Abstract
Local bone erosion and systemic bone loss are hallmarks of rheumatoid arthritis and cause progressive disability. Tumor necrosis factor (TNF) is a key mediator of arthritis and acts catabolically on bone by stimulating bone resorption and inhibiting bone formation. We hypothesized that the concerted action of anti-TNF, which reduces inflammation and parathyroid hormone (PTH), which stimulates bone formation, or osteoprotegerin (OPG), which blocks bone resorption and could lead to repair of local bone erosions and reversal of systemic bone loss. To test this, human TNF-transgenic mice with established erosive arthritis and systemic bone loss were treated with PTH, OPG, and anti-TNF, alone or in combination. Local bone erosions almost fully regressed, on combined treatment with anti-TNF and PTH and/or OPG, suggesting repair of inflammatory skeletal lesions. In contrast, OPG and anti-TNF alone led to arrest of bone erosions but did not achieve repair. Treatment with PTH alone had no influence on the progression of bone erosions. Local bone erosions all showed signs of new bone formation such as the presence of osteoblasts, osteoid formation, and mineralization. Furthermore, systemic bone loss was completely reversed on combined treatment and this effect was mediated by osteoblast stimulation and osteoclast blockade. In summary, we conclude that local joint destruction and systemic inflammatory bone loss because of TNF can regress and that repair requires a combined approach by reducing inflammation, blocking bone resorption, or stimulating bone formation.
Chronic inflammation and bone loss are closely linked. This is impressively illustrated in rheumatoid arthritis (RA), a chronic inflammatory disease of the joints, which is characterized by synovial inflammation and cartilage and bone destruction. Tumor necrosis factor (TNF) is a central inflammatory mediator of RA and its therapeutic inhibition leads to dramatic improvement in signs and symptoms of RA.1–4 In addition, TNF has profound effects on bone: overexpression of TNF is not only involved in local bone erosion, but also induces generalized bone loss.5,6 Therefore, TNF can be considered as an important link between chronic inflammation and bone loss.
At a cellular level, TNF affects bone by its potential to inhibit osteoblast function and its ability to stimulate osteoclast formation.7,8 The role of TNF as a potent stimulator of osteoclasts directed attention to the role of osteoclasts in local bone erosion of arthritis. It has been shown that inflammatory bone erosions in human RA and experimental arthritis contain abundant amounts of osteoclasts.9,10 Furthermore, osteoclasts play an essential role in the development of such lesions, as unequivocally demonstrated in arthritis models, which lack osteoclasts. Thus, mice lacking essential mediators of osteoclastogenesis, such as c-Fos or receptor-activator of nuclear factor-κB ligand (RANKL), develop synovial inflammation, but no bone erosions on induction of arthritis by immune complexes or TNF overexpression, respectively.11,12
Therapeutic interventions to slow or arrest local bone erosions are important because joint destruction is a major determinant of functional disability in human RA.13 Therapies that inhibit bone erosions target molecules involved in the differentiation and activation of osteoclasts.3,4,14 Inhibition of TNF and interleukin-1 in human RA by antibodies, soluble receptors, or receptor antagonists are important in currently used clinical examples,2–4,14 and experimental therapy with osteoprotegerin (OPG), a decoy receptor of RANKL, slows or even completely blocks bone erosion in animal models of arthritis.10,15,16
Although there is occasional radiological evidence for healing of erosions, it is unclear, however, whether bone erosions, once they have been formed, can regress and be restored by normal bone.17–19 Such healing may require osteoblasts, which is the cell competent to build up new bone. Blockade of osteoclasts slows or, at best, blocks bone erosion, but may not be sufficient in repair of erosions, which likely requires the synthesis and mineralization of bone matrix. Thus, repair of local bone erosions may require new approaches, such as combination of agents that lead to 1) reduction of the inflammatory process of arthritis that fuels bone erosion, 2) blockade of the resorptive capacity of arthritis by inhibiting osteoclasts, and 3) formation of new bone by stimulation of osteoblasts.
We addressed this question using human tumor necrosis factor transgenic (hTNFtg) mice that develop erosive arthritis.5 Treatment was initiated long after local bone erosions and systemic bone loss had already developed. Following the above hypothesis, we applied a TNF-blocker (for interference with the inflammatory response), OPG (to directly affect osteoclast differentiation and activation), and parathyroid hormone (PTH) to stimulate osteoblast activity as single therapies or in combination.
Materials and Methods
Animals and Treatments
The heterozygous Tg197 transgenic mice have been described previously.5 Briefly, mice were made transgenic for the human TNF-α gene construct with an unmodified 5′promotor region, but allowing deregulated human TNF-α gene expression in vivo. Preparation of the hTNF gene constructs used has been described in detail.5 These mice develop a chronic inflammatory and destructive arthritis within 5 weeks after birth. All mice were bred and maintained in a CBAxC57BL/6 genetic background. Mice were fed a standard diet with water ad libitum. All animal procedures were approved by the local ethical committee.
A total number of 68 hTNFtg mice were assessed for clinical signs of arthritis (see below) up to 10 weeks after birth, ie, 5 weeks after initiation of the arthritic process. At this time point mice were matched according to clinical signs of arthritis into six groups as follows: group 1 (n = 12) was sacrificed at the beginning of treatment (week 10) and served as baseline control. Group 2 (n = 8) was left untreated up to week 14 and served as positive control. Group 3 (n = 8) received 10 mg/kg of OPG intraperitoneally every third day. Group 4 (n = 8) received 80 μg/kg of PTH 5 days per week subcutaneously. Group 5 (n = 8) received 10 mg/kg of infliximab (anti-TNF) intraperitoneally every third day. Groups 6, 7, and 8 (each n = 4) received a combination of OPG+PTH, anti-TNF+OPG, and anti-TNF+PTH, respectively, applied as indicated above. Group 9 (n = 12) received anti-TNF+OPG+PTH (combination). Two independent experiments were performed. All mice were littermate controlled. Treatment was applied between weeks 10 and 14 and mice were then sacrificed. Doses of OPG, PTH, and anti-TNF were based on those successfully applied in previous studies on blocking bone resorption, stimulation of bone formation, and anti-inflammatory activity, respectively.10,20,21
Reagents
Fc-OPG and PTH were kindly provided by Amgen (Thousand Oaks, CA). Fc-OPG is a truncated form of human OPG (amino acids 22 to 194) fused to the Fc domain of human IgG1 and produced in Escherichia coli.22 PTH is the 1 to 34 amino terminal peptide of PTH, which contains all functional properties of the full-length hormone. The chimeric monoclonal anti-TNF antibody (infliximab) was from Centocor (Leiden, The Netherlands).
Cell Culture
Cultures of osteoblasts and osteoclasts were performed as previously described.8,23 In brief, bone marrow was removed from femoral bones by flushing with α-minimal essential medium. For culturing osteoblasts, cells were plated at 104 cells/cm2 and cultivated in α-minimal essential medium supplemented with 10% fetal calf serum, 5 mmol/L glycerophosphate, and 100 μg/ml ascorbic acid. After 10 days, histochemical staining of alkaline phosphatase (AP) was performed. Briefly, cells were fixed with neutral-buffered formalin for 20 minutes at 4°C, incubated with substrate solution at room temperature for 15 minutes, rinsed five times with distilled water, and photographed (F601; Nikon, Tokyo, Japan). AP-substrate was prepared from solution A (8 mg naphtol-AS-TR phosphate/300 μl N,N′-dimethylformamide; both from Sigma, St. Louis, MO) and solution B (24 mg fast blue BB salt/30 ml of 100 mmol/L Tris-HCl, pH 9.6). Both solutions were mixed, 10 mg of MgCl2 was added, and used immediately after sterile filtration. Enzymatic activity was determined in cell lysates that were solubilized with 0.1% Triton X-100. Aliquots (20 μl) of each sample were incubated with 100 μl of AP substrate buffer (100 mmol/L diethanolamine, 150 mmol/L NaCl, 2 mmol/L MgCl2, p-nitrophenylphosphate at 2.5 μg/ml) for 5 to 30 minutes at room temperature. Total cellular protein was determined using the bicinchoninic method (Pierce Chemical Co., Rockford, IL). AP activity is expressed as U/mg protein with 1 U defined as enzymatic activity that released 1 nmol of p-nitrophenol/minute. Bone nodule formation was assessed after 21 days of culture using alizarin red staining (Sigma).
For osteoclast culture, bone marrow mononuclear cells were isolated by Ficoll gradient centrifugation. Cells were plated and cultivated in α-minimal essential medium supplemented with 20 ng/ml of M-CSF (R&D Diagnostics, Minneapolis, MN) and 50 ng/ml RANKL (R&D). Tartrate-resistant acid phosphatase (TRAP) staining was performed after 5 days of culture using a leukocyte acid phosphatase kit (Sigma).
Clinical Assessment of Arthritis
Clinical evaluation was started 6 weeks after birth and performed weekly up to week 14. Arthritis was evaluated in a blinded manner as described previously.11 Briefly, joint swelling was examined using a clinical score graded from 0 to 3 (0, no swelling; 1, mild-; 2, moderate-; or 3, severe swelling of toes and ankle). In addition, grip strength of each paw was analyzed on a wire of 3 mm in diameter using a score from 0 to −4 (0, normal grip strength; −1, mildly-; −2, moderately-; −3, severely reduced grip strength; −4, no grip strength at all). The last evaluation was performed 14 weeks after birth. Animals were sacrificed by cervical dislocation, the blood was withdrawn by heart puncture and the paws of all animals were dislocated for histology.
Joint Histology
Paws were fixed in 70% ethanol and embedded undecalcified in methylmetacrylate (K-Plast; Medim, Buseck, Germany). After polymerization sections of 3 μm were made on a Jung microtome (Jung, Heidelberg, Germany), deplastinated, and stained by Goldner trichrome, von Kossa, and toluidine blue. Bone erosions were assessed at a magnification of ×200 using a Zeiss microscope (Axioskop 2; Zeiss, Marburg, Germany) and quantitated by OsteoMeasure program 2.2 (Osteometrics, Atlanta, GA). Numbers of osteoclasts (N.Oc) and osteoblasts (N.Ob) within inflammatory lesions were identified by their staining and morphology using histomorphometry of Goldner trichrome-stained sections. Osteoid-covered surface was also assessed using Goldner trichrome-stained sections and was related to area of erosions. Furthermore, the fraction of osteoid area per osteoblast (O.Ar./OB) was calculated. For dynamic histomorphometry mice were injected with calcein-3 1 day before sacrificing. Undecalcified paw sections were then analyzed for fluorescence labeling at 495 nm.
Densitometry
As previously described,6 bone mineral density (BMD) was quantified in ethanol-fixed, undecalcified bones by peripheral quantitative computed tomography (pQCT) analysis (XCT-960 mol/L tomograph; Norland Medical Systems, Ft. Atkinson, WI; XMICE 5.2 statistical software; Stratec, Pforzheim, Germany). BMD was measured in the tibia for the total zone (ie, the entire bone), the trabecular (ie, the inner 20% of bone mass), and the cortical zone. For the total and trabecular zones, values from two 1.25-mm sections, located 0.5 mm apart, taken 1.5 mm distal to the proximal articular surface were averaged. In contrast, BMD in the cortical zone of the tibia was measured in a 1.25-mm section located 4.0 mm distal to the articular surface. The total and trabecular regions were differentiated from soft tissue using a separation threshold of 1500, whereas for delineating the cortical zone a threshold of 2000 was used.
Bone Histomorphometry
Left tibial bones were fixed in 70% ethanol and embedded undecalcified in methylmetacrylate as described above and stained by Goldner trichrome. Histomorphometry was performed at a magnification of ×200 using a Zeiss microscope (Axioskop 2) and the OsteoMeasure program 2.2 (Osteometrics). The following parameters were measured according to international standards:24 fraction of bone volume of total sample volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), numbers of osteoclasts per bone perimeter (N. OC/B.Pm), and numbers of osteoblasts per bone perimeter (N. OB/B. Pm). In addition, the width of primary spongiosa (Wi.PS) was assessed.
Serum Parameters of Bone Metabolism and hTNF Levels
Serum levels of deoxypyridinoline (DPD) crosslinks were measured by enzyme immunoassay (Quidel, San Diego, CA) after previous hydrolysis of serum samples according to the manufacturer’s recommendations. Osteocalcin was measured by immunoradiometric assay (Immutopics, San Clemente, CA). Serum levels of human TNF were assessed by enzyme immunoassay (R&D).
Statistical Analysis
Data are shown as means ± SEM. Group mean values were compared by two-tailed Student’s t-test.
Results
hTNFtg Mice Show Increased Formation of Osteoclasts but Decreased Formation of Osteoblasts
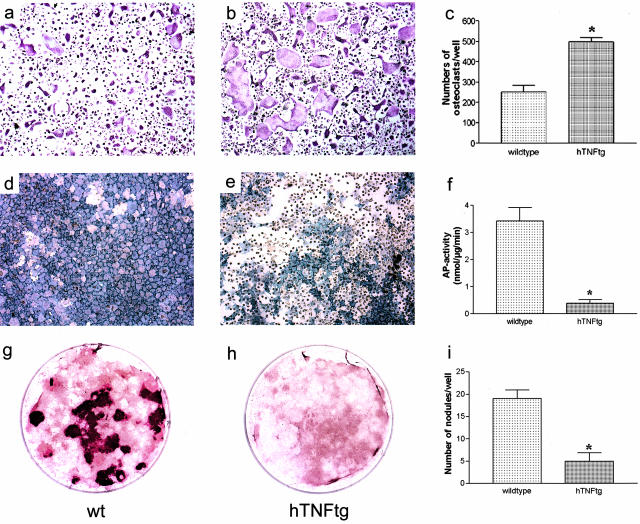
To address the potential of hTNFtg mice to generate osteoclasts and osteoblasts, we investigated differentiation of bone marrow mononuclear cells into osteoclasts and of bone marrow stromal cells into osteoblasts. Formation of TRAP-positive cells on stimulation with M-CSF and RANKL was increased in hTNFtg mice compared to wild-type controls (Figure 1; a to c). Furthermore, size of osteoclasts was significantly (P < 0.01) increased in hTNFtg mice compared to wild-type controls (mean ± SEM, 0.0021 mm2 ± 0.0001 versus 0.0014 mm2 ± 0.00007) indicating increased osteoclastogenesis. In contrast, AP production (Figure 1; d to f) and bone nodule formation (Figure 1; g to i) by bone marrow stromal cells of hTNFtg mice was decreased, suggesting impaired osteoblast differentiation. These data not only confirmed the basis underlying the destructive process namely osteoclast hyperactivity, but also provide an explanation for the lack of repair process in TNF-mediated arthritis, namely reduced osteoblast activity.
Figure 1.
hTNFtg mice show increased ex vivo osteoclastogenesis but decreased osteoblast formation. Bone marrow mononuclear cells derived from wild-type (a) and hTNFtg (b) mice were cultured in the presence of 20 ng/ml of M-CSF and 50 ng/ml of RANKL for 5 days. c: TRAP staining revealed significantly increased formation of osteoclasts in hTNFtg mice compared to wild-type (wt) mice. Bone marrow stromal cells derived from wild-type (d, g) and hTNFtg (e, h) mice were cultivated in the presence of 5 mmol/L of glycerophosphate and 100 μg/ml of ascorbic acid. AP staining (d, e), measurement of AP enzyme activity (f), as well as Alizarin Red S-stained bone nodule formation (g, h, i) revealed significantly impaired formation of osteoblasts in hTNFtg mice compared to wild-type mice. Data are mean ± SEM. Asterisks indicate significant (P < 0.01) difference. Original magnifications: ×100 (a, b, d, e); ×4 (g, h).
Joint Inflammation Is Only Affected by Anti-TNF but Not OPG or PTH
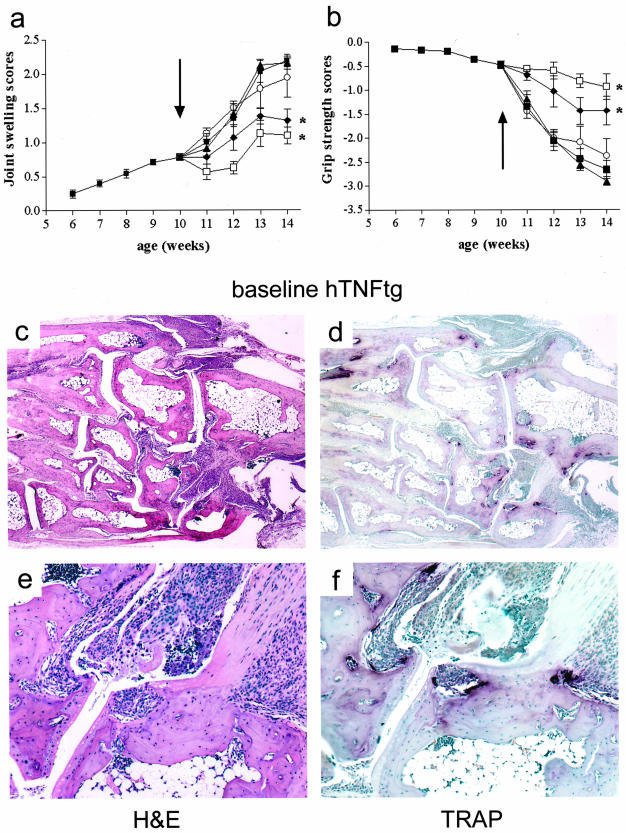
At baseline (week 10), all hTNFtg mice had already undergone 5 weeks of active arthritis as seen clinically (Figure 2, a and b) and histologically (Figure 2, c and e). Moreover, local bone erosions containing numerous osteoclasts were detectable (Figure 2, d and f). At this time point treatment was initiated and continued until week 14. Although hTNFtg control mice, as well as mice treated with OPG or PTH alone (Figure 2, a and b) or together (data not shown) progressed in paw swelling and continued to lose grip strength, mice treated with anti-TNF showed a significantly improved course of disease (Figure 2, a and b). Anti-TNF along with OPG (data not shown) or PTH (data not shown) or OPG+PTH (Figure 2, a and b), was equally effective, suggesting that PTH and OPG, alone or in combination, did not significantly alter the efficacy of anti-TNF on joint inflammation.
Figure 2.
Joint inflammation is only affected by anti-TNF but not OPG or PTH. Clinical course of arthritis indicated by joint swelling (a) and grip strength (b) was assessed in hTNFtg mice between weeks 6 and 10 (pretreatment period, black squares) and after randomization (weeks 10 to 14) into the following treatment groups: untreated (black squares), OPG (black triangles), PTH (white circles), anti-TNF (black diamonds), and OPG+PTH+anti-TNF (white squares). Asterisks indicate a significant (P < 0.01) decrease in joint swelling and increase in grip strength in anti-TNF-treated mice as well as in OPG+PTH+anti-TNF-treated mice compared to untreated hTNFtg controls (black squares). Arrow indicates start of treatment. hTNFtg mice develop progressive arthritis between weeks 5 and 10 as indicated by joint swelling (a) and loss of grip strength (b). c, d, e, f: At baseline assessment (week 10) hTNFtg mice show not only signs of synovial inflammation, but also subchondral bone erosions as indicated by H&E staining (c, e) and TRAP-positive multinucleated cells representing osteoclasts as shown by TRAP-stained (d, f) histological sections of hind paws (tarsal area). Original magnifications: ×25 (c, d); ×100 (e, f).
Anti-TNF in Combination with OPG or PTH Leads to Repair of Bone Erosions
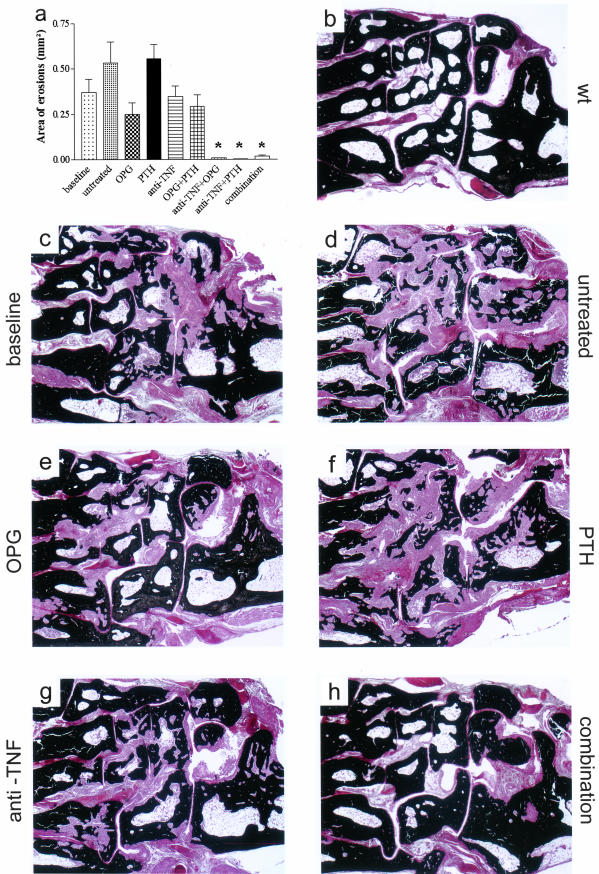
As illustrated before (Figure 2, d and f), histological analyses revealed bone erosions in all hTNFtg mice at baseline, confirming a severely destructive disease state at the onset of treatment. The natural course of disease showed a further increase in bone damage, as evident from mice left untreated from week 10 to week 14 (Figure 3, a, c, and d). This is depicted in Figure 3, c and d, which reveals significant loss of juxtaarticular bone (stained black by van Kossa labeling) between baseline and week 14. Treatment with PTH alone (Figure 3, a and f) did not affect this progression, showing a similar degree of bone erosion as found in untreated mice. In contrast, OPG, anti-TNF, and OPG+PTH led to an arrest of bone erosion, indicated by an amount of bone damage similar to that observed in the baseline control group (Figure 3; a, e, and g). However, mice treated with anti-TNF along with OPG, PTH, or OPG+PTH, respectively, had virtually no bone erosions (Figure 3, a and h). This indicated that bone erosions already present at the onset of therapy at week 10 had regressed and that, therefore, local repair processes had been induced by the therapeutic regimens.
Figure 3.
Repair of bone erosions on treatment with anti-TNF combined with OPG and/or PTH. a: Mean areas of local bone erosions revealed erosive disease in hTNFtg mice at baseline (week 10); progression of erosions in untreated and PTH-treated mice; and arrest in OPG, OPG+PTH, and anti-TNF-treated mice during the following 4 weeks. Mice treated with anti-TNF in combination with OPG, PTH, or OPG+PTH showed almost complete repair of bone erosions during this period. Asterisks indicate significant (P < 0.01) differences between treatment groups and baseline. b–h: Degree of bone destruction is illustrated by von Kossa-stained histological sections (tarsal areas) of wild-type (wt) mice (b), baseline (10 weeks) hTNFtg mice (c), as well as in untreated (d), osteoprotegerin (OPG) (e), parathyroid hormone (PTH) (f), infliximab (anti-TNF) (g), and OPG+PTH+anti-TNF (combination)-treated hTNFtg mice (h). Note the fragmentation of bone (black) by synovial inflammatory tissue (red). Original magnifications, ×25.
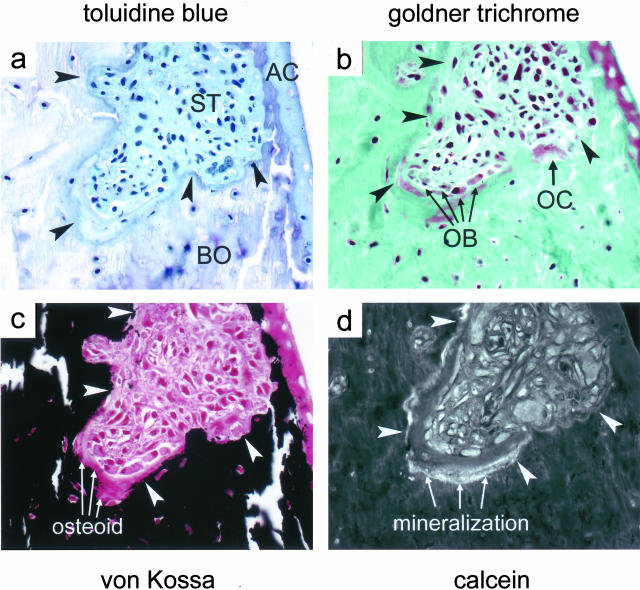
On the basis of our observations that bone erosions can regress, we searched for evidence of bone formation within erosions. As depicted in serial sections (Figure 4), bone erosions showed all signs of new bone formation in vicinity to osteoclast-mediated bone resorption. Figure 4a shows subchondral bone erosion stained with toluidine blue. The area below the articular cartilage, which normally contains subchondral bone, is filled with invasive inflammatory tissue, while articular cartilage shows signs of proteoglycan loss. Goldner-trichrome staining of the same lesion shows that this subchondral bone erosion consists of two subcompartments with different bone metabolism. (Figure 4b). Whereas one compartment (adjacent to cartilage) shows a resorption lacuna with an osteoclast, the other subcompartment shows numerous osteoblasts and osteoid formation, but no osteoclasts. This is further illustrated by von Kossa staining of the same lesion. The area between osteoblasts and mineralized bone is filled by unmineralized osteoid (Figure 4c). In contrast, mineralized bone is directly attached by inflammatory tissue at the area where osteoclasts form a resorption lacuna. Finally, as illustrated by calcein fluorescence, mineralization and thus completion of new bone formation occurs only in areas of bone erosions that are covered by osteoblasts and osteoid, but not next to osteoclast-mediated resorption lacunae (Figure 4d). These observations suggest that eroded bone maintained capacity to recover.
Figure 4.
Local bone erosions show all signs of functional bone turnover. In serial sections of a single subchondral bone erosion (arrowheads) signs of bone resorption as well as new bone formation are detectable. a: Staining with toluidine blue reveals proteoglycan loss, indicated by destaining of the AC, articular cartilage; ST, inflammatory synovial tissue; and BO, erosion of subchondral bone. b: Goldner-trichrome staining reveals multinucleated osteoclasts (OCs) as well as cubic mononuclear osteoblasts (OBs) at the front of erosions. c: Von Kossa staining shows an osteoid seam between osteoblasts and bone surface. d: Calcein labeling indicates a mineralization front underneath the osteoid seam. Original magnifications, ×200.
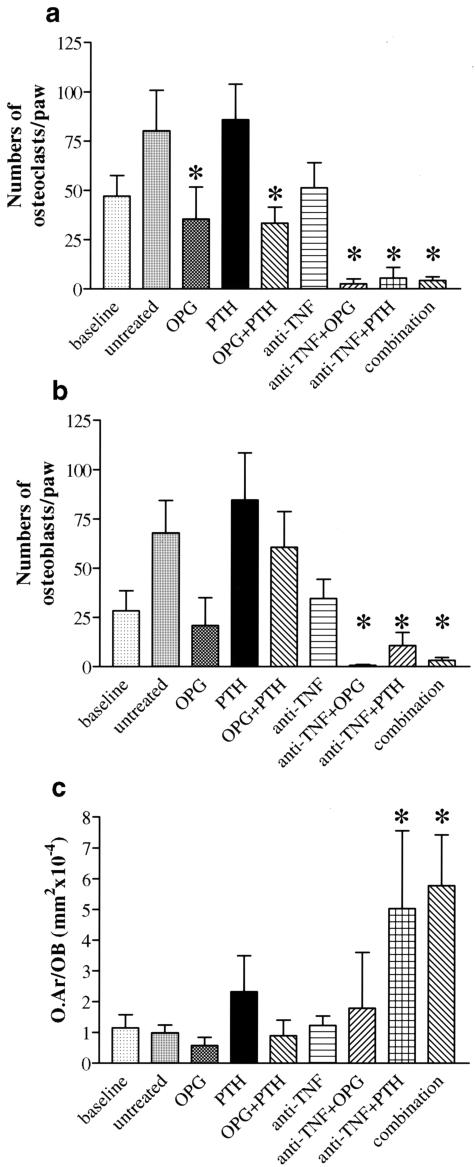
OPG Reduces Osteoclasts and PTH Increases Osteoblasts and Osteoid Formation in Local Bone Erosions
To test whether the observed therapeutic effects on bone erosions (Figure 3) are related to changes in local bone turnover, we quantitatively assessed osteoclast and osteoblast numbers as well as osteoid formation by osteoblasts within erosions. Osteoclast numbers increased from baseline in untreated hTNFtg mice, reflecting the progression of erosion size (Figure 5a). In parallel, however, osteoblast numbers also increased, indicating skeletal responsiveness to increased bone resorption (Figure 5b). The numbers of osteoclasts and osteoblasts were low in mice subjected to anti-TNF along with either OPG, PTH, or OPG+PTH, which was because of almost complete regression of bone erosions. Thus, osteoclast and osteoblast numbers were closely linked. To evaluate the potential of osteoblasts for repair of bone erosions, we quantified the amount of osteoid produced by osteoblasts within bone erosions as an indicator for new bone formation (osteoid area per osteoblast). Osteoid production was increased on therapy with PTH and particularly on treatment with anti-TNF+PTH or triple (combination) therapy, which suggests that osteoblast stimulation by PTH effectively leads to new bone formation within arthritic bone erosions (Figure 5c).
Figure 5.
OPG reduces osteoclast numbers and PTH increases osteoblast numbers and formation of osteoid in local bone erosions. Quantitative assessment of osteoclast (a) and osteoblast (b) numbers as well as osteoid formation (c) within local bone erosions in baseline (10 weeks) hTNFtg mice, untreated, osteoprotegerin (OPG), parathyroid hormone (PTH), infliximab (anti-TNF), OPG+PTH, anti-TNF+OPG, anti-TNF+PTH, and anti-TNF+OPG+PTH (combination)-treated hTNFtg mice. Asterisks indicate a significant (P < 0.05) difference to untreated hTNFtg control mice. O.Ar/OB (mm2 × 10−4) = area of osteoid per osteoblast.
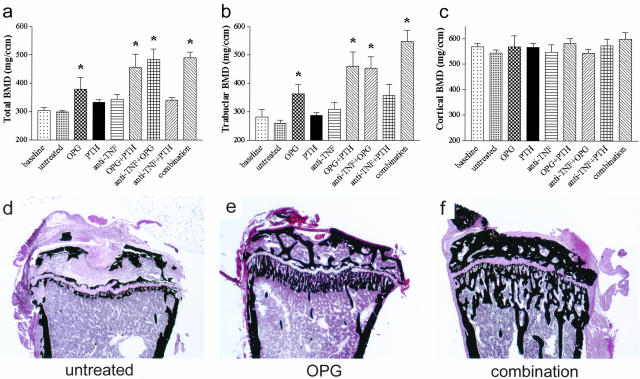
Osteoclast Blockade Is Essential for Reversal of Systemic Bone Loss Mediated by TNF
We have previously reported that hTNFtg mice, similar to patients with RA, suffer from systemic bone loss.6 To address the question if the local changes described above were accompanied by systemic bone loss we evaluated the effects of the different treatment regimens on prolonged osteoporosis. TNF-blockade, when started at week 10, had no effect on BMD, neither total (Figure 6a) nor trabecular (Figure 6b), compared to untreated hTNFtg control mice (Figure 6, a and b). Likewise PTH alone or in combination with anti-TNF had no effect. In contrast, a significant increase of BMD was observed with OPG therapy (Figure 6; a, b, and e). However, there was an even more dramatic increase in BMD when OPG was combined anti-TNF, PTH, or anti-TNF+PTH (Figure 6; a, b, and f). Gain in bone density was because of an increase of trabecular bone, whereas cortical bone remained almost unaffected (Figure 6, b and c). These data together indicate that even longstanding inflammatory bone loss is reversible by osteoclast blockade with OPG, or more effectively, if osteoblasts are stimulated or inflammation is blocked simultaneously. Histological correlates of changes in bone density are depicted in Figure 6, d, e, and f, showing reappearance of trabecular bone in the metaphyses of mice treated with OPG (Figure 6e) and, most prominently with combination therapy (Figure 6f).
Figure 6.
Osteoclast blockade is essential for reversal of systemic bone loss mediated by TNF. Quantitative computed tomography of hTNFtg mice showing total (a), trabecular (b), and cortical (c) BMD. hTNFtg mice of the following groups were analyzed: baseline control (week 10); untreated control (week 14); and osteoprotegerin (OPG), parathyroid hormone (PTH), infliximab (anti-TNF), OPG+PTH, anti-TNF+OPG, anti-TNF+PTH, or anti-TNF+OPG+PTH (combination) treatment each from weeks 10 to 14. A significant increase (asterisk; P < 0.05) of total as well as trabecular BMD was observed after treatment with OPG alone or in combination with PTH, anti-TNF, or anti-TNF+PTH, whereas cortical BMD remained unchanged. Histological correlates are depicted in sections of tibial heads stained by von Kossa (d–f). Untreated hTNFtg mice show severe osteoporosis (d). Trabecular bone reappears in the metaphyses of mice receiving OPG alone (e) and, more pronounced, after combination therapy (f). Original magnifications, ×25.
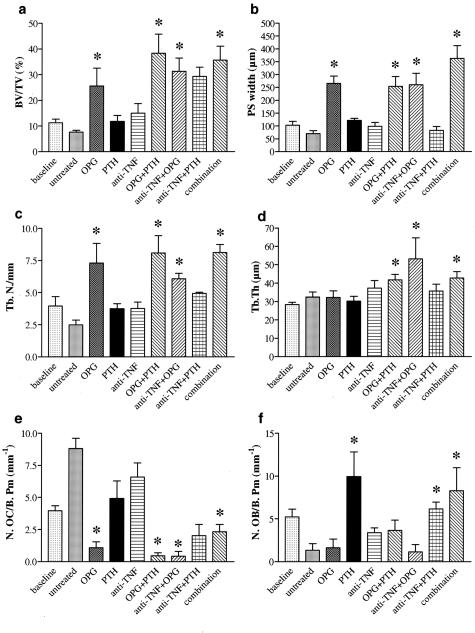
Combinations of PTH, OPG, and Anti-TNF Increase Trabecular Bone Mass by Reducing the Number of Osteoclasts and Stimulating Osteoblasts
We next specified the morphological basis of changes in bone density in hTNFtg mice. Bone volume increased significantly on treatment of hTNFtg mice with OPG alone or most prominently together with anti-TNF, PTH, or anti-TNF+PTH (Figure 7a). Similar results were obtained in respect to the width of primary spongiosa and trabecular number (Figure 7, b and c), whereas trabecular thickness only increased in mice treated with combination therapy (Figure 7d). Taken together these data indicate a recovering of bone microarchitecture after OPG. Changes in bone mass were accompanied by decreased numbers of osteoclasts in therapies containing OPG as well as increased osteoblast numbers in therapies, which included PTH (Figure 7, e and f). Thus, combination treatment is highly effective because of synergy of osteoclast blockade with osteoblast stimulation.
Figure 7.
Combinations of PTH, OPG, and anti-TNF increase trabecular bone mass by reducing the number of osteoclasts and stimulating osteoblasts. Bone histomorphometry was performed in baseline (week 10) hTNFtg mice as well as in untreated-, osteoprotegerin (OPG)-, parathyroid hormone (PTH)-, infliximab (anti-TNF)-, OPG+PTH-, anti-TNF+OPG-, anti-TNF+PTH-, or anti-TNF+OPG+PTH (combination)-treated hTNFtg mice. The fraction of bone volume of total sample volume (BV/TV) (a), width of the primary spongiosa (PS) (b), and trabecular number (Tb.N) (c) were significantly increased in mice treated with OPG alone or along with PTH, anti-TNF, or anti-TNF+PTH. Trabecular thickness (Tb.Th) (d) was increased in the same groups except OPG alone. This was accompanied by a decreased number of osteoclasts per bone perimeter (N.OC/B.Pm) (e). In contrast, the number of osteoblasts per bone perimeter (N.OB/B. Pm) was increased in hTNFtg mice receiving PTH alone or along with anti-TNF or anti-TNF+OPG (f). Asterisks indicate significant (P < 0.05) difference to untreated hTNFtg controls.
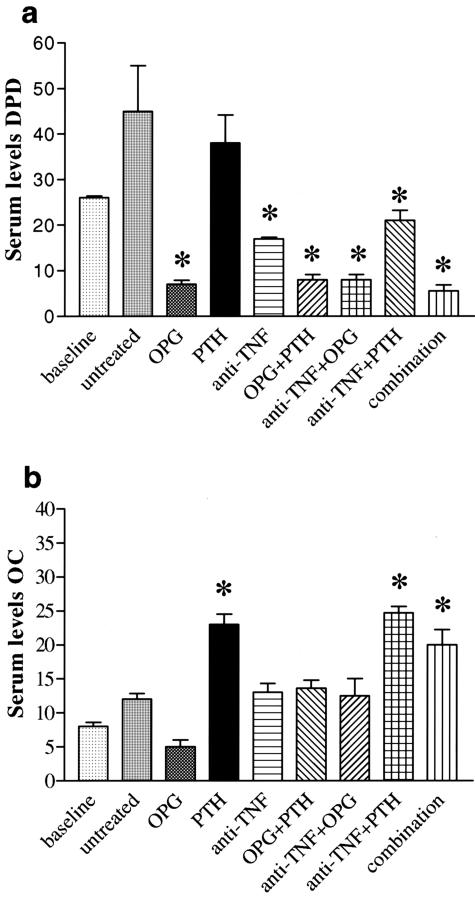
Finally, to address whether the effects observed in densitometry and histomorphometry correlate with systemic changes of bone metabolism in hTNFtg mice, we analyzed serum levels of DPD and osteocalcin. Levels of DPD, a marker for bone resorption, were significantly decreased after treatment with anti-TNF alone or in combination with PTH. Treatments containing OPG, however were even more effective, indicating successful blockade of systemic osteoclast activity (Figure 8a). Conversely, PTH, alone or in combination, significantly increased levels of osteocalcin, a marker of osteoblast activation and bone formation (Figure 8b). In contrast, serum levels of human TNF were not altered by treatments (data not shown). Thus, the effects of OPG and PTH on bone are reflected by profound changes in systemic bone metabolism. The combination of osteoclast blockade with osteoblast stimulation has a positive net effect on bone mineralization, in addition to that on local bone erosions.
Figure 8.
Decrease of systemic bone resorption by OPG and increase of systemic bone formation by PTH. Serum levels of DPD (a) and osteocalcin (OC) (b) were measured in baseline (10 weeks) hTNFtg mice, as well as in untreated, osteoprotegerin (OPG)-, parathyroid hormone (PTH)-, infliximab (anti-TNF)-, OPG+PTH-, anti-TNF+OPG-, anti-TNF+PTH-, or anti-TNF+OPG+PTH (combination)-treated hTNFtg mice. DPD levels, a marker of bone resorption most prominently decreased after treatments containing OPG, indicating effective systemic blockade of osteoclast activity. Conversely, treatments containing PTH increased OC levels, a marker of osteoblast activation and bone formation. Asterisks indicate a significant (P < 0.05) difference to untreated hTNFtg control mice.
Discussion
Local and generalized bone loss represent a serious problem for patients with RA. Local bone erosion is a hallmark of RA patients and is considered as irreversible damage associated with increased morbidity and loss of joint function.13 Generalized bone loss in RA patients is a consequence of high inflammatory disease activity, functional impairment, and corticosteroid use, and leads to an increased risk of osteoporotic fracture.25,26 Repair of bone damage, rather than slowing or at best blocking structural damage should therefore be an ambitious aim of anti-rheumatic therapy. Previous data from in vivo experiments and clinical studies have shown that pure blockade of TNF is not sufficient to repair or even just arrest local and systemic inflammatory bone loss,2–4,6,10,21 suggesting that a more profound intervention in the bone remodeling process is necessary to achieve this ambitious goal. This study demonstrates that therapeutic regulation of bone metabolism, as achieved with the combination of PTH or OPG, dramatically improves structural joint damage when combined with an agent that controls the chronic inflammatory process, such as anti-TNF antibody. Moreover, combination therapy also led to a dramatic increase of systemic bone mass in mice suffering from longstanding inflammatory disease and severe osteoporosis, ultimately reversing it to the physiological levels of healthy mice.
The rationale for combining TNF-blockade with osteoclast inhibition or osteoblast stimulation to overcome TNF-mediated bone damage comes from in vitro observations on the effects of TNF on osteoclasts and osteoblasts.7 TNF is a potent stimulator of osteoclast differentiation and activation, which is illustrated by increased formation of osteoclasts from mononuclear precursors.27 The effect is mediated by induction of RANKL expression and a synergistic effect with RANKL on nuclear factor-κB and stress-activated/c-Jun NH(2)-terminal protein kinase, two signaling pathways essential for osteoclastogenesis.8 With respect to the osteoblast lineage, however, TNF is considered a negative regulator that inhibits osteoblast differentiation and function. Decreased in vitro differentiation of bone marrow stromal cells into osteoblasts supports this idea.7,28 Several mechanisms have been described, which explain the regulatory effect of TNF on osteoblasts. TNF inhibits IGF-1 and the transcription factor Runx-2, which are involved in the differentiation process of osteoblasts.29 In addition, TNF inhibits the synthesis of matrix proteins, such as osteocalcin30 and AP,31,32 which are considered to be indicators for osteoblast function. This is illustrated by TNF-transgenic mice, which show a negative net effect of TNF on bone resulting in local bone erosions and generalized bone loss.5,6 Thus, a combined intervention, which slows bone resorption and induces bone formation, seems to be a highly effective strategy to achieve repair of TNF-induced bone damage.
OPG can be considered to be a feasible means of blocking TNF-mediated bone resorption, because it interferes with RANKL, which is essential for TNF-mediated osteoclastogenesis.33 OPG, given in pharmacological doses, effectively blocks bone resorption and increases systemic bone mass.34 This was because of a predominant effect of OPG on trabecular bone, which exhibits a more active turnover than cortical bone.6 OPG also slows local bone erosion in arthritic joints, indicating that TNF and RANKL induce formation of osteoclasts in the synovial membrane, which ultimately erode subchondral bone.10,15,35 Indeed, the fact that in our model OPG treatment results in a positive net effect on bone, suggests that the primary effect of OPG is an effective blockade of bone resorption. However, an inhibitory effect of OPG on osteoblasts also could be observed, attributing to recent experimental findings, which identified RANKL as stimulator of osteoblasts and supporting observations of blunted bone formation after OPG treatment in laboratory animals and humans.6,34,36 In contrast, PTH primarily stimulates osteoblasts and its effects on osteoclasts are of indirect nature.37 PTH, when given cyclically, stimulates bone formation and leads to increased bone mass.38 Because TNF inhibits PTH-mediated effects on osteoblasts,39 therapeutic substitution of PTH seems to be a feasible strategy to stimulate bone formation in the presence of TNF.
Most importantly, local bone erosions regressed after TNF blockade combined with osteoclast inhibition or osteoblast stimulation. Local erosions are mediated by osteoclasts derived from mononuclear precursor cells within the inflamed synovial tissue. Current therapies for RA, such as blockade of TNF can slow or, at best, arrest progression of local bone erosion.2,3,40 Experimental blockade of osteoclasts by OPG or bisphosphonates also slows this process, but does not induce the repair of such lesions. So far there has been no convincing evidence of repair mechanisms occurring in local bone erosions.10,15,35 Recently, cells expressing PTH receptor have been described within bone erosions and these cells are attached to bone at the erosion front.41 Because of their localization these cells most likely represent osteoblasts, although PTH receptors are expressed by synovial lining cells and fibroblast-like cells within the synovial pannus as well.42 In this study we demonstrate that local bone erosions are an area of dynamic bone turnover. Apart from resorption lacunae formed by osteoclasts, all signs of bone formation are found within such lesions. These include 1) cells with the typical morphological characteristics of osteoblasts localized between erosion front and bone, 2) osteoid formation next to osteoblast at the interface of pannus and bone, and 3) mineralization of newly formed bone. Although there is the potential of repair mechanisms within local bone erosion, bone formation can obviously not completely compensate the erosive process. This may have two reasons: firstly, factors synthesized by synovial inflammatory tissue such as TNF may hamper osteoblast activity and, secondly, excessive osteoclast activity may prevent the recruitment of osteoblasts and thus remodeling of resorption lacunae. Thus, a combined therapeutic approach, which reduces the inflammatory activity of synovial tissue and blocks either osteoclast activity or stimulates bone formation, is necessary to repair local bone erosions in vivo.
Whether these findings of hTNFtg mice are also valid for human RA remains to be determined. Strengths of this model are 1) its chronic progressive course leading to a high burden of disease, 2) a well-defined pathomechanism based on cytokine overexpression, and 3) an excellent possibility to study the local and systemic skeletal effects of inflammation. Although hTNFtg mice represent an attractive model for human RA its limitations should be considered: especially, the model is not an autoimmune driven model of arthritis and hence does not depend on autoantibodies and T cells. Because human RA exhibits autoimmune features, similar studies in autoimmune arthritis models may thus allow gaining further insight in the mechanisms of bone repair during inflammation. Nevertheless, these data not only describe repair mechanisms of inflammatory bone damage but also highlight differences between local and systemic bone loss. Thus, local bone erosion results from a tight interplay between inflammation and unfavorable bone-remodeling processes. Reversal is only possible if these two mechanisms are targeted simultaneously. In fact, both of them appear equally important because neither pure blockade of inflammation nor even complex interference with bone remodeling is sufficient to achieve regression of local bone erosion. In contrast, systemic bone loss is much more a result of osteoclast-mediated bone resorption, which is supported by the fact that OPG per se increases systemic bone mass and by the high efficacy of all OPG-containing combination therapies.
In summary, our data show that joint destruction and generalized inflammatory bone loss are reversible. Bone repair requires therapeutic intervention, which not only controls inflammation but also shifts the balance of osteoclasts to osteoblasts in favor of the latter. These findings open new perspectives in the treatment of RA by demonstrating that bone damage is of reversible nature rather than end-stage damage associated with loss of joint function.
Footnotes
Address reprint requests to Georg Schett, M.D., Department of Internal Medicine III, Division of Rheumatology, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria. E-mail: georg.schett@akh-wien.ac.at.
Supported by the Austrian Science Fund (START prize to G.S.); the Center of Molecular Medicine of the Austrian Federal Ministry for Education, Science, and Culture and the City of Vienna; and the Austrian National Bank (project 8715 to G.S.).
References
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St. Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Redlich K, Hayer S, Zwerina J, Bolon B, Dunstan CR, Görtz B, Schulz A, Bergmeister H, Kollias G, Steiner G, Smolen JS. Osteoprotegerin protects from generalized bone loss in TNF-transgenic mice. Arthritis Rheum. 2003;48:2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast, Akrad M, Lang S, Türk B, Pietschmann P, Woloszczuk W, Kollias G, Steiner G, Smolen J, Schett G. Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46:785–792. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit AR, Ji H, von Stechow D, Müller R, Goldring SR, Choi Y, Benoist C, Gravallese EM. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, Hieke K. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 2000;39:122–132. doi: 10.1093/rheumatology/39.2.122. [DOI] [PubMed] [Google Scholar]
- Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, Watt I, Williams B, Aitchison R, McCabe D, Musikic P. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- Rau R, Wassenberg S, Herborn G, Perschel WT, Freitag G. Identification of radiologic healing phenomena in patients with rheumatoid arthritis. J Rheumatol. 2001;28:2608–2615. [PubMed] [Google Scholar]
- Sharp JT, Van Der Heijde D, Boers M, Boonen A, Bruynesteyn K, Emery P, Genant HK, Herborn G, Jurik A, Lassere M, McQueen F, Ostergaard M, Peterfy C, Rau R, Strand V, Wassenberg S, Weissman B. Repair of erosions in rheumatoid arthritis does occur. Results from 2 studies by the OMERACT Subcommittee on Healing of Erosions. J Rheumatol. 2003;30:1102–1107. [PubMed] [Google Scholar]
- Sokka T, Hannonen P. Healing of erosions in rheumatoid arthritis. Ann Rheum Dis. 2000;59:647–649. doi: 10.1136/ard.59.8.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenuik PJ, Capparelli C, Morony S, Adamu S, Shimamoto G, Shen V, Lacey DL, Dunstan CR. OPG and PTH-(1-34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology. 2001;142:4295–4304. doi: 10.1210/endo.142.10.8437. [DOI] [PubMed] [Google Scholar]
- Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, Dunstan CR, Kollias G, Steiner G, Smolen JS, Schett G: Isolated and combined inhibition of TNF-, IL-1 and RANKL-pathways in TNF-induced arthritis: effects on synovial inflammation, bone erosion and cartilage destruction. Arthritis Rheum (in press) [DOI] [PubMed] [Google Scholar]
- Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL, Dunstan CR. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha PTH, PTHrP, and 1,25(OH)2D3. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- Halstead LR, Scott MJ, Rifas L, Avioli LV. Characterization of osteoblast-like cells from normal adult rat femoral trabecular bone. Calcif Tissue Int. 1992;50:93–95. doi: 10.1007/BF00297304. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Woolf AD. Osteoporosis in rheumatoid arthritis—the clinical viewpoint. Br J Rheumatol. 1991;30:82–84. doi: 10.1093/rheumatology/30.2.82. [DOI] [PubMed] [Google Scholar]
- Spector TD, Hall GM, McCloskey EV, Kanis JA. Risk of vertebral fracture in women with rheumatoid arthritis. BMJ. 1993;306:558. doi: 10.1136/bmj.306.6877.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Hauschka PV. Effects of interleukin-1 beta and tumor necrosis factor-alpha on osteoblastic expression of osteocalcin and mineralized extracellular matrix in vitro. Inflammation. 1992;16:587–601. doi: 10.1007/BF00919342. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Shingu M, Koshihara Y, Nobunaga M. Effects of cytokines on alkaline phosphatase and osteocalcin production, calcification and calcium release by human osteoblastic cells. Br J Rheumatol. 1994;33:224–230. doi: 10.1093/rheumatology/33.3.224. [DOI] [PubMed] [Google Scholar]
- Nakase T, Takaoka K, Masuhara K, Shimizu K, Yoshikawa H, Ochi T. Interleukin-1 beta enhances and tumor necrosis factor-alpha inhibits bone morphogenetic protein-2-induced alkaline phosphatase activity in MC3T3–E1 osteoblastic cells. Bone. 1997;21:17–21. doi: 10.1016/s8756-3282(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw GL, Hughes TM, Hill D, Pattison W, Campbell P, Sanders S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density [see comments]. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001;16:348–360. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- Romas E, Bakharevski O, Hards DK, Kartsogiannis V, Quinn JM, Ryan PF, Martin TJ, Gillespie MT. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000;43:821–826. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lam J, Ross FP, Teitelbaum SL. RANK ligand stimulates anabolic bone formation. J Bone Miner Res. 2001;16(Suppl 1):1053. [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Hanevold CD, Yamaguchi DT, Jordan SC. Tumor necrosis factor alpha modulates parathyroid hormone action in UMR-106-01 osteoblastic cells. J Bone Miner Res. 1993;8:1191–1200. doi: 10.1002/jbmr.5650081006. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Genant HK, Watt I, Cobby M, Bresnihan B, Aitchison R, McCabe D. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Funk JL, Cordaro LA, Wei H, Benjamin JB, Yocum DE. Synovium as a source of increased amino-terminal parathyroid hormone-related protein expression in rheumatoid arthritis. A possible role for locally produced parathyroid hormone-related protein in the pathogenesis of rheumatoid arthritis. J Clin Invest. 1998;101:1362–1371. doi: 10.1172/JCI484. [DOI] [PMC free article] [PubMed] [Google Scholar]