Abstract
Thioredoxin and apurinic/apyrimidinic excision (APE)/ref-1 are important redox mediators in biochemical pathways that promote cell survival under adverse conditions including hypoxia and oxidative stress. For example, elevated levels occur surrounding vascular infarcts and protect from reperfusion injury. Because elevated thioredoxin or APE/ref-1 is also associated with resistance to certain forms of cancer treatment, we examined their tissue distribution in a series of 110 cervical carcinoma biopsies. Analysis was done using a quadruple fluorescence imaging technique, incorporating carbonic anhydrase IX (CAIX) immunofluorescence to outline hypoxic microregions and 4′,6-diamidino-2-phenylindole to localize nuclear staining of thioredoxin and APE/ref-1. A scanning autostage was used to image the entire tissue section. Thioredoxin and APE/ref-1 levels were expressed as the average pixel brightness in tumor tissue, subdivided based on CAIX and 4′,6-diamidino-2-phenylindole staining. Results showed that the nuclear and cytoplasmic levels of thioredoxin were similar, whereas APE/ref-1 expression was greater in nuclei. Neither of these markers was predictive of outcome in this series of patients treated with radical radiotherapy. Both proteins showed highly significant elevations in CAIX-positive regions compared to CAIX-negative regions, and there was a nonsignificant trend for this effect to be greater in adenocarcinomas compared to squamous cell carcinomas. Levels of APE/ref-1 decreased with increasing tumor grade, but the expression was similar in CAIX-positive regions of poorly differentiated tumors compared to moderately or well-differentiated tumors. Elevated expression of thioredoxin and APE/ref-1 might promote cancer cell survival in hypoxic microenvironments of cervical carcinomas.
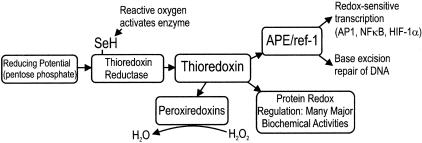
The redox-active protein thioredoxin plays a critical role in cellular antioxidant systems.1 This action is partially mediated via a family of enzymes called peroxiredoxins that are able to reduce hydrogen peroxide.2,3 During this process thioredoxin is oxidized, and the reduced protein is replenished under the action of the selenium-dependent thioredoxin reductase, using the pentose-phosphate pathway as the source of metabolic energy.4 Thioredoxin also efficiently reduces disulfides that can be formed as a result of oxidative stress.1,5 It is responsible for the maintenance of many important cellular processes that are dependent on protein thiol redox states, including ribonucleotide reduction and the suppression of apoptosis. One of these processes involves the regulation of gene transcription under the action of redox-sensitive transcription factors including nuclear factor-κB, AP1, hypoxia-inducible factor-1, and p53.6–9 This is via an interaction with the redox effector factor 1 (ref-1) that maintains cysteine residues in the appropriate redox state needed for the transcription factors to bind to DNA. Thioredoxin provides the reducing potential needed for ref-1 activity. Transcription factors that are dependent on the thioredoxin/ref-1 interaction are responsible for the activation of many genes that have the overall effect of promoting cell viability in response to adverse conditions including hypoxia and oxidative stress. These effects include production of cellular antioxidants and angiogenic factors, and p53-mediated cell-cycle arrest.7–9
The activities of thioredoxin and ref-1 are sensitive to environmental factors including hypoxia and oxidative stress.6,7,10,11 These responses include alterations in the nuclear to cytoplasmic distributions, increases in the total amounts of thioredoxin and ref-1 protein, and activating phosphorylation of ref-1.12 Furthermore, thioredoxin-dependent pathways are subject to metabolic regulation at the level of thioredoxin reductase, which becomes activated when reactive oxygen species interact with a critical selenocysteine residue in the regulatory C-terminal domain.4,6 Thus complex cellular processes act to regulate thioredoxin and ref-1 activities in response to hypoxia or oxidative stress, and these proteins are in turn able to effect many cytoprotective responses, as summarized in Figure 1. These responses have been extensively studied in ischemic reperfusion injury involving brain and myocardium, and in organ transplants. They protect organ function from hypoxia, and from additional damage because of oxidative stress that occurs during reoxygenation after a period of ischemia.
Figure 1.
Schematic of thioredoxin and APE/ref-1 biochemical pathways.
In addition to its redox effector function, ref-1 is also a key enzyme involved in DNA base excision repair,13 and for this reason is also known as APE (apurinic/apyrimidinic excision). Base excision repair is needed to maintain genomic integrity in the face of base damage that can occur naturally, for example as a result of oxidative stress, or after treatment with cytotoxic drugs or radiotherapy.14 Elevated levels of APE/ref-1 have been previously shown to occur in cervical carcinomas, and to correlate inversely with their intrinsic radiosensitivity.15,16
Considering the large number of actions that have the overall effect of maintaining cell viability, enhanced thioredoxin or APE/ref-1 activity could potentially be linked to biological aggression or treatment failure in cancers. This is supported by in vitro experiments, which have shown enhanced growth potential, suppression of apoptosis, and increased resistance to radiation or cytotoxic agents because of increased thioredoxin or APE/ref-1 levels.13,17,18 Several studies involving various types of human cancer have reported that both thioredoxin and APE/ref-1 levels are increased in cancers relative to normal tissues, and many have found a worse prognosis in patients with higher expression levels.19–24
Hypoxia occurs in human solid tumors and has important effects on cancer biology that are the subject of considerable current interest.25–27 In light of the increases in thioredoxin and APE/ref-1 activities that can occur in response to hypoxia and oxidative stress in vitro and in response to ischemia in normal tissues, we developed a fluorescence image analysis technique to examine the tissue and nuclear/cytoplasmic distributions of thioredoxin and APE/ref-1 in relation to the intrinsic hypoxia marker carbonic anhydrase IX (CAIX)25,28 in human cancer biopsies.
Materials and Methods
Patients
The patients had locally advanced cervical carcinoma (FIGO stages 1B to IIIB), and were enrolled in a translational research program funded by the Terry Fox Foundation examining the biology and clinical effects of tumor hypoxia. During examination under anesthesia, patients had measurements of tumor pO2 and interstitial fluid pressure made using an Eppendorf probe and a wick-in-needle technique, respectively,29,30 and had biopsies taken from the surface of the tumor according to institutional ethical guidelines. The biopsies were placed in vials containing OCT embedding medium, and stored in a liquid nitrogen freezer. The patients were treated using a standard protocol consisting of external beam radiotherapy to a planned dose of 45 to 50 Gy in 1.8- to 2-Gy daily fractions, followed by a single intracavitary brachytherapy application of 40 Gy, as previously described. In 1999 the treatment protocol was modified by the addition of cis-platin chemotherapy at a weekly dose of 40 mg/m2 during external beam radiotherapy after the publication of clinical trials showing improved patient outcome with combined modality therapy.31,32
Immunofluorescence Staining
Preliminary experiments done in our laboratory using human cervical carcinoma cell lines confirmed that all three of the monoclonal antibodies used gave single bands of the appropriate molecular size on Western blot analysis. All antibody dilutions used were based on preliminary dose-response curves done in-house to determine optimal signal to noise. Frozen sections were cut 5 μm thick, and allowed to air dry. They were then fixed in 4% methanol-free formaldehyde at 4°C for 20 minutes, and washed in phosphate-buffered saline (PBS). Sections were stained overnight with a mouse monoclonal anti-ref-1 (Novus) at 1/5000 dilution, washed in PBS twice, then treated with an Alexa 546-conjugated goat anti-mouse IgG2a isotype-specific secondary antibody (1/1000 dilution; Molecular Probes, Eugene, OR). After further washes, the sections were next stained with a mixture of mouse monoclonal antibodies to thioredoxin (1/300; PharMingen) and CAIX (M75, 1/50). The anti-CAIX used in this study was obtained from Dr. Adrian Harris, University of Oxford, Oxford, UK, and is now available from Bayer. Slides were incubated with primary antibodies for 1 hour, washed, and then treated with a mixture of Alexa 488-conjugated goat anti-mouse IgG1 (1/400, Molecular Probes) and Alexa 647-conjugated goat anti-mouse IgG2b (1/300, Molecular Probes) for 30 minutes. After two further washes in PBS, the sections were counterstained with the DNA dye 4′,6-diamidino-2-phenylindole (DAPI) at a concentration of 1 μg/ml for 5 minutes. Stained sections were stored at 4°C in the dark if not examined immediately.
Image Capture
Fluorescence microscopy was done with a 20 × 0.5 n.a. objective lens on an Olympus BX50 microscope, fitted with a cooled charge-coupled device camera (Quantix; Photometics Inc.). Composite 12-bit images of the entire cut surface were obtained using a Ludl XYZ motorized stage with MAC 5000 controller (Ludl, Hawthorne, NY) and a miniprogram running under the Elite image analysis package from Imaging Research Inc. (St. Catharines, Ontario, Canada). Each of the four colors was scanned separately, using appropriate filter cubes obtained from Chroma (Brattleboro, VT). The background because of tissue autofluorescence, reflected light, instrument noise, and nonspecific secondary antibody binding was determined for the thioredoxin and APE/ref-1 images by measuring the fluorescence intensities of parallel sections labeled with the secondary antibodies only. The mean value for each color was obtained from four ×20 fields selected at random within the viable tumor area.
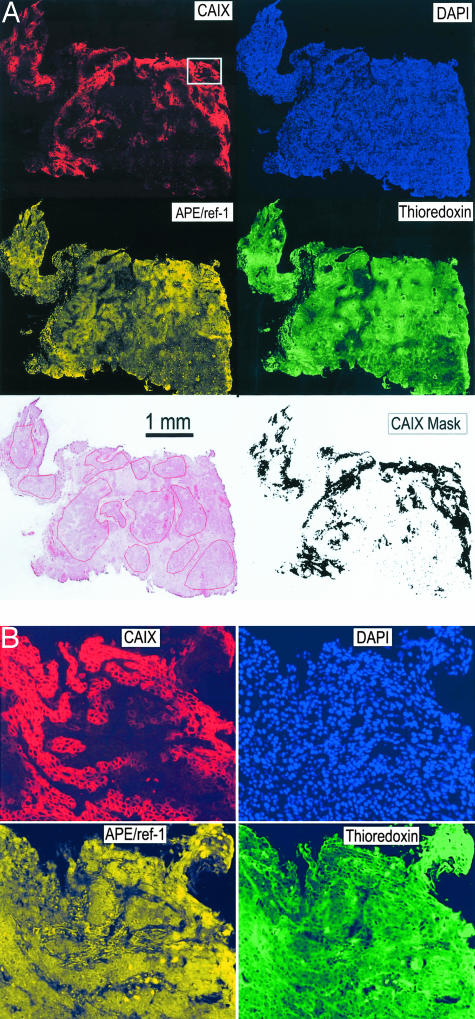
A serial section was stained using hematoxylin and eosin (H&E) and a digital image obtained using a 4000 dpi slide scanner (Polaroid). Using the original slide for fine detail, a pathologist (DB) outlined areas of viable tumor tissue on this image, blinded to information about the antibody-labeling patterns (Figure 2A).
Figure 2.
A: Representative composite, quadruple fluorescence images obtained from a patient sample. The images have been contrast-enhanced for visual inspection. The H&E image was obtained from a serial section using a slide scanner, and the areas of viable tumor tissue analyzed are drawn on this image. The CAIX gray-scale image was converted to a binary image, filled using a dilation/erosion technique, and used as a mask to identify hypoxic regions for measurements of thioredoxin and APE/ref-1 (bottom right). B: Higher power view of the area indicated by the rectangle on the CAIX image in A. Original pixel resolution.
Image Analysis
The digital image files, consisting typically of 50 to 100 million pixels, were written to DVD as TIFF files, and the quadruple overlaid images analyzed on a separate computer using software modules written in Interactive Data Language (IDL 5.6; Research Systems Inc., Boulder, CO). The images were first manually edited with reference to the H&E image so as to outline the areas of viable tumor tissue, and artifacts and noncellular regions were excluded. The extent of thioredoxin and APE/ref-1 staining in the tumor tissue was expressed as the mean integrated optical density (IOD), or average pixel brightness, corrected for background. Because high-affinity, specific antibodies were used at saturating concentrations, this value in principle correlates with the actual concentration of target protein in tissue.
To measure protein expression in hypoxic versus nonhypoxic tumor tissue, the CAIX images were first converted to binary images by thresholding each image at the same brightness area. Negative pixels within CAIX-positive tumor regions were filled in using a dilation/erosion technique, so as to create a mask outlining CAIX-positive areas including cytoplasm and nuclei that were not usually stained with anti-CAIX (Figure 2A). The IOD values for thioredoxin and ref-1 were then obtained for the CAIX-positive and CAIX-negative regions of tumor tissue.
The DAPI-stained images were then converted to binary images to create a mask of the nuclear area. The IOD values for thioredoxin and APE/ref-1 were obtained for tumor tissue in the DAPI-positive and DAPI-negative areas. Because neither of these proteins was found to be expressed in significant amounts in the cell membrane, the DAPI-negative staining was termed cytoplasmic. Finally, the nuclear and cytoplasmic levels of thioredoxin and APE/ref-1 were obtained for the CAIX-positive and CAIX-negative regions.
Statistical Analysis
The summary statistics and the routine examination of the data suggested that the levels of thioredoxin and APE/ref-1 did not follow a normal distribution. Therefore, whenever possible the analysis was done using nonparametric statistics tools. The comparisons between the nuclear and cytoplasmic values and between the levels in CAIX-positive and the in CAIX-negative areas were performed using the signed rank test. The comparisons between the two main types of histology (squamous cell carcinoma and adenocarcinoma) were performed using Wilcoxon rank-sum test, and for the comparisons between the three groups of tumor grade the Kruskal-Wallis test was used.
The main outcome was disease-free survival, defined as the time between diagnosis and the first event, any relapse, or death. To investigate the association between the thioredoxin and APE/ref-1 levels and outcome the Cox proportional hazards regression was used keeping the values as continuous. The linearity assumption was checked using the plot of the Martingale residuals against the levels of the factor analyzed. The illustration of the lack of association was done by dichotomizing the values at the median and drawing a Kaplan-Meier curve for each of the two groups.
Results
Expression of Thioredoxin and APE/Ref-1 in Relation to Histological Features
Biopsies were available from 123 patients, but 13 did not have viable tumor tissue present. Details of the remaining 110 cases are shown in Table 1. Of these, eight were excluded from analysis of outcome because of the presence of distant metastases at presentation (four cases), surgical treatment (two cases), small cell histology (one case), and patient not treated at our institution (one case). Of the 102 patients with follow-up data, 56 were treated with radiotherapy alone, and 46 with combined radiotherapy and cis-platin chemotherapy. Using human cervical carcinoma xenografts, we confirmed previous reports that CAIX staining is closely correlated with that of the nitroimidazole hypoxia probe EF5 and the hypoxia-inducible factor-1α protein expression (data not shown). Figure 2 shows a representative tissue section from one of the patient samples, illustrating the quadruple fluorescence image and the image processing used to obtain thioredoxin and ref-1 protein levels in CAIX-positive versus -negative tumor tissue, and their nuclear versus cytoplasmic distributions. Staining for CAIX showed this to be predominantly at the cell surface. As reported by others,25,28,33,34 positive CAIX staining was seen in almost all cases, but there was considerable intertumoral heterogeneity in the extent of staining. Unlike some authors, we did not see a worse prognosis for patients with high levels of CAIX expression.35
Table 1.
Patient Characteristics
| N | 110 |
| Age | |
| Median | 51 |
| Range | 23–80 |
| Histology | |
| Adenocarcinoma | 14 |
| Squamous cell carcinoma | 90 |
| Adenosquamous carcinoma | 5 |
| Other | 1 |
| Histological grade | |
| Well differentiated | 9 |
| Moderately differentiated | 55 |
| Poorly differentiated | 33 |
| FIGO | |
| IB/IIA | 30 |
| IIB | 41 |
| III | 36 |
| Pelvic nodal status | |
| Negative | 51 |
| Equivocal | 24 |
| Positive | 35 |
| Para-aortic nodal status | |
| Negative | 89 |
| Equivocal | 9 |
| Positive | 9 |
| Tumor size (cm) | |
| Median | 5 |
| Range | 2–15 |
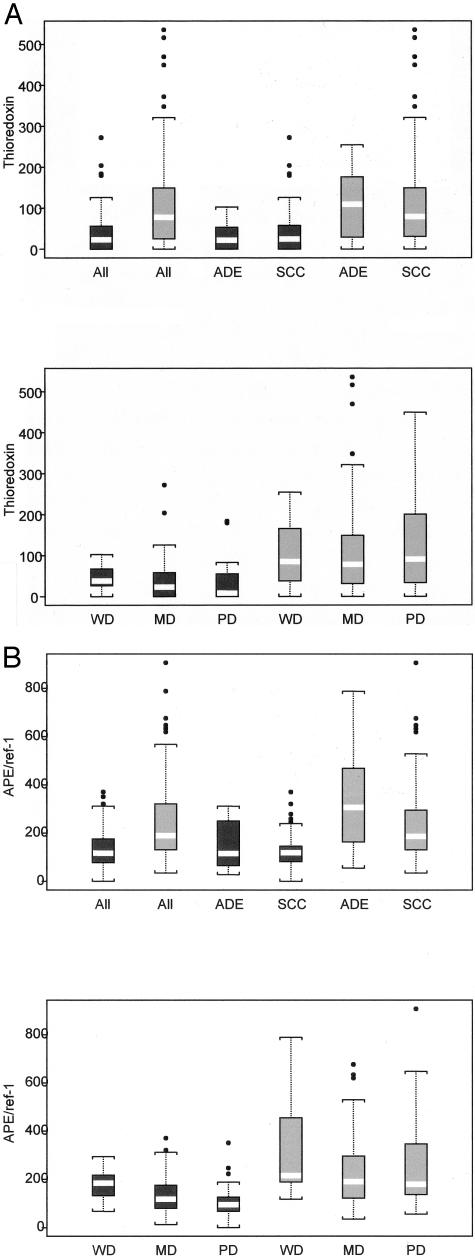
Visual inspection of the images showed considerable inter- and intratumoral heterogeneity in fluorescence staining for both thioredoxin and APE/ref-1. As previously reported by others,16,20–23 the proteins were expressed in the nucleus and cytoplasm, and the extent of distribution within the two compartments varied both within and between individual tumors. Table 2 summarizes the median IOD values for thioredoxin and APE/ref-1. Both proteins showed wide ranges of values, including cases in which the proteins were below the limit of detection. The median IOD values for thioredoxin in nuclei and cytoplasm were similar, whereas nuclear APE/ref-1 was higher than cytoplasmic (Table 2, P < 0.0001), consistent with previously published work. There was a highly significant increase in both thioredoxin and APE/ref-1 in CAIX-positive tissue (Table 2 and Figure 3; P < 0.0001 for both), and the magnitude of this effect was similar in nuclei and cytoplasm. The data suggest that the increases in thioredoxin and APE/ref-1 in association with the hypoxia marker CAIX were greater in adenocarcinomas compared to squamous cell carcinomas (Table 3 and Figure 3), although not statistically significant (P = 0.31 and P = 0.085, respectively).
Table 2.
Thioredoxin and APE/Ref-1 Expression (IOD)
| Median | Range | |
|---|---|---|
| Thioredoxin | 26.2 | 0–289 |
| Thioredoxin nuclear level | 29.5 | 0–308 |
| Thioredoxin cytoplasmic level | 22.5 | 0–289 |
| Thioredoxin in CAIX-negative | 23.1 | 0–273 |
| Thioredoxin in CAIX-positive | 77.7 | 0–536 |
| APE/ref-1 | 121.4 | 0–412 |
| APE/ref-1 nuclear level | 139.6 | 0–497 |
| APE/ref-1 cytoplasmic level | 99.5 | 0–391 |
| APE/ref-1 in CAIX-negative | 117.1 | 0–371 |
| APE/ref-1 in CAIX-positive | 189.7 | 34–907 |
Figure 3.
Box plots of thioredoxin (A) and APE/ref-1 (B) levels in CAIX-positive (gray boxes) and -negative (black boxes) areas of 110 patient biopsies. The white bars indicate the median values, and the bottom and top edges of the rectangles represent the first and third quartiles. The length of the lines drawn outside the boxes is 1.5 × the interquartile range. Tumors are subdivided based on adenocarcinomas (ADEs) versus squamous cell carcinomas (SCCs), and according to histological grade: well differentiated (WD), moderately differentiated (MD), and poorly differentiated (PD).
Table 3.
Thioredoxin and APE/Ref-1 Levels According to Histological Type
| Adenocarcinoma (median IOD) | SCC (median IOD) | P value | |
|---|---|---|---|
| Thioredoxin | 26.2 | 29.0 | 0.81 |
| Thioredoxin nuclear level | 38.4 | 31.3 | 0.72 |
| Thioredoxin in CAIX-negative | 22.2 | 24.8 | 0.76 |
| Thioredoxin in CAIX-positive | 109.3 | 79.4 | 0.62 |
| APE/ref-1 | 126.9 | 121.1 | 0.49 |
| APE/ref-1 nuclear level | 156.1 | 138.1 | 0.3 |
| APE/ref-1 in CAIX-negative | 115.3 | 118.7 | 0.65 |
| APE/ref-1 in CAIX-positive | 307.2 | 190.5 | 0.082 |
There were significant decreases in the levels of cytoplasmic and nuclear APE/ref-1 with increasing tumor grade (Table 4 and Figure 3; P < 0.01 and P < 0.003, respectively), and a nonsignificant trend for thioredoxin levels to decrease with increasing grade (Table 4 and Figure 3). These effects were only seen in the CAIX-negative regions of the tumors, whereas the APE/ref-1 and thioredoxin levels in the CAIX-positive regions of poorly differentiated tumors were similar to those found in well-differentiated tumors.
Table 4.
Thioredoxin and APE/Ref-1 Levels According to Tumor Grade
| Well differentiated (median IOD) | Moderately differentiated (median IOD) | Poorly differentiated (median IOD) | P value | |
|---|---|---|---|---|
| Thioredoxin | 41.9 | 30.6 | 15.5 | 0.34 |
| Thioredoxin nuclear level | 43.4 | 31.9 | 16.5 | 0.18 |
| Thioredoxin in CAIX-negative | 38.0 | 23.0 | 8.2 | 0.40 |
| Thioredoxin in CAIX-positive | 85.3 | 78.2 | 90.7 | 0.78 |
| APE/ref-1 | 185.5 | 120.6 | 96.5 | 0.0045 |
| APE/ref-1 nuclear level | 191.9 | 142.7 | 107.9 | 0.002 |
| APE/ref-1 in CAIX-negative | 184.7 | 117.8 | 95.0 | 0.007 |
| APE/ref-1 in CAIX-positive | 215.4 | 190.5 | 179.7 | 0.56 |
Expression of Thioredoxin and APE/Ref-1 in Relation to Clinical Features
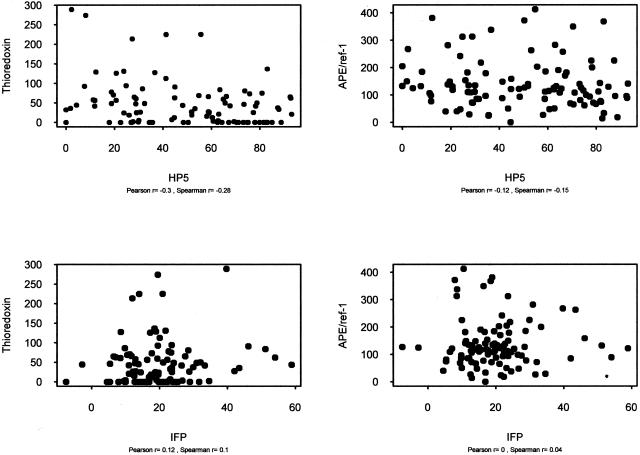
The associations between the expression levels of thioredoxin and APE/ref-1 with standard clinical features were investigated. Among the clinical factors examined were patient age, tumor size, and the presence of lymph node metastases at diagnosis (as detected by CT/MRI imaging). In addition, results were compared to direct tumor hypoxia measurements obtained using the Eppendorf probe, and to interstitial fluid pressure. There were no statistically significant correlations between these features and the protein expression levels (Figure 4). The expression of thioredoxin and APE/ref-1 was examined as the overall level as well as the nuclear level or the level in CAIX-positive and -negative areas.
Figure 4.
Bivariate plots of mean IOD values for thioredoxin (left) or APE/ref-1 (right) in relation to tumor hypoxia measurements (HP5, top) and interstitial fluid pressure (IFP, bottom). Tumor oxygenation was measured directly using an Eppendorf polarographic probe and the term HP5 signifies the proportion of measurements made within a tumor that were less than 5 mm/Hg. Interstitial fluid pressure was measured directly using a wick-in-needle technique, and the results are in mm/Hg.
Expression of Thioredoxin and APE/Ref-1 in Relation to Patient Outcome
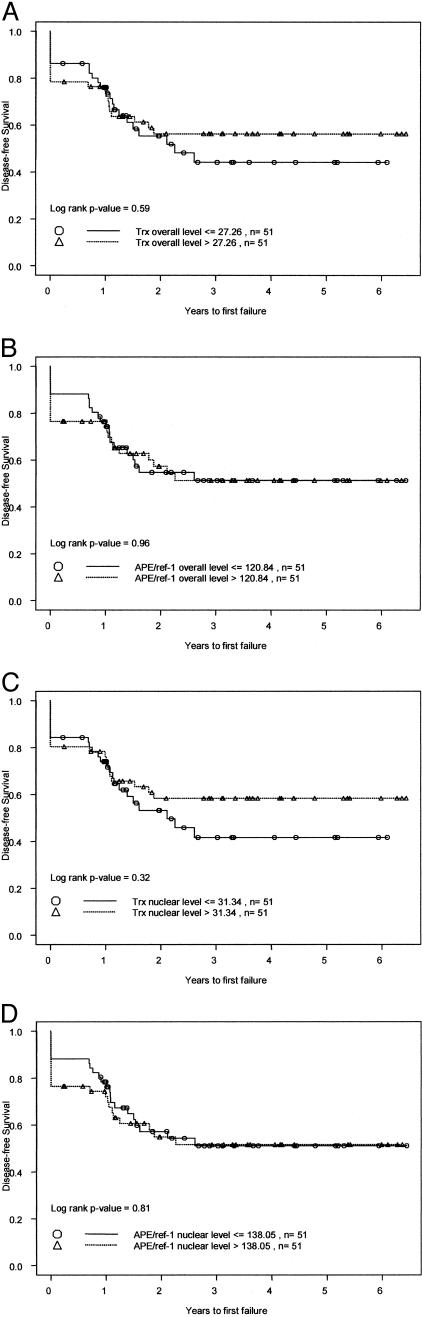
The expression levels of thioredoxin and APE/ref-1, including the values obtained from tumor areas defined by CAIX and DAPI staining, were dichotomized at the median values and the results plotted in relation to patient survival. As shown in Figure 5, none of the measures of thioredoxin or APE/ref-1 expression was correlated with patient outcome.
Figure 5.
Kaplan-Meier curves for survival of 102 evaluable patients, dichotomized at the median values for overall tumor thioredoxin mean IOD (A); overall tumor APE/ref-1 mean IOD (B); nuclear thioredoxin values (C); and nuclear APE/ref-1 values (D).
Discussion
This article describes the development and application of a wide-field, multicolor fluorescence image analysis technique able to map the spatial distributions and nuclear-to-cytoplasmic ratios of major antioxidant proteins in relation to tumor hypoxia. Important features of this technique are the use of fluorescence imaging, which provides quantitative data over a wide dynamic range as well as allowing the measurement of multiple markers on the same tissue section, and the use of a scanning autostage to image the entire section. The images were then edited in a blinded manner by a pathologist’s evaluation of a parallel H&E section. Thus to a large degree the data obtained are unbiased, quantitative, and address the problem of intratumoral heterogeneity to the extent that the entire tissue section is analyzed.
Hypoxia occurs in solid tumors because of their inefficient neovasculature, and the extent of hypoxia, determined by direct tumor pO2 measurements, is an important prognostic feature in a number of types of cancer including cervical carcinomas.26,27,29,36,37 Cells exposed to hypoxia in tissue culture are resistant to radiation because of well-characterized radiobiological principles, but it is now clear that the poor prognosis for cancer patients with hypoxic tumors cannot be explained entirely by radioresistance, because hypoxia is also associated with poor prognosis in surgically treated patients.38,39 Results from recent investigations using animal models as well as cancer patients indicate that hypoxic tumors show greater metastatic potential40,41 and are more resistant to apoptosis than better oxygenated tumors.42,43 Hence there is currently more general interest in the molecular mechanisms associated with hypoxia.
It is now recognized that tumor hypoxia can occur chronically because of the anatomical distance from the nearest blood vessel or intermittently because of the instability of blood flow through the tumor vascular bed.25,26 Similar to reperfusion injury seen surrounding vascular infarcts involving, for example, myocardium or brain, intermittent hypoxia can produce oxidative stress that is injurious to cells but is capable of eliciting an antioxidant response. Increased expression levels of several important antioxidant elements including thioredoxin and APE/ref-1 have been extensively documented in normal tissues in response to ischemic injury and protect from organ damage because of oxidative stress.44–48 Similar responses in tumors are predicted to promote cancer cell survival within hypoxic microenvironments and might also enhance resistance to some anti-cancer treatments.
Research into the biology of tumor hypoxia has been facilitated by the development of probes that can be applied to histological sections, allowing the spatial distribution of tumor hypoxia to be mapped in relation to other cellular markers. CAIX seems to be a useful intrinsic marker of tumor hypoxia that is highly expressed on the cell membrane after transcriptional activation by hypoxia-inducible factor-1.25,28
Using CAIX expression as a surrogate marker for tumor hypoxia, we found highly significant increases in thioredoxin and APE/ref-1 expression within the hypoxic microenvironments of invasive cervical carcinomas. This effect is reminiscent of the findings previously reported in association with vascular infarcts involving normal tissues, and is consistent with the idea that thioredoxin and APE/ref-1 act in a coordinated manner to promote cell survival as illustrated in Figure 1. Unlike previous work showing accumulation of thioredoxin in the nuclei of cells exposed to hypoxia in tissue culture,49,50 we did not observe consistent alterations in the relative amounts of thioredoxin and APE/ref-1 in the nuclear and cytoplasmic compartments within CAIX-positive versus CAIX-negative regions of the cervix cancer biopsies.
Although we often observed greater thioredoxin and APE/ref-1 expression in cancer tissue relative to adjacent dysplastic epithelium, as has been reported by others, the expression levels of APE/ref-1 showed a progressive decrease with increasing tumor grade, with a similar trend for thioredoxin. Interestingly, there was no decrease in thioredoxin or APE/ref-1 seen in the CAIX-positive regions of poorly differentiated relative to moderate or well-differentiated tumors, suggesting that the mechanisms up-regulating these proteins in response to hypoxia remain functional with the progressive loss of histological grade.
Although elevated levels of thioredoxin and APE/ref-1 are associated with enhanced tumor cell survival mechanisms, and have been found in other series to predict worse prognosis for some types of cancer, in the present series we did not see associations between the levels of either protein and patient outcome. However, the predominant anti-cancer effect of radiotherapy in solid tumors is believed to be the formation of DNA double-strand breaks, against which thioredoxin and APE/ref-1 probably exert little protection. No statistically significant prognostic effect was seen in the subset of 46 patients who also received cis-platin chemotherapy, but the follow-up for these patients is relatively short. It will be of interest to examine a series of chemotherapy-treated cancer patients in which thioredoxin and APE/ref-1 are predicted to exert significant cytoprotective effects.
Given that tumor hypoxia promotes cell death, and that tumor cells are known to adapt to hypoxia so as to maintain their viability in the hostile environment, the large increases in thioredoxin and APE/ref-1 that occur in response to hypoxia in cervical carcinomas suggest an adaptive response that promotes survival under conditions of hypoxic stress. Novel treatments that disrupt thioredoxin and APE/ref-1 might therefore show selective anti-cancer effects in hypoxic regions of cancers, and this is currently under investigation in our laboratory.
In summary, using a sophisticated image analysis technique we show large increases in the expression levels of thioredoxin and APE/ref-1 in relation to tumor hypoxia in invasive cervical carcinomas. This effect was more apparent in poorly differentiated tumors, because of a relatively large decrease in thioredoxin and APE/ref-1 expression in nonhypoxic regions. This work illustrates the analytical potential of wide-field multicolor imaging to examine complex biological processes using histological sections.
Acknowledgments
We thank James Ho and Kelvin So, research immunohistochemistry facility, Ontario Cancer Institute, for the immunofluorescence staining done by using techniques originally developed by Dr. Hans Kristian Haugland.
Footnotes
Address reprint requests to Dr. David W. Hedley, Department of Medical Oncology and Hematology, Princess Margaret Hospital, 610 University Ave., Toronto, Ontario M5G 2M9, Canada. E-mail: david.hedley@uhn.on.ca.
Supported by the National Cancer Institute of Canada using funds raised by the Terry Fox Run.
References
- Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- Butterfield LH, Merino A, Golub SH, Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signal. 1999;1:385–402. doi: 10.1089/ars.1999.1.4-385. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Robin HJ, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- Flaherty DM, Monick MM, Hunninghake GW. AP endonucleases and the many functions of Ref-1. Am J Respir Cell Mol Biol. 2001;25:664–667. doi: 10.1165/ajrcmb.25.6.f220. [DOI] [PubMed] [Google Scholar]
- Grosch S, Fritz G, Kaina B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 1998;58:4410–4416. [PubMed] [Google Scholar]
- Hsieh MM, Hegde V, Kelley MR, Deutsch WA. Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 2001;29:3116–3122. doi: 10.1093/nar/29.14.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 2002;8:3008–3018. [PubMed] [Google Scholar]
- Herring CJ, West CM, Wilks DP, Davidson SE, Hunter RD, Berry P, Forster G, MacKinnon J, Rafferty JA, Elder RH, Hendry JH, Margison GP. Levels of the DNA repair enzyme human apurinic/apyrimidinic endonuclease (APE1, APEX, Ref-1) are associated with the intrinsic radiosensitivity of cervical cancers. Br J Cancer. 1998;78:1128–1133. doi: 10.1038/bjc.1998.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl M, Oberhuber G, Pichlbauer EG, Obermair A, Birner P, Kelley MR. DNA repair-redox enzyme apurinic endonuclease in cervical cancer: evaluation of redox control of HIF-1alpha and prognostic significance. Int J Oncol. 2001;19:799–802. doi: 10.3892/ijo.19.4.799. [DOI] [PubMed] [Google Scholar]
- Robertson KA, Bullock HA, Xu Y, Tritt R, Zimmerman E, Ulbright TM, Foster RS, Einhorn LH, Kelley MR. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 2001;61:2220–2225. [PubMed] [Google Scholar]
- Sasada T, Iwata S, Sato N, Kitaoka Y, Hirota K, Nakamura K, Nishiyama A, Taniguchi Y, Takabayashi A, Yodoi J. Redox control of resistance to cis-diamminedichloroplatinum (II) (CDDP): protective effect of human thioredoxin against CDDP-induced cytotoxicity. J Clin Invest. 1996;97:2268–2276. doi: 10.1172/JCI118668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin Cancer Res. 2001;7:3510–3518. [PubMed] [Google Scholar]
- Kahlos K, Soini Y, Saily M, Koistinen P, Kakko S, Paakko P, Holmgren A, Kinnula VL. Up-regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma. Int J Cancer. 2001;95:198–204. doi: 10.1002/1097-0215(20010520)95:3<198::aid-ijc1034>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kakolyris S, Giatromanolaki A, Koukourakis M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC, Harris AL. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res. 2001;7:3087–3091. [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Kakolyris S, Sivridis E, Georgoulias V, Funtzilas G, Hickson ID, Gatter KC, Harris AL. Nuclear expression of human apurinic/apyrimidinic endonuclease (HAP1/Ref-1) in head-and-neck cancer is associated with resistance to chemoradiotherapy and poor outcome. Int J Radiat Oncol Biol Phys. 2001;50:27–36. doi: 10.1016/s0360-3016(00)01561-3. [DOI] [PubMed] [Google Scholar]
- Moore DH, Michael H, Tritt R, Parsons SH, Kelley MR. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers. Clin Cancer Res. 2000;6:602–609. [PubMed] [Google Scholar]
- Soini Y, Kahlos K, Napankangas U, Kaarteenaho-Wiik R, Saily M, Koistinen P, Paaakko P, Holmgren A, Kinnula VL. Widespread expression of thioredoxin and thioredoxin reductase in non-small cell lung carcinoma. Clin Cancer Res. 2001;7:1750–1757. [PubMed] [Google Scholar]
- Bussink J, Kaanders JH, van der Kogel AJ. Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol. 2003;67:3–15. doi: 10.1016/s0167-8140(03)00011-2. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, Hill RP. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- Milosevic M, Fyles A, Hedley D, Pintilie M, Levin W, Manchul L, Hill R. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res. 2001;61:6400–6405. [PubMed] [Google Scholar]
- Thomas GM. Improved treatment for cervical cancer—concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–1200. doi: 10.1056/NEJM199904153401509. [DOI] [PubMed] [Google Scholar]
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- Airley RE, Loncaster J, Raleigh JA, Harris AL, Davidson SE, Hunter RD, West CM, Stratford IJ. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int J Cancer. 2003;104:85–91. doi: 10.1002/ijc.10904. [DOI] [PubMed] [Google Scholar]
- Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- Hedley D, Pintilie M, Woo J, Morrison A, Birle D, Fyles A, Milosevic M, Hill R. Carbonic anhydrase IX expression, hypoxia, and prognosis in patients with uterine cervical carcinomas. Clin Cancer Res. 2003;9:5666–5674. [PubMed] [Google Scholar]
- Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7:928–934. [PubMed] [Google Scholar]
- Rofstad EK, Sundfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354–359. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8908. [PubMed] [Google Scholar]
- Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M, Chiu RK, Theys J, Buijsen J, Lambin P. Modulation of cell death in the tumor microenvironment. Semin Radiat Oncol. 2003;13:31–41. doi: 10.1053/srao.2003.50004. [DOI] [PubMed] [Google Scholar]
- Andoh T, Chock PB, Chiueh CC. Preconditioning-mediated neuroprotection: role of nitric oxide, cGMP, and new protein expression. Ann NY Acad Sci. 2002;962:1–7. doi: 10.1111/j.1749-6632.2002.tb04051.x. [DOI] [PubMed] [Google Scholar]
- Araki M, Nanri H, Ejima K, Murasato Y, Fujiwara T, Nakashima Y, Ikeda M. Antioxidant function of the mitochondrial protein SP-22 in the cardiovascular system. J Biol Chem. 1999;274:2271–2278. doi: 10.1074/jbc.274.4.2271. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Bottiger B, Hossmann KA. Expression of nuclear redox factor ref-1 in the rat hippocampus following global ischemia induced by cardiac arrest. Brain Res Mol Brain Res. 1997;52:194–200. doi: 10.1016/s0169-328x(97)00237-4. [DOI] [PubMed] [Google Scholar]
- Hattori I, Takagi Y, Nozaki K, Kondo N, Bai J, Nakamura H, Hashimoto N, Yodoi J. Hypoxia-ischemia induces thioredoxin expression and nitrotyrosine formation in new-born rat brain. Redox Rep. 2002;7:256–259. doi: 10.1179/135100002125000749. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci USA. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]