Abstract
Amyloid β protein (Aβ) accumulates in the brains of aging humans, amyloid precursor protein (APP) transgenic mouse lines, and rhesus monkeys. We tested the hypothesis that aging was associated with increased activity of the β-site amyloid precursor protein cleaving enzyme (β-secretase, BACE) in brain. We evaluated BACE activity, BACE protein, and formic acid-extractable Aβ levels in cohorts of young (4 months old) and old (14 to 18 months old) nontransgenic mice (n = 16) and Tg2576 APP transgenic mice (n = 17), young (4.4 to 12.7 years old) and old (20.9 to 30.4 years old) rhesus monkeys (n = 17), and a wide age range (18 to 92 years old) of nondemented human brains (n = 25). Aging was associated with increased brain Aβ levels in each cohort. Furthermore BACE activity increased significantly with age in mouse, monkey, and human brains, independent of brain region. BACE protein levels, however, were unchanged with age. BACE activity correlated with formic acid-extractable Aβ levels in transgenic mouse, nontransgenic mouse, and human cortex, but not in monkey brain. These data suggest that an age-related increase of BACE activity contributes to the increased production and accumulation of brain Αβ, and potentially predisposes to Alzheimer’s disease in humans.
Aging is by far the major risk factor for Alzheimer’s disease (AD),1 but the cause of this association is unknown. A characteristic neuropathological feature of the aging brain is deposition of amyloid β protein (Aβ), initially as diffuse plaques, and more significantly as senile plaques in AD.2 Aβ levels increase in the brains of amyloid precursor protein (APP) transgenic mouse lines, monkeys, and humans with aging.3–8 Again, the reasons for the accumulation of Aβ species in the aging brain are not well characterized. Aβ is derived from processing of APP by two enzymatic activities: β-secretase (β-site APP-cleaving enzyme, BACE) that releases the N-terminus of Aβ from APP, and a presenilin-dependent γ-secretase complex that releases the C-terminus of Aβ from the membrane. The increased levels of Aβ in most autosomal-dominant inherited forms of familial AD are attributed to mutations in presenilin or APP.9,10 In the majority of sporadic AD cases, without these mutations, increased levels may be because of increased production11–13 or reduced catabolism14 of Aβ. However, levels of APP substrate or γ-secretase components do not seem to increase with age: APP expression is uniform with aging, and presenilin mRNA levels are actually highest in the embryonic brain, and then markedly decline to remain stable with increasing age.15,16
Tg2576 transgenic mice, which express mutant human APP (KM670-1NL) under the hamster prion protein promoter, develop cortical and limbic amyloid deposits only with aging;4 cognitive deficits occur coincident with increasing Aβ levels.17 Similar to the case in human aging and sporadic AD, increased levels of mouse Aβ with age are not associated with increased levels of APP or presenilin.15,18–20
Nonhuman primates have also served as models of age-associated neuropathological changes and cognitive impairment. Aβ deposition occurs in the brains of aged nonhuman primates,6,7,21 although Aβ deposition in monkeys does not correlate with cognitive decline.7 Age-related alterations in the subcellular distribution of presenilin have been reported in cynomolgus monkey brain.22
Although the level of BACE mRNA also does not appear to be altered in AD or transgenic mouse models of AD,20,23 BACE enzymatic activity is elevated in AD brain, particularly in cortical regions susceptible to degeneration.11–13 Interestingly, BACE activity seems to increase out of proportion to BACE protein levels in AD temporal cortex.12
We tested the hypothesis that the accumulation of Aβ in the aging brain is associated with an age-related increase of BACE enzymatic activity. We studied BACE protein, BACE activity, and Aβ protein levels in the postmortem brains of a wide age-range of APP transgenic and nontransgenic mice (line Tg2576), rhesus monkeys, and humans.
Materials and Methods
Mouse Brain
In the Tg2576 mouse line, the human APP695 KM670-1NL transgene was expressed under the hamster prion protein promoter in C57B6/SJL F1 mice backcrossed to C57B6/SJL breeders.4 Age-matched nontransgenic littermates served as controls.
To determine the effects of aging and transgene status on BACE protein and activity, male Tg2576 transgenic mice (young at 4 months, n = 8; aged at 14 to 18 months, n = 9) and nontransgenic littermates (young at 4 months, n = 9; aged at 14 to 18 months, n = 7) were studied. The right hemisphere was dissected for BACE assays and the left for Aβ determination.
Monkey Brain
We analyzed archived fresh-frozen frontal and temporal cortex from a cohort of young (4.4 to 12.7 years) and old (20.9 to 30.4 years) rhesus monkeys. All monkeys were carefully screened to exclude any with a history of experimental manipulation or disease that could affect the brain, and they were part of an ongoing study of aging in monkeys.7 The samples were of the frontal cortex of 6 young and 11 old rhesus monkeys and the temporal cortex of 4 young and 7 old rhesus monkeys. All samples were flash-frozen with a postmortem interval of 20 minutes or less and stored at −80°C until processed.
Human Brain
Frozen temporal cortex slices [Brodman Area (BA) 20, 21, 22], frontal association cortex (BA 9, 10), and cerebellum were obtained from control brains in the Massachusetts Alzheimer Disease Research Center brain bank (n = 2), the Harvard Brain Tissue Resource Center (n = 5). and the University of Maryland Developmental Brain and Tissue Bank for Developmental Disorders (n = 18). Control brains had no history of dementia ante-mortem and did not satisfy National Institute of Aging and the Reagan Institute Working Group pathological criteria for AD,24 although three brains had diffuse cortical amyloid deposits without cortical neurofibrillary tangles. The cases ranged in age from 18 to 92 years (average ± SD, 46.5 ± 21.1 years), with postmortem intervals of 4 to 26 hours (13.6 ± 5.8 hours).
Tissue Processing
For the BACE protein enzyme-linked immunosorbent assay (ELISA) and BACE activity assay, frozen tissues were homogenized with a mechanical homogenizer in 10 μl/mg (volume per wet weight) of Tris buffer (Tris 50 mmol/L, pH 7.4, 400 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid) containing 0.1% Triton X-100, protease inhibitors (Complete; Roche, Indianapolis, IN) and 2% protease-free bovine serum albumin. The soluble fraction was extracted after centrifugation at 15,000 rpm for 5 minutes at 4°C. For the formic acid-extractable Aβ ELISA, each tissue was homogenized in Tris buffer with 0.1% Triton X-100 (human, monkey) or without Triton X-100 (mouse), with protease inhibitors and 2% bovine serum albumin. The homogenate was centrifuged at 15,000 rpm for 5 minutes at 4°C. The resulting pellet was homogenized in 70% formic acid then recentrifuged at 22,000 rpm for 5 minutes at 4°C. The supernatant was neutralized with 1 mol/L Tris buffer, pH 11, and used for assay of formic acid-extractable Aβ.
BACE Activity Assay
Black plates (MaxiSorp; Nunc, Rochester, NY) were coated with MAB5308 (monoclonal mouse anti-BACE C-terminus; Chemicon, Temecula, CA) 1:4000 in carbonate buffer, pH 9.6, overnight at 4°C, then washed three times with phosphate-buffered saline (PBS), and blocked with BlockAce (25% in PBS; Dai-Nippon, Osaka, Japan) for more than 6 hours at 4°C. After three washes with PBS, samples (50 μl of 0.01 or 0.005 dilution, wet weight in mg to volume of homogenization buffer in μl) were added to each well containing Superblock (50 μl; Pierce, Rockford, IL). Plates were incubated for 1 hour at 37°C and then washed six times with PBS. The enzymatic reaction was performed by incubation with 10 μmol/L of fluorescent quenching substrate for BACE [H-RE(EDANS)EVNLD-AEFK(DABCYL)R-OH (β-secretase substrate IV; Calbiochem, San Diego, CA)] in acetate buffer/100 mmol/L NaCl (pH 4.1) containing 0.025% bovine serum albumin for 16 to 24 hours at 37°C. The enzymatic product was measured on a Wallac Victor V2 plate reader (Perkin-Elmer, Wellesley, MA) using a 355-nm excitation filter and a 510-nm emission filter. Serial dilutions of the same temporal cortex homogenate were used as the standard for each plate. The operating characteristics of the BACE activity assay have been published.12
ELISAs
The BACE protein ELISA used capture antibody MAB5308 and detector antibody PA1-756 (polyclonal rabbit anti-BACE N-terminus; Affinity Bioreagents, Golden, CO), as detailed in Fukumoto and colleagues.12 We developed a new ELISA specific for pro-BACE. Pro-BACE protein ELISA was performed under the same conditions using capture antibody MAB5308 and a rabbit polyclonal detector antibody directed against amino acid residues 26 to 45, specific for the prodomain of BACE (Calbiochem). Aβ ELISAs were performed with BNT77 (monoclonal anti-Aβ11-28; Takeda, Osaka, Japan) as the capture and horseradish peroxidase-conjugated BA27 (monoclonal anti-Aβ40, Takeda) or BC05 (monoclonal anti-Aβ42, Takeda) as the detector antibodies, using human Aβ40 and Aβ42 as standards (Bachem, King of Prussia, PA).12,25
Western Blot
To evaluate gross changes in migration pattern and processing of BACE in the aging cohort, we performed Western blot of the brain homogenates of human aging brain and monkey brain. Homogenates were performed as above in Triton buffer without bovine serum albumin, and protein measured by Bio-Rad Protein Assay (Hercules, CA). Protein (20 to 40 μg) was run on 10 to 20% and 8% Tris-glycine gels (Invitrogen, Carlsbad, CA) under denaturing and reducing conditions and transferred to Immobilon polyvinylidene difluoride membrane at 22 V overnight at 5°C. The membrane was blocked with 5% nonfat dry milk in TBS-T and sequentially probed with primary antibody (mouse anti-BACE C-terminus MAB5308, 1:1000 or rabbit anti-BACE 26-45, 1:1000) and secondary antibody (horseradish peroxidase anti-mouse or anti-rabbit IgG, 1:2000; Jackson, West Grove, PA). Protein binding was visualized by enhanced chemiluminescence (Perkin-Elmer) on X-ray film (Kodak, Rochester, NY), scanned by densitometer (PowerLook III; UMAX, Dallas, TX), with relative optical density quantitated using Molecular Analyst Software (Bio-Rad).
Statistical Analysis
For the mouse and monkey studies, analysis of variance was performed with BACE or Aβ measures as the dependent variables and age (young versus aged) and brain region as the independent variables. For human studies, with a range of ages, correlation analysis between age, BACE, and Aβ measures were performed for each brain region (StatView; Abacus Concepts, Berkeley, CA).
Results
Formic Acid-Extractable Aβ Increases in the Brain with Aging
Consistent with previous studies of pathological measures in the aging brain, we found that biochemical measures of formic acid-extractable Aβ increased with aging in all cohorts studied (Figure 1). In nontransgenic mice, Aβ increased with age [F(1,26) = 18.2, P = 0.0002], by fourfold in the cerebral cortex [t(13) = 2.9, P = 0.01] and 14-fold in the cerebellum [t(13) = 3.8, P = 0.002] (Figure 1A). Aβ measures were several orders of magnitude greater in the APP transgenic mice, increasing to an even greater extent with age [F(1,30) = 34.8, P < 0.0001], reflecting a 155-fold increase in the cerebral cortex [t(15) = 5.6, P < 0.0001] and a 37-fold increase in the cerebellum [t(15) = 5.3, P < 0.0001] between the ages of 4 months and 14 to 18 months (Figure 1B).
Figure 1.
Formic acid-extractable Aβ measures in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mice (A) and transgenic mice (B), Aβ levels were increased in 14- to 18-month-old mice (old) relative to 4-month-old mice (young) (*, analysis of variance; P < 0.001), reflecting significant increases in cortex (ctx) and cerebellum (cb), (†, posthoc t-test; P < 0.02). C: In rhesus monkey frontal (fr) and temporal (temp) cortices, there was a trend toward increased Aβ in the older monkeys (**, analysis of variance; P = 0.05). Aβ levels increased exponentially with age in human temporal cortex (D) and frontal cortex (E) (‡, P < 0.02) but not cerebellum (F).
In monkeys, there was a trend toward increased Aβ in the older monkeys that approached significance [F(1,24) = 4.1, P = 0.054], with an 11-fold increase in average frontal cortex levels and threefold increase in average temporal cortex levels in the old cohort relative to the young cohort (Figure 1C).
In humans, formic acid-extractable Aβ increased exponentially with age in temporal and frontal cortex but not cerebellum (Figure 1; D to F). There were significant correlations between the log of formic acid Aβ levels with age in the neocortical regions (Figure 1; D to E), reflecting significant age-related increases in temporal Aβ40 [r2(25) = 0.34, P = 0.002] and Aβ42 [r2(25) = 0.48, P = 0.0001] (Figure 1D) as well as frontal Aβ40 [r2(23) = 0.27, P = 0.01] and Aβ42 [r2(23) = 0.47, P = 0.0002; Figure 1E].
BACE Activity Increases in the Brain with Aging
BACE activity is increased in AD neocortex.11–13 Because the primary risk factor for AD is aging,1 we investigated BACE activity in aging human brain, in transgenic mouse models of AD, in which amyloid deposits also only occur with increasing age,18,19 as well as in rhesus monkeys that develop diffuse amyloid deposits with aging.6,26,27 The study of nontransgenic mouse littermates further allowed us to determine whether BACE activity was altered by overexpression of its substrate, APP, in the transgenic mice.
BACE activity increased with age in both nontransgenic [F(1,28) = 21.1, P < 0.0001] and transgenic mice [F(1,30) = 58.8, P < 0.0001; Figure 2, A and B]; however, amyloid plaques only form in the transgenic mice, which express high levels of the mutant human APP substrate susceptible to β-secretase cleavage.19 In transgenic mice, BACE activity increased by 21% in cortex [t(15) = 5.4, P < 0.0001], an area susceptible to Aβ deposition in APP transgenic mice, and by 66% in cerebellum [t(15) = 5.9, P < 0.0001], an area usually spared of amyloid deposits except as amyloid angiopathy (Figure 2B). There were similar changes in the nontransgenic mice, in which BACE activity increased by 16% in cortex [t(14) = 3.5, P = 0.004] and 63% in cerebellum [t(14) = 3.6, P = 0.003; Figure 2A]. BACE activity levels did not differ by genotype (transgenic versus nontransgenic) at either age.
Figure 2.
BACE activity in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mouse (A), transgenic mouse (B), and monkey (C) brains, BACE activity was increased in the old animals relative to the young animals (*, analysis of variance; P < 0.001), reflecting significant increases in each brain region (†, P < 0.05). BACE activity levels also increased with age in human temporal cortex (D), frontal cortex (E), and cerebellum (F) (‡, P < 0.03).
BACE activity levels also increased in the cortex of aged rhesus monkeys [F(1,24) = 21.4, P = 0.0001], with an 18% increase in frontal BACE activity [t(15) = 2.5, P = 0.03] and a 56% increase in temporal cortex [t(9) = 3.6, P = 0.006; Figure 2C].
In human brain samples, age was significantly associated with increasing BACE activity in temporal [r2(25) = 0.29, P = 0.005], frontal [r2(22) = 0.34, P = 0.004], and cerebellar cortex [r2(25) = 0.21, P = 0.02; Figure 2; D to F]. There was no association of BACE activity levels with postmortem interval in the range of 4 to 26 hours in any of the brain regions evaluated (r2 < 0.09, P > 0.28).
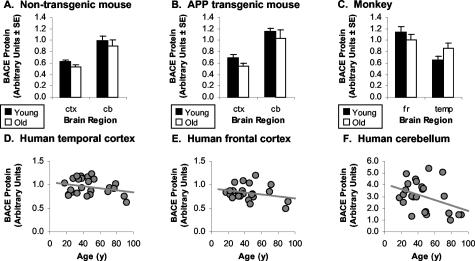
There Are No Significant Changes in Brain BACE Protein Levels with Aging
To determine whether the age-related increase in BACE enzymatic activity was because of elevation in BACE protein levels with age, BACE protein levels were determined in the brain specimens by ELISA. BACE protein levels were not affected by aging in nontransgenic mice [F(1,28) = 1.8, P = 0.19; Figure 3A], Tg2576 APP transgenic mice [F(1,30) = 2.0, P = 0.17; Figure 3B], rhesus monkey [F(1,24) = 0.65, P = 0.43; Figure 3C], or human brain regions (r2 < 0.15, P > 0.05; Figure 3; D to F). There was no association of BACE protein levels with postmortem interval in any of the brain regions studied (r2 < 0.01, P > 0.80). ELISA specific for the proprotein form of BACE did not show any association of pro-BACE levels with aging in humans (r2 < 0.11, P > 0.11). Pro-BACE levels correlated with BACE levels in human temporal cortex [r2(25) = 0.49, P = 0.0001] and human cerebellum [r2(25) = 0.44, P = 0.0003], but not frontal cortex [r2(23) = 0.05, P = 0.29].
Figure 3.
BACE protein levels in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. There was no significant association of BACE protein levels with age in any of the cohorts.
To evaluate posttranslational changes in the BACE protein with aging, Western blot was performed in homogenates from human and monkey brain. Western blot with MAB5308 demonstrated a broad doublet between the 50- and 64-kd molecular weight markers12,28 on 10 to 20% Tris-glycine gels, that could be further resolved in 8% Tris-glycine gels (Figure 4), likely representing posttranslational modifications of BACE. Western blot with the pro-BACE antibody in the human samples demonstrated a doublet at ∼64 kd and a weak broad band at ∼58 kd (Figure 4). There were no consistent changes in the pattern of bands with aging in human or monkey frontal or temporal cortex. Quantitation of the Western blot bands did not reveal any increase in BACE or pro-BACE protein with aging to account for the increased BACE activity in human or monkey frontal and temporal cortex.
Figure 4.
Western blot of BACE (A) and pro-BACE (B) in human frontal cortices. There was no consistent change in the pattern of migration with aging.
BACE Activity Normalized to BACE Protein Increases in the Brain with Aging
Because BACE activity increased out of proportion to changes in BACE protein with aging in each cohort and in AD temporal cortex,12 we evaluated the ratio of BACE activity to BACE protein (BACE act/prt) for each case in the mouse, monkey, and human brain cohorts (Figure 5). The ratio increased in nontransgenic mouse brain [F(1,28) = 24.6, P < 0.0001] with a 41% increase in cortex [t(14) = 3.2, P = 0.006] and a 101% increase in cerebellum [t(14) = 3.8, P = 0.002; Figure 5A]. Comparable increases with aging were seen in the transgenic mouse brains [F(1,30) = 21.6, P < 0.0001] with a 62% increase in cortex [t(15) = 3.1, P = 0.008] and a 116% increase in cerebellum [t(15) = 3.9, P = 0.001; Figure 5B].
Figure 5.
The ratio of BACE activity to BACE protein (BACE act/prt) in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mice (A) and transgenic mice (B), the BACE act/prt ratio was increased in the old mice relative to the young mice (*, analysis of variance; P < 0.001) reflecting significant increases in both cortex and cerebellum (†, posthoc; P < 0.01). In rhesus monkey (C), there was a trend toward increased BACE act/prt in the old monkeys relative to the young monkeys (**, analysis of variance; P = 0.07). In human brain, BACE act/prt increased with age in temporal cortex (D), frontal cortex (E), and cerebellum (F) (‡, P < 0.001).
In the monkey brains, there was a trend toward increased BACE act/prt ratio [F(1,24) = 3.5, P = 0.07] with a 37% increase in frontal cortex and 14% increase in temporal cortex of the old cohort relative to the young cohort (Figure 5C).
In the human cohort, BACE act/prt increased with aging in temporal cortex [r2(25) = 0.48, P = 0.0001], frontal cortex [r2(22) = 0.46, P = 0.0005], and cerebellum [r2(25) = 0.44, P = 0.0003; Figure 5; D to F]. These data and the Western blot data suggest that the changes in BACE activity levels with aging are not simply because of changes in BACE protein levels, and that age-related posttranslational changes or allosteric modulation of BACE activity occur.
BACE Activity Correlates Inconsistently with Formic Acid-Extractable Aβ in the Brain
Because BACE is the initial enzyme in Aβ synthesis, we investigated whether BACE activity correlated with insoluble Aβ measures. In both transgenic and nontransgenic mice, total formic acid-extractable Aβ increased exponentially relative to BACE activity, and correlations were performed relative to the log total formic acid Aβ level. Significant correlations were found in nontransgenic cortex [r2(15) = 0.41, P = 0.01] and cerebellum [r2(15) = 0.59, P = 0.0008] as well as in transgenic cortex [r2(17) = 0.60, P = 0.0002] and cerebellum [r2(17) = 0.78, P < 0.0001; Figure 6, A and B]. In the monkey brains, there were no significant associations between formic acid-extractable Aβ measures and BACE activity (Figure 6C). In human brain, formic acid-extractable Aβ levels increased exponentially relative to BACE activity levels in neocortex, with weak associations between BACE activity and log Aβ measures for temporal cortex Aβ40 [r2(25) = 0.17, P = 0.04], frontal cortex Aβ40 [r2(22) = 0.28, P = 0.01], and frontal cortex Aβ42 [r2(22) = 0.44, P = 0.0008; Figure 6; D to F].
Figure 6.
BACE activity levels and Aβ levels in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains. In nontransgenic mice (A) and transgenic mice (B), Aβ increased exponentially with increasing BACE activity levels in cortex and cerebellum (‡, P < 0.05). In monkey (C), there was no significant association between BACE activity levels and formic acid-extractable Aβ levels. In human brain, there were weak associations of BACE activity levels with Aβ40 levels in temporal cortex (D), and with Aβ40 and Aβ42 levels in frontal cortex (‡, P < 0.05) (E), but no association with Aβ levels in cerebellum (F).
Discussion
Aging humans, APP transgenic mice, and nonhuman primates develop Aβ deposits in cortical and hippocampal regions, and allow the study of Aβ-related pathological changes in the absence of additional features of AD such as neocortical neurofibrillary tangles and prominent cortical neuronal loss. Our data confirm that formic acid-extractable Aβ species accumulate in the brain with aging, not only in humans, rhesus monkeys, and transgenic mice, which develop immunohistochemically detectable Aβ deposits, but also in nontransgenic mice, which do not develop plaques. The reason for this increase in Aβ with aging is puzzling. Nonetheless, it seems likely that similar processes also underlie the more dramatic age-related increased Aβ observed in sporadic AD brain. The idea that age-associated Aβ accumulation represents a presymptomatic stage of AD emphasizes the importance of understanding the biological mechanisms of this phenomenon.
BACE is a pivotal enzyme for the initial cleavage of full-length APP to generate the β-secretase cleavage fragment APPβ, which is subsequently cleaved by γ-secretase to generate Aβ. We find that levels of BACE activity increase with aging in each of the species studied, potentially contributing to increased synthesis of Aβ by BACE. We postulate that the elevation of BACE activity (up to twofold in the human neocortex) may be sufficient throughout time to alter the balance of Aβ generation and clearance, leading to Aβ oligomerization, fibrillization, and ultimately deposition as senile plaques in AD.
There are only weak associations of BACE activity with Aβ levels, which are variable across species. The strongest associations are in the mice, consistent with findings demonstrating increased levels of Aβ with BACE overexpression,29 and elimination of Aβ with BACE knockout in mice.30,31 However, other factors affect Aβ levels in brain. The absolute Aβ levels that we measured reflect the sum of multiple complex processes including Aβ production, oligomerization, fibrillization, deposition, uptake, clearance, and degradation. We anticipate that these processes vary in different brain regions, leading to the observed pattern of Aβ deposition in neocortex with relative sparing of the cerebellum (despite Aβ generation in all brain regions.) For example, chaperone proteins (apolipoprotein E, apolipoprotein J), Aβ receptors (receptor for advanced glycation end-products, RAGE; macrophage scavenger receptor, MSR; low-density lipoprotein receptor related protein, LRP), proteolytic enzymes (neprilysin, endothelin converting enzyme, and insulin degradation enzyme), and extracellular matrix composition, likely contribute to the absolute levels and regional distribution of Aβ,32 modifying quantitative correlations of BACE activity with Aβ across species.
The increased BACE activity associated with aging and AD is distinct from any changes in BACE protein or mRNA levels. BACE activity is optimal at pH 3.5 to 4.5,12,33 therefore sorting of BACE into acidic endosomal/lysosomal compartment may regulate BACE activity.34,35 Other factors, including endoproteolysis,36 oxidative stress,37 membrane cholesterol composition,38,39 interactions with nicastrin,40 phospholipid scramblase 1,39 and chronic inflammation41,42 have been reported to affect BACE expression or activity in neuronal and astrocytic cells. However, the activity assay we report measures BACE isolated from brain homogenates, so the age-related increases in BACE activity cannot be explained by changes in subcellular distribution and interactions alone.
This suggests that posttranslational modification, allosteric modulation, or co-factor association augments the enzymatic activity of BACE during aging. BACE is produced as an active proprotein with different enzyme kinetics relative to BACE.43,44 Splice variants of BACE possess reduced enzymatic activity.45 BACE also undergoes phosphorylation, glycosylation, and palmitoylation with unknown effects on enzymatic activity.34,46–48 The effects of aging on the relative distributions of these differentially active forms of BACE remains to be clarified. Although we could identify no gross changes in the pattern of BACE migration in aging human and monkey brains by Western blot, and no increases in total BACE and pro-BACE protein levels in aging human brain, more detailed proteomic analysis of BACE will prove critical for understanding these pathophysiological events. Because aging is the strongest risk factor for AD, and BACE activity increases with aging and to a greater extent in sporadic AD, AD may reflect an exaggeration of age-related changes in BACE activity. These data support BACE as a pharmacological target to prevent the age-associated increased synthesis of Aβ that may serve as a harbinger of AD.
Footnotes
Address reprint requests to Michael C. Irizarry, M.D., Alzheimer Disease Research Unit, Massachusetts General Hospital–East, 114 16th St., Charlestown, MA 02129. E-mail: mirizarry@partners.org.
Supported by the National Institutes of Health (AG15453 to B.T.H. and AG05134 to Massachusetts Alzheimer Disease Research Center; AG00001 to Boston University, RR00165 to Emory University, PHS MH/NS 31862 to Harvard Brain Tissue Resource Center); the University of Maryland Developmental Brain and Tissue Bank for Developmental Disorders; the American Federation for Aging Research Beeson Award (to M.C.I.); the Harvard University W.F. Milton Fund (to M.C.I.); the J.D. French Foundation, Los Angeles, CA (to M.C.I.); and Takeda Chemical Industries, Osaka, Japan (to H.F.).
References
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Price DL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagoplan S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973;32:566–584. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol (Berl) 1997;94:471–478. doi: 10.1007/s004010050735. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol. 1996;148:259–265. [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Imagawa M, Seki K, Arai H, Sasaki H, Tsuji S, Asami-Odaka A, Fukushima T, Imai K, Iwatsubo T. The beta APP717 Alzheimer mutation increases the percentage of plasma amyloid-beta protein ending at A beta42(43). Neurology. 1997;48:741–745. doi: 10.1212/wnl.48.3.741. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Holsinger D, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of amyloid precursor β-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- Li-Bang Y, Lindholm K, Yan R, Citron M, Xia W, Xiao-Li Y, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Iwata N, Takaki Y, Fukami S, Tsubuki S, Saido TC. Region-specific reduction of A beta-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. J Neurosci Res. 2002;70:493–500. doi: 10.1002/jnr.10390. [DOI] [PubMed] [Google Scholar]
- Berezovska O, Xia MQ, Page K, Wasco W, Tanzi RE, Hyman BT. Developmental regulation of presenilin mRNA expression parallels notch expression. J Neuropathol Exp Neurol. 1997;56:40–44. doi: 10.1097/00005072-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Flood FM, Cowburn RF, Johnston JA. Presenilin-1, amyloid precursor protein and amyloid precursor-like protein 2 mRNA levels in human superior frontal cortex during aging. Neurosci Lett. 1997;235:17–20. doi: 10.1016/s0304-3940(97)00697-6. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the PDAPP transgenic mice. J Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APP-Sw transgenic mice develop age related amyloid deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Locascio JJ, Hyman BT. β-Site APP cleaving enzyme mRNA expression in APP transgenic mice: anatomic overlap with transgene expression and static levels with aging. Am J Pathol. 2001;158:173–177. doi: 10.1016/s0002-9440(10)63955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Struble RG, Wagster MV, Price DL, Cork LC. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988;9:567–666. doi: 10.1016/s0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- Kimura N, Nakamura SI, Honda T, Takashima A, Nakayama H, Ono F, Sakakibara I, Doi K, Kawamura S, Yoshikawa Y. Age-related changes in the localization of presenilin-1 in cynomolgus monkey brain. Brain Res. 2001;922:30–41. doi: 10.1016/s0006-8993(01)03146-8. [DOI] [PubMed] [Google Scholar]
- Yasojima K, McGeer EG, McGeer PL. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta precursor (APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Struble RG, Price DL, Jr, Cork LC, Price DL. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985;361:267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science. 1987;235:873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Sun A, Koelsch G, Tang J, Bing G. Localization of β-secretase memapsin 2 in the brain of Alzheimer’s patients and normal aged controls. Exp Neurol. 2002;175:10–22. doi: 10.1006/exnr.2002.7875. [DOI] [PubMed] [Google Scholar]
- Bodendorf U, Simone D, Fischer F, Stefani M, Sturchler-Pierrat C, Wiederhold K-H, Staufenbiel M, Paganetti P. Expression of human β-secretase in mouse brain increases the steady-state level of β-amyloid. J Neurochem. 2002;80:799–806. doi: 10.1046/j.0022-3042.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. J Biol Chem. 2000;275:33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 2003;116:3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- Huse JT, Bryant D, Yang Y, Pijak DS, D’Souza I, Lah JJ, Lee VM, Doms RW, Cook DG. Endoproteolysis of beta-secretase (BACE) within its catalytic domain: a potential mechanism for regulation. J Biol Chem. 2003;278:17141–17149. doi: 10.1074/jbc.M213303200. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of β-secretase (Asp2) into low-buoyant density, non-caveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Kametaka S, Shibata M, Moroe K, Kanamori S, Ohsawa Y, Waguri S, Sims PJ, Emoto K, Umeda M, Uchiyama Y. Identification of phospholipid scramblase 1 as a novel interacting molecule with beta-secretase (BACE). J Biol Chem. 2003;278:15239–15245. doi: 10.1074/jbc.M208611200. [DOI] [PubMed] [Google Scholar]
- Hattori C, Asai M, Oma Y, Kino Y, Sasagawa N, Saido TC, Maruyama K, Ishiura S. BACE1 interacts with nicastrin. Biochem Biophys Res Commun. 2002;293:1228–1232. doi: 10.1016/S0006-291X(02)00351-0. [DOI] [PubMed] [Google Scholar]
- Hartlage-Rubsamen M, Zeitschel U, Apelt J, Gartner U, Franke H, Stahl T, Gunther A, Schliebs R, Penkowa M, Bigl V, Rossner S. Astrocytic expression of the Alzheimer’s disease beta-secretase (BACE1) is stimulus-dependent. Glia. 2003;41:169–179. doi: 10.1002/glia.10178. [DOI] [PubMed] [Google Scholar]
- Rossner S, Apelt J, Schliebs R, Perez-Polo JR, Bigl V. Neuronal and glial beta-secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology. J Neurosci Res. 2001;64:437–446. doi: 10.1002/jnr.1095. [DOI] [PubMed] [Google Scholar]
- Ermolieff J, Loy JA, Koelsch G, Tang J. Proteolytic activation of recombinant pro-memapsin 2 (pro-beta-secretase) studied with new fluorogenic substrates. Biochemistry. 2000;39:12450–12456. doi: 10.1021/bi001494f. [DOI] [PubMed] [Google Scholar]
- Shi XP, Chen E, Yin KC, Na S, Garsky VM, Lai MT, Li YM, Platchek M, Register RB, Sardana MK, Tang MJ, Thiebeau J, Wood T, Shafer JA, Gardell SJ. The pro domain of beta-secretase does not confer strict zymogen-like properties but does assist proper folding of the protease domain. J Biol Chem. 2001;276:10366–10373. doi: 10.1074/jbc.m009200200. [DOI] [PubMed] [Google Scholar]
- Tanahashi H, Tabira T. Three novel alternatively spliced isoforms of the human beta-site amyloid precursor protein cleaving enzyme (BACE) and their effect on amyloid beta-peptide production. Neurosci Lett. 2001;307:9–12. doi: 10.1016/s0304-3940(01)01912-7. [DOI] [PubMed] [Google Scholar]
- Capell A, Steiner H, Willem M, Kaiser H, Meyer C, Walter J, Lammich S, Multhaup G, Haass C. Maturation and pro-peptide cleavage of beta-secretase. J Biol Chem. 2000;275:30849–30854. doi: 10.1074/jbc.M003202200. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chretien M, Seidah NG. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem. 2001;276:10879–10887. doi: 10.1074/jbc.M009899200. [DOI] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]