was significantly lower in female rats. At 12 weeks after renal mass reduction, male RK rats gained significantly lower BW compared to male sham rats (153.6 versus 207.4 g, P < 0.05) whereas comparable BW gain was noted between female RK and sham rats (93.6 versus 99.1 g, P = NS).
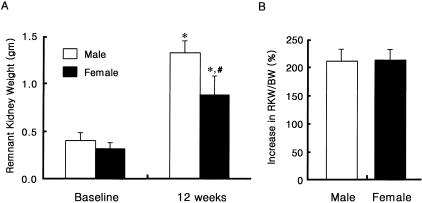
The absolute increase in remnant kidney weight (RKW) was higher in male RK rats, but the percent increase in RKW/BW was comparable in male and female RK rats (211.9 ± 22.5% versus 215.6 ± 21.6%, P = NS) (Figure 1).
Figure 1.
Comparison of RKW and percent increase of RKW/body weight (BW) in male and female RK rats. *, P < 0.05 versus baseline; #, P < 0.05 versus male RK rats. Data are expressed as mean ± SD.
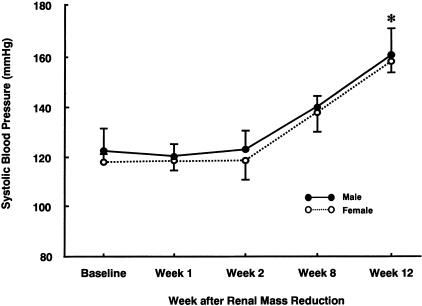
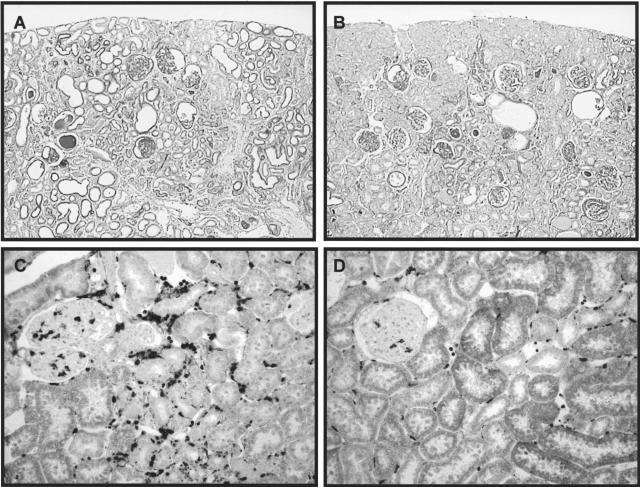
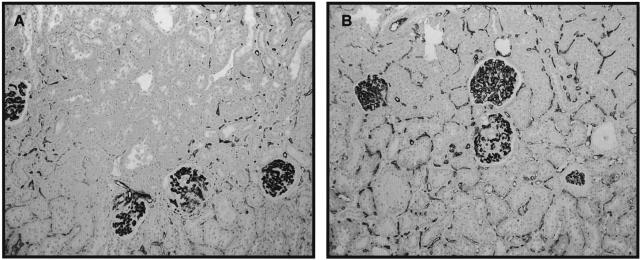
At 1, 2, and 12 weeks after renal mass reduction, there were no significant differences in blood pressure between male and female RK rats (Figure 2). Glomerular hypertrophy assessed by measuring glomerular tuft area was also comparable. However, renal scarring as assessed by percent glomerulosclerosis and tubulointerstitial fibrosis was more severe with higher serum creatinines and more proteinuria in male RK rats (Table 1 and Figure 3, A and B). Macrophage infiltration in glomerular and tubulointerstitial area and vessel wall were also more prominent in male RK rats at 12 weeks. (Figure 3, C and D) (glomerular macrophage, 6.0 ± 2.4 versus 4.1 ± 1.5/glomerulus; tubulointerstitial macrophage, 154.0 ± 33.2 versus 90.2 ± 25.8/mm2, male versus female; P < 0.05).
Figure 2.
Changes in systolic blood pressure in male and female RK model. After renal mass reduction, there was a gradual increase in blood pressure in both male and female RK rats that reached statistical significance at 12 weeks. At each time point, there were no differences in blood pressure between groups. *, P < 0.05 versus baseline and other time points of corresponding gender. Data are expressed as mean ± SD.
Table 1.
Renal Function in Male and Female Remnant Kidney Rats at 12 Weeks
| Male
|
Female
|
|||
|---|---|---|---|---|
| Sham (n = 6) | RK (n = 8) | Sham (n = 6) | RK (n = 8) | |
| BUN (mg/dl) | 14.2 ± 2.5 | 134.5 ± 29.0 | 12.2 ± 3.5 | 101.3 ± 32.1* |
| Cr (mg/dl) | 0.9 ± 0.1 | 2.1 ± 0.8 | 0.8 ± 0.2 | 1.4 ± 0.6* |
| Urine protein (mg/day) | 3.3 ± 2.7 | 159.0 ± 45.4 | 4.4 ± 1.3 | 96.4 ± 20.6* |
| GS (% glomeruli) | 1.8 ± 1.5 | 19.6 ± 10.5 | 0.9 ± 0.6† | 7.8 ± 5.4* |
| TI fibrosis score (0–5) | 0.09 ± 0.04 | 3.06 ± 1.42 | 0.04 ± 0.05 | 1.22 ± 1.03* |
Data is expressed as mean ± SD.
P <0.05 vs. male RK.
P <0.05 vs. male sham.
Abbreviations: GS, glomerulosclerosis; TI, tubulointerstitial.
Figure 3.
Comparison of renal scarring and macrophage infiltration in male and female RK rats. Compared to male RK rats (A, PAS; C, ED-1 immunostaining), there is less renal damage and less macrophage infiltration both in glomerular and tubulointerstitial areas in female RK rats (B and D). Original magnifications: ×100 (A and B); ×200 (C and D).
Microvascular Changes
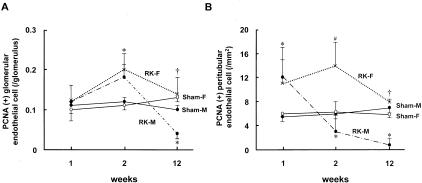
Glomerular and PTC EC proliferation at 1 and 2 weeks of renal mass reduction were comparable between male and female RK rats and both groups were significantly increased compared to sham-operated rats (Figure 4). However, the initial EC proliferation in renal microvasculature was not maintained in male RK rats, and fell to levels below sham rats at 12 weeks of RK. Interestingly, in female RK rats, the number of proliferating endothelial (PCNA- and RECA-positive) cells in glomeruli and PTC were comparable with sham rats and were significantly higher than male RK rats, suggesting a better preserved angiogenic response in female RK rats (Figure 4). At 12 weeks male RK rats had more severe EC loss than that observed in female RK, with a greater loss of glomerular capillary density and more PTC rarefaction (Table 2 and Figure 5).
Figure 4.
Mircovascular EC proliferation in male and female RK rats. The kinetics of proliferating glomerular (A) and PTC (B) ECs (defined as PCNA+, RECA+ cells) throughout the 12-week period after surgery are shown in male (RK-M) and female RK (RK-F) rats and compared to sham rats. An initial early proliferation of glomerular (week 2) and peritubular (week 1) ECs is not maintained in male RK rats whereas it persists in female RK rats. At week 12, both glomerular and PTC proliferation are lower than sham-operated controls in male RK rats, but they are still comparable to sham and female RK rats. *, p < 0.05 vs. corresponding sham; †, p < 0.05 vs. male RK; #, p < 0.05 vs. corresponding sham and male RK. Data are expressed as mean ± SD.
Table 2.
Microvascular Changes in Male and Female Remnant Kidney Rats
| Male
|
Female
|
|||
|---|---|---|---|---|
| Sham (n = 6) | RK (n = 8) | Sham (n = 6) | RK (n = 8) | |
| Glomerular capillary loops (/glomerulus) | 38.1 ± 7.4 | 28.2 ± 18.2 | 35.2 ± 10.2 | 32.0 ± 7.5 |
| Glomerular capillary density (/0.01 mm2) | 27.2 ± 7.9 | 10.6 ± 4.5 | 25.4 ± 10.7 | 18.3 ± 3.6* |
| Proliferating endothelial cell (/glomerulus) | 0.11 ± 0.01 | 0.03 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.05* |
| Positive area of PTC (%/100 tubules) | 10.5 ± 1.4 | 0.57 ± 0.20 | 9.5 ± 2.2 | 4.3 ± 1.5* |
| PTC rarefaction index | 0 | 25.1 ± 7.0 | 0 | 6.9 ± 1.5* |
| Proliferating endothelial cell (/mm2) | 10.2 ± 2.5 | 0.70 ± 1.34 | 6.0 ± 1.5 | 6.4 ± 1.4* |
Data is expressed as mean ± SD.
Abbreviations: PTC, peritubular capillary.
P <0.05 vs. male RK.
Figure 5.
Comparison of renal microvasculature in male and female RK rats. Shown are the changes in glomerular and peritubular capillaries in male (A) and female (B) RK rats (RECA) at 12 weeks of RK surgery. Male RK rats had a greater loss of glomerular capillary density and more PTC rarefaction compared to female RK rats. Original magnifications, ×100.
Changes in Preglomerular (Arterial) Vasculature
Although EC loss characterized the glomerular and postglomerular vasculature, the endothelium in the preglomerular vessels (afferent arteriole and more proximal vessels) in the RK rats was preserved in association with a significant increase in the vascular medial region, similar to previous reports.7 There was no significant difference in EC number in afferent arteriole, interlobular, or arcuate artery between male and female RK rats (Table 3). Male RK rats also showed more prominent vascular injury with greater medial wall thickness, higher numbers of smooth muscle cells, and more SMC proliferation when compared to the female RK group (Table 3).
Table 3.
Changes in Macrovasculature in Male and Female Remnant Kidney Rats at 12 Weeks
| Male
|
Female
|
|||
|---|---|---|---|---|
| Sham (n = 6) | RK (n = 8) | Sham (n = 6) | RK (n = 8) | |
| Interlobular artery | ||||
| Number of SMC (/cross-section) | 6.2 ± 1.0 | 11.2 ± 4.2 | 6.8 ± 1.1 | 8.6 ± 5.1* |
| SMC proliferation (/each vessel) | 0.17 ± 0.03 | 0.38 ± 0.04 | 0.15 ± 0.02 | 0.18 ± 0.02* |
| Number of EC (/cross-section) | 6.1 ± 0.8 | 7.0 ± 0.7 | 6.3 ± 0.4 | 6.4 ± 2.9 |
| EC proliferation (/each vessel) | 0.12 ± 0.04 | 0.20 ± 0.03 | 0.11 ± 0.01 | 0.12 ± 0.02 |
| Wall area (μm2) | 659.3 ± 203.2 | 1190.4 ± 237.2 | 620.4 ± 253.0 | 875.7 ± 334.6* |
| Arcuate artery | ||||
| Number of SMC (/cross-section) | 17.8 ± 1.7 | 29.5 ± 6.7 | 15.2 ± 1.4 | 20.5 ± 6.0* |
| SMC proliferation (/each vessel) | 0.20 ± 0.10 | 0.35 ± 0.06 | 0.13 ± 0.02 | 0.22 ± 0.08* |
| Number of EC (/cross-section) | 9.0 ± 1.0 | 14.5 ± 2.4 | 10.4 ± 1.6 | 15.5 ± 2.6 |
| EC proliferation (/each vessel) | 0.32 ± 0.11 | 0.40 ± 0.12 | 0.11 ± 0.01 | 0.28 ± 0.09 |
| Wall area (μm2) | 2876.2 ± 840.5 | 5737.6 ± 3206.9 | 2180.2 ± 1001.3 | 3690.1 ± 1440.3* |
Data is expressed as mean ± SD.
Abbreviations: SMC, smooth muscle cell, EC, endothelial cell.
P <0.05 vs. male RK.
VEGF and VEGFR-2 Expression in Male and Female RK Rats
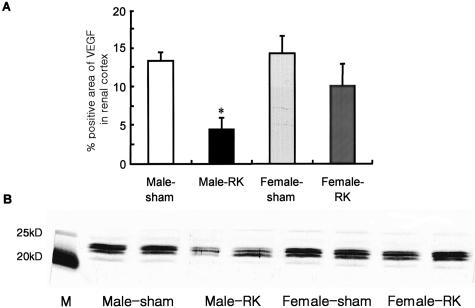
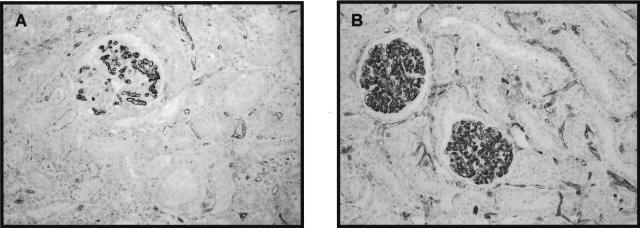
Consistent with our previous study,4–6 we documented a loss in VEGF expression in both podocytes and in tubular epithelial cells in RK rats. In female RK rats, the percent positive area of cortical tubular VEGF expression by immunostaining was increased compared to male RK rats (Figure 6A). VEGF expression by Western blotting (Figure 6B) was also significantly higher in female rats at both 1 and 12 weeks after RK surgery. As shown in other models of progressive renal disease, VEGF expression in tubules of renal cortex was correlated with PTC density (data not shown). Interestingly, in male RK rats, there was some de novo VEGF staining in arteriolar SMC at 12 weeks, which contrasted with the uniformly negative staining for VEGF in smooth muscle cells of sham or female RK rats. The expression of type 2 receptor of VEGF or a kinase insert domain-containing receptor, KDR, was significantly higher in female RK rats (Figure 7).
Figure 6.
Renal cortical VEGF expression in male and female RK rats. A: Quantification of cortical VEGF expression assessed by immunohistochemistry and computer image analysis is shown for RK and sham rats. In male RK rats, there is a marked decrease in VEGF expression in renal cortex. Data are expressed as mean ± SD. B: Western blotting for VEGF also shows similar findings. Shown is a representative blot of two renal cortical tissue animals in each group at 12 weeks. *, P < 0.05 versus other groups.
Figure 7.
VEGFR-2 or KDR expression in male and female RK rats. Immunohistochemistry with VEGFR-2 or KDR (see Material and Methods) shows a better preservation of VEGF receptor staining along the ECs of glomerular and peritubular capillaries in female RK rats (B) compared to male RK rats (A). Original magnification, ×200 (B).
Differential In Vitro Effects of Estrogen on VEGF Expression in Renal Tubules and VSMCs
Because we observed de novo VSMC VEGF staining in male RK rats whereas decreased VEGF expression was observed in renal tubular cell in this group, we hypothesized that VEGF expression regulated by gender-specific factors might be different in renal tubular cells and VSMCs. Estrogen is known to influence cell growth and differentiation and to induce cardiorenal protective effects via genomic and nongenomic mechanisms.3,14,15 We therefore examined the effect of 17βE on the expression of VEGF in cultured renal proximal tubular cells (NRK52E cells) and rat VSMCs.
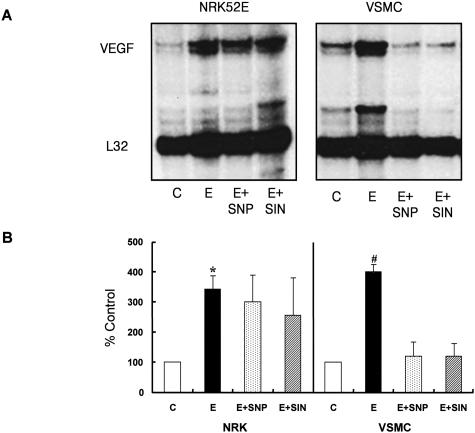
17βE (10 nmol/L) induced VEGF mRNA expression both in NRK52E cells and VSMCs as early as 6 hours after exposure. To simulate the in vivo condition in which VEGF expression of VSMCs is constitutively controlled by NO produced by ECs,11 we pretreated cells with NO donors. Interestingly, in VSMCs pretreated with SNP or SIN-1, 17βE inhibited VEGF expression whereas there was no effect of NO pretreatment on 17βE-induced VEGF expression in NRK52E cells (Figure 8A). This is consistent with the in vivo finding that showed preservation of tubular VEGF expression in female RK rats and de novo expression of VEGF in VSMCs of male RK rats. VEGF protein levels (by enzyme-linked immunosorbent assay) in the cell-culture supernatants were consistent with the changes in VEGF mRNA expression (Figure 8B).
Figure 8.
Differential effect of estrogen on VEGF expression in renal tubular cells and VSMCs. 17βE induced an up-regulation of VEGF mRNA and protein both in NRK52E cells and VSMCs assessed by RNase protection assay (A, n = 6) and enzyme-linked immunosorbent assay (B, n = 4), respectively. However, pretreatment with a NO donor resulted in attenuation of 17βE-induced VEGF expression only in VSMCs. A: Shown is a representative RNase protection assay blot. E, 17βE; SNP, sodium nitroprusside; SIN, 3-morpholinosydnonomine. *, P < 0.05 versus control; #, P < 0.05 versus control, E+SNP, and E+SIN. Data are expressed as mean ± SD.
Differential In Vitro Effect of Estradiol on the Proliferation of ECs and SMCs
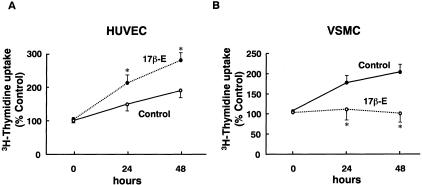
17βE stimulated DNA synthesis of HUVECs time dependently as assessed by 3H-thymidine uptake while 17βE inhibited serum-induced proliferation of VSMCs (Figure 9). This is also consistent with in vivo findings showing more microvascular EC proliferation and less smooth muscle cell proliferation in female RK rats.
Figure 9.
Differential effect of estrogen on the proliferation of HUVECs and VSMCs. 17βE stimulated DNA synthesis of HUVECs time-dependently assessed by 3H-thymidine uptake (n = 6) while 17βE inhibited serum-induced proliferation of VSMCs (n = 6).
Discussion
The rate of progression of chronic renal disease is more rapid in men than women, independent of other risk factors such as systemic blood pressure or serum lipid levels.1,2,14 Experimental data suggest that the impact of gender on renal disease progression may reflect both genetically determined differences between the sexes in renal structure and function as well as receptor-mediated effects of sex hormones.
Lombet and colleagues13 examined sex vulnerability of glomerulosclerosis in the 5/6 RK model at early time points from 1 to 5 weeks after renal ablation. They used a renal infarction model that differs from the polectomy model in resulting in more renin activation and faster rates of progression. Their primary finding was that male RK rats had increased proteinuria and more than 10 times greater segmental glomerulosclerosis despite comparable systemic hypertension and glomerular hypertrophy as female RK rats. Our studies in the polectomy form of the RK model are consistent with their findings. Additionally, in our experiment, tubulointerstitial fibrosis and vascular changes were more prominent in male RK rats. A diminished susceptibility to glomerular injury in females is also supported by the fact that aging female rats are less likely to develop the glomerulosclerosis compared to aging males16,17 although it was not fully confirmed in aging humans.18 Clinical prospective studies in donors for kidney transplantation have also reported that men have a higher rate for developing proteinuria and hematuria as compared to women.19,20
The underlying mechanisms responsible for the relative protection observed in the female remains obscure. Because renal vascular changes are one of the key factors in progressive renal disease,6 we hypothesized that gender-related difference in the progression of kidney diseases may be because of better preservation of the intrarenal vasculature, possibly by effects on the angiogenic factor, VEGF. Vascular remodeling in progressive renal disease is a complex process that involves dysfunction and damage of microvascular ECs and abnormal proliferation of VSMCs. This process is usually accompanied by loss of both glomerular and PTC, vascular and interstitial inflammatory cell infiltration, and lumenal narrowing and obliteration of preglomerular arterial vessels. Consistent with this hypothesis, we observed better preservation of microvasculature with more endothelial proliferation in female RK rats, which suggests there is less impaired angiogenesis in female rats.
The better preservation of angiogenesis in female rats can be explained by several different mechanisms. One of the potential mechanisms for gender-related difference in vascular remodeling is both direct and indirect effects of estrogen. Antus and colleagues21 reported the beneficial effect of exogenous estradiol administration on renal scarring in oophorectomized female RK rats. They found that estradiol replacement reduced proteinuria, which was paralleled by a diminished glomerular and tubulointerstitial injury. We found that estrogen directly stimulated EC proliferation in the in vitro study, which could explain the relatively greater glomerular and PTC EC proliferation observed in the female RK rats. As we have already reported,5 in RK models of male rats, the initial proliferative response by the glomerular and PTC endothelium was not sustained and there was a progressive capillary loss, which was related to progression of renal disease and renal scarring. Failure of maintenance of endothelial proliferation observed in the late stage of renal scarring in male RK rats was prevented or attenuated in female RK rats. In addition, estrogen directly inhibited VSMC proliferation, which is a major finding of pathological vascular remodeling and atherosclerosis. The inhibitory effect of estrogen on VSMC proliferation has also been reported by others.22–24 Estradiol is known to have some beneficial effects on vasculature by inhibiting processes that initiate or mediate vascular remodeling associated with neointima formation and the vaso-occlusive process. In this regard, 17βE stimulates EC-derived NO synthesis and inhibits VSMC proliferation. There is also an in vitro experiment with glomerular endothelial and mesangial cells in which estradiol induced NO synthesis and inhibited glomerular mesangial cell proliferation, which the authors suggested as a mechanism of estrogen-related attenuation of glomerulosclerosis.25
An indirect effect of estrogen that could explain microvascular preservation and the inhibition of pathological macrovascular remodeling in female rats is to regulate VEGF expression differentially in renal tubular cells and VSMCs. There have been several reports that estradiol up- or down-regulates VEGF expression in different cell lines. Because we observed more preserved VEGF in renal tubules of female RK rats and more VEGF expression in vessel wall of male RK rats, we hypothesized that 17βE would regulate VEGF in these cells differently and this might be related to gender-related differences in the progression of renal disease. However, different from our hypothesis, we found that 17βE increased VEGF expression in both renal tubular cells and VSMCs. Given the fact that VEGF expression in VSMCs in vivo is primarily controlled by NO from adjacent ECs,11 we examined whether the presence of NO would affect estradiol-induced VEGF expression in VSMCs. Interestingly, both the NO donors we used (SNP and SIN) inhibited estradiol-induced up-regulation of VEGF of VSMCs, which was consistent with the in vivo finding in female RK rats. Because estradiol is also known to increase NO production from ECs, this increased NO in female rats may further down-regulate VEGF expression in VSMCs. Increased VEGF expression in VSMCs is now considered as an important factor in the development of atherosclerosis.26 This proatherosclerosis effect of VEGF may be related to the chemotactic effect of VEGF to amplify the vascular inflammatory reaction and promote atherosclerosis. Recently, Celletti and colleagues27,28 have published data demonstrating that VEGF promotes atherosclerosis using double-knockout mice (apoE/apoB100) and cholesterol-fed rabbits. The investigators showed that VEGF increased the total number of blood and plaque monocytes/macrophages. We also observed medial wall thickening, cell proliferation, and de novo expression of VEGF in VSMCs of preglomerular vessels, which was associated with more severe renal fibrosis in the RK model with NO inhibition.7
Another indirect mechanism to explain more preservation of renal tubular VEGF and microvasculature in females is related to less macrophage infiltration in these animals. We have reported that the loss of VEGF expression by podocytes and tubular cells in animal models of progressive renal disease strongly correlated both spatially and quantitatively with macrophage infiltration.5 Additionally, in in vitro experiments, macrophage-associated cytokines (interleukin-1β, interleukin-6, and tumor necrosis factor-α) inhibited VEGF mRNA expression and protein secretion by cultured renal tubular cells. Therefore, less macrophage infiltration can explain more preserved tubular VEGF expression in female rats.
An additional important finding was preservation of type 2 VEGF receptor (or KDR) in female RK rats, which is responsible for VEGF-stimulated EC proliferation and migration.29 Although it is not known whether more flk-1 expression is a primary or secondary event of more preserved microvasculature in female rats, this can be beneficial for slowing down the progression of renal disease in association with preservation of renal tubular VEGF expression. There was also a report showing estradiol increased the synthesis and expression of VEGF receptor-2 in microvascular ECs.30
There are several other potential mechanisms for gender-related difference in progressive renal disease such as differences in renal hemodynamics, which was extensively studied by Baylis and co-workers,31–33 and alterations in the renin-angiotensin system,34 lipid and lipoprotein metabolism,35 intravascular coagulation, arachidonate metabolism and oxidative stress. Although our study did not include intraglomerular hemodynamic measurements, neither glomerular area nor percent increase in the RK weight, as markers of glomerular hypertension, were significantly different in male and female rats after renal mass reduction.
We have observed both early- and long-term effects of gender in systemic hypertension, renal function, and pathology in an animal model of progressive renal disease. Although the changes in systemic and renal hypertension were comparable at early (1 and 2 weeks after renal ablation) and late (12 weeks) time points, accelerated endothelial proliferation at capillary levels with an increased tubular VEGF expression at the early phase in female rats was associated with better renal function and pathology at later phase. Taken together, our findings provide the first evidence that estradiol induced VEGF expression in renal tubular cells. Estradiol-induced NO may also play an important role in regulating VEGF expression in VSMCs in addition to other beneficial effects of NO on glomerular hemodynamics, thrombosis, and fibrosis.
In conclusion, our data show that favorable renal vascular remodeling such as microvascular proliferation and neoangiogenesis with increased VEGF expression (which can relieve renal ischemia) is more prominent in female RK rats whereas unfavorable renal vascular remodeling such as afferent arteriopathy and vascular wall thickening (which can decrease renal perfusion, activate RAS, and aggravate renal ischemia) is less evident in female RK rats. To the best of our knowledge, this is the first observation suggesting that intrarenal vascular changes with differential expression of VEGF and its receptor can be one of the mechanisms for better renal outcome in female rats. These effects may be mediated by both direct and indirect effects of estradiol to preserve endothelium and maintain VSMCs in quiescence.
Footnotes
Address reprint requests to Duk-Hee Kang, M.D., Division of Nephrology, Ewha Women’s University Hospital, 70 Chongno 6-ka Chongno-ku Seoul 110-126, Korea. E-mail: dhkang@ewha.ac.kr.
Supported by the Korea Research Foundation (grant KRF-2002-041-E00123) and the United States Public Health Service (grant DK-52121).
References
- Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol. 2001;280:F365–F388. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: potential role of VEGF and TSP-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model (I): potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- Kang D-H, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- Kang DH, Nakagawa T, Feng L, Johnson RJ. Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol. 2002;161:239–248. doi: 10.1016/S0002-9440(10)64175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunag RD, Butterfield J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension. 1982;4:898–903. doi: 10.1161/01.hyp.4.6.898. [DOI] [PubMed] [Google Scholar]
- Kim Y-G, Suga S, Kang D-H, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int. 2000;58:2390–2399. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model (II): VEGF administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol. 1988;254:F223–F231. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- Lombet JR, Adler SG, Anderson PS, Nast CC, Olsen DR, Glassock RJ. Sex vulnerability in the subtotal nephrectomy model of glomerulosclerosis in the rat. J Lab Clin Med. 1989;114:66–74. [PubMed] [Google Scholar]
- Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Renal Replace Ther. 2003;10:3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- Tolbert T, Oparil S. Cardiovascular effects of estrogen. Am J Hypertens. 2001;14:186S–193S. doi: 10.1016/s0895-7061(01)02087-8. [DOI] [PubMed] [Google Scholar]
- Elema JD, Arends A. Focal and segmental glomerular hyalinosis and sclerosis in the rat. Lab Invest. 1975;33:554–561. [PubMed] [Google Scholar]
- Kreisberg JI, Karnovsky MJ. Focal glomerular sclerosis in the fawn-hooded rat. Am J Pathol. 1978;92:637–652. [PMC free article] [PubMed] [Google Scholar]
- Neugarten J, Gallo G, Silbiger S, Kasiske B. Glomerulosclerosis in aging humans is not influenced by gender. Am J Kidney Dis. 1999;34:884–888. doi: 10.1016/S0272-6386(99)70046-6. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Suthanthiran M, Riggio RR, Williams JJ, Riehle RA, Vaughan ED, Stubenbord WT, Mouradian J, Cheigh JS, Stenzel KH. Impact of renal donation. Long-term clinical and biochemical follow-up of living donors in a single center. Am J Med. 1985;79:201–208. doi: 10.1016/0002-9343(85)90010-5. [DOI] [PubMed] [Google Scholar]
- Hakim RM, Goldszer RC, Brenner BM. Hypertension and proteinuria: long-term sequelae of uninephrectomy in humans. Kidney Int. 1984;25:930–936. doi: 10.1038/ki.1984.112. [DOI] [PubMed] [Google Scholar]
- Antus B, Hamar P, Kokeny G, Szollosi Z, Mucsi I, Nemes Z, Rosivall L. Estradiol is nephroprotective in the rat remnant kidney. Nephrol Dial Transplant. 2003;18:54–61. doi: 10.1093/ndt/18.1.54. [DOI] [PubMed] [Google Scholar]
- Krasinski K, Spyridopoulos S, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- Xiao S, Gillespie DG, Baylis C, Jackson EK, Dubey RK. Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension. 2001;37:645–650. doi: 10.1161/01.hyp.37.2.645. [DOI] [PubMed] [Google Scholar]
- Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- Celletti FL, Hilfiker PR, Ghafouri P, Dake MD. Effect of human recombinant vascular endothelial growth factor165 on progression of atherosclerotic plaque. J Am Coll Cardiol. 2001;37:2126–2130. doi: 10.1016/s0735-1097(01)01301-8. [DOI] [PubMed] [Google Scholar]
- Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) receptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–26979. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, Oh H, Kobayashi K, Honda Y. 17 Beta-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol. 1988;254:F223–F231. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- Baylis C. Renal effects of cyclooxygenase inhibition in the pregnant rat. Am J Physiol. 1987;253:F158–F163. doi: 10.1152/ajprenal.1987.253.1.F158. [DOI] [PubMed] [Google Scholar]
- Baylis C, Wilson CB. Sex and the single kidney. Am J Kidney Dis. 1989;13:290–298. doi: 10.1016/s0272-6386(89)80035-6. [DOI] [PubMed] [Google Scholar]
- Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JM, Edwards IJ, Wagner WD, Wagner JD, Adams MR, Parks JS. Effects of contraceptive estrogen and progestin on the atherogenic potential of plasma LDLs in cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17:1216–1223. doi: 10.1161/01.atv.17.7.1216. [DOI] [PubMed] [Google Scholar]